Influence of Beam Angle on Normal Tissue Complication Probability of Knowledge-Based Head and Neck Cancer Proton Planning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
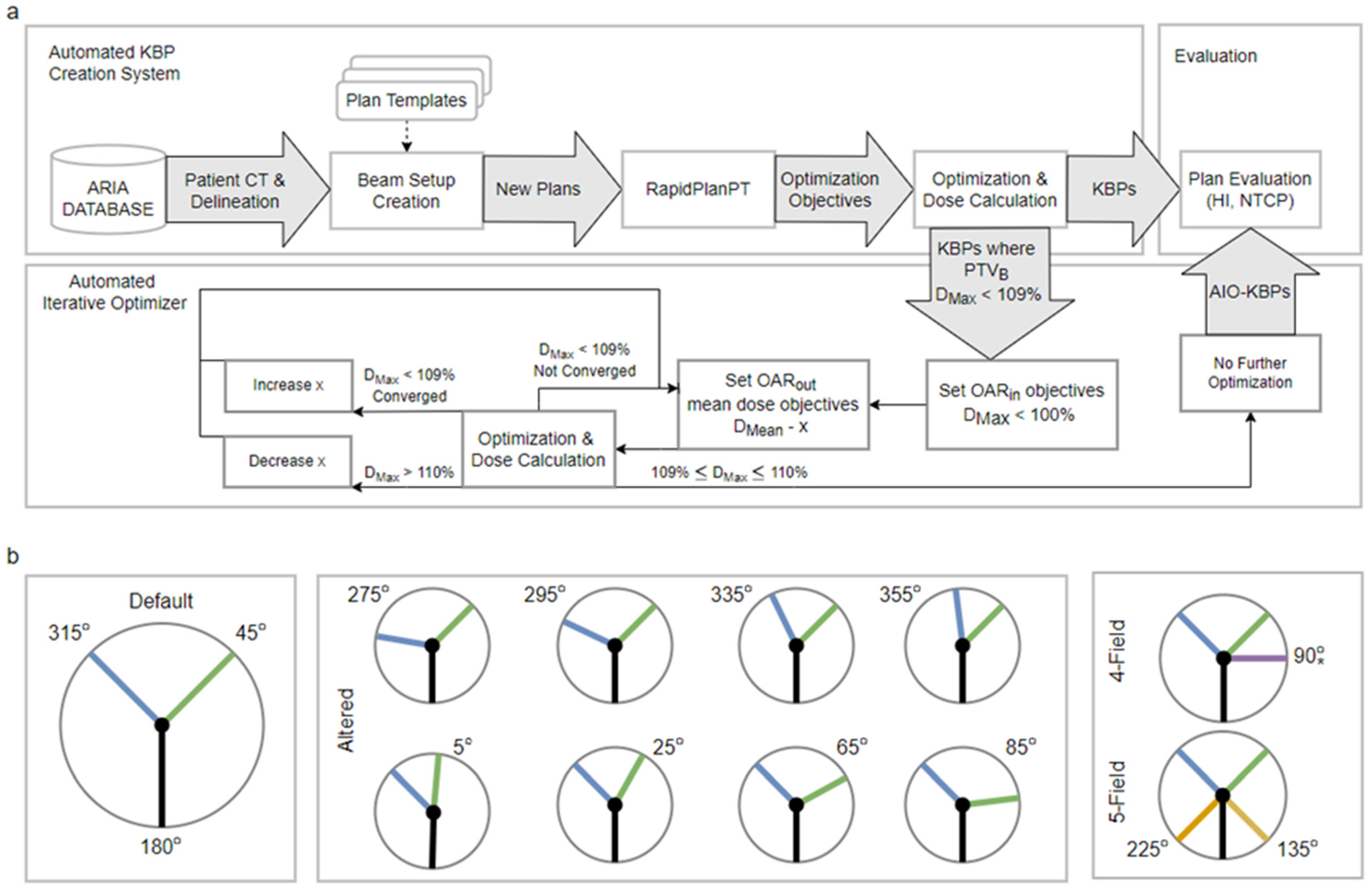
2.1. Patient Cohort and KBP Creation
2.2. Automatic Iterative Optimizer
2.3. Plan Evaluation
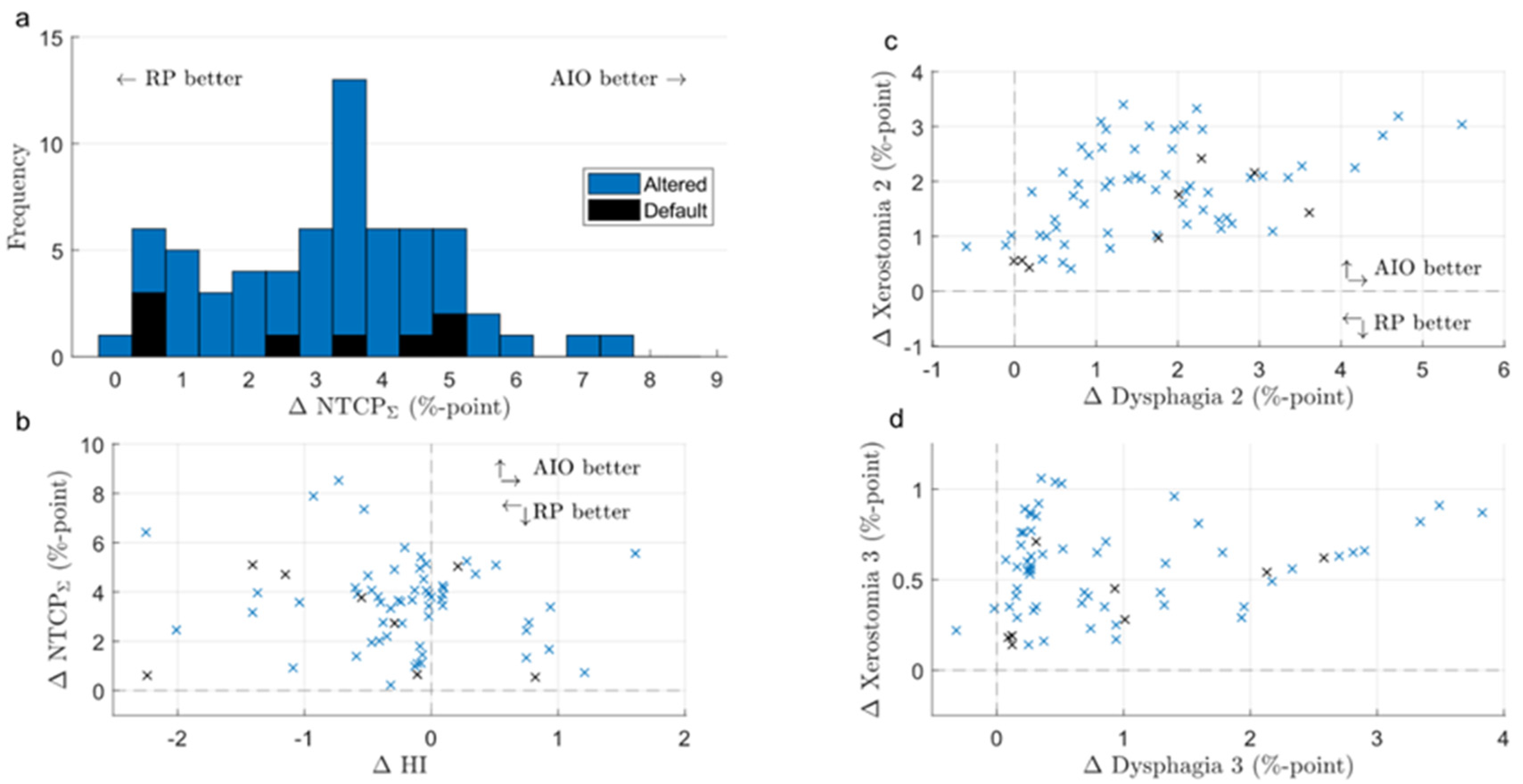
3. Results
3.1. Default- and Altered-Angle KBPs
3.2. KBPs and AIO Plans
3.3. Default- and Altered-Angle AIO Plans
3.4. Increased Number of Beams
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelms, B.E.; Robinson, G.; Markham, J.; Velasco, K.; Boyd, S.; Narayan, S.; Wheeler, J.; Sobczak, M.L. Variation in external beam treatment plan quality: An inter-institutional study of planners and planning systems. Pract. Radiat. Oncol. 2012, 2, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, W.F.A.R.; Doornaert, P.A.H.; Raaijmakers, C.P.J.; Bos, L.J.; Essers, M.; van de Kamer, J.B.; Dahele, M.; Terhaard, C.H.J.; Kaanders, J.H.A.M. Targeted Intervention to Improve the Quality of Head and Neck Radiation Therapy Treatment Planning in the Netherlands: Short and Long-Term Impact. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Heijmen, B.J.M.; Verellen, D.; Nisbet, A. Automation in intensity modulated radiotherapy treatment planning-a review of recent innovations. Br. J. Radiol. 2018, 91, 20180270. [Google Scholar] [CrossRef]
- Tol, J.P.; Delaney, A.R.; Dahele, M.; Slotman, B.J.; Verbakel, W.F.A.R. Evaluation of a knowledge-based planning solution for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Fogliata, A.; Wang, P.M.; Belosi, F.; Clivio, A.; Nicolini, G.; Vanetti, E.; Cozzi, L. Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat. Oncol. 2014, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Fogliata, A.; Belosi, F.; Clivio, A.; Navarria, P.; Nicolini, G.; Scorsetti, M.; Vanetti, E.; Cozzi, L. On the pre-clinical validation of a commercial model-based optimisation engine: Application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother. Oncol. 2014, 113, 385–391. [Google Scholar] [CrossRef]
- Fogliata, A.; Nicolini, G.; Bourgier, C.; Clivio, A.; De Rose, F.; Fenoglietto, P.; Lobefalo, F.; Mancosu, P.; Tomatis, S.; Vanetti, E.; et al. Performance of a Knowledge-Based Model for Optimization of Volumetric Modulated Arc Therapy Plans for Single and Bilateral Breast Irradiation. PLoS ONE 2015, 10, e0145137. [Google Scholar] [CrossRef]
- Moore, K.L. Automated Radiotherapy Treatment Planning. Semin. Radiat. Oncol. 2019, 29, 209–218. [Google Scholar] [CrossRef]
- Delaney, A.; Dong, L.; Mascia, A.; Zou, W.; Zhang, Y.; Yin, L.; Rosas, S.; Hrbacek, J.; Lomax, A.; Slotman, B.; et al. Automated Knowledge-Based Intensity-Modulated Proton Planning: An International Multicenter Benchmarking Study. Cancers. 2018, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Cyriac, J.; De Ornelas, M.; Bossart, E.; Padgett, K.; Butkus, M.; Diwanji, T.; Samuels, S.; Samuels, M.A.; Dogan, N. Knowledge-Based Planning for Robustly Optimized Intensity-Modulated Proton Therapy of Head and Neck Cancer Patients. Front. Oncol. 2021, 11, 737901. [Google Scholar] [CrossRef]
- Delaney, A.R.; Verbakel, W.F.; Lindberg, J.; Koponen, T.K.; Slotman, B.J.; Dahele, M. Evaluation of an Automated Proton Planning Solution. Cureus 2018, 10, e3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hytönen, R.; Vergeer, M.R.; Vanderstraeten, R.; Koponen, T.K.; Smith, C.; Verbakel, W.F.A.R. Fast, Automated, Knowledge-Based Treatment Planning for Selecting Patients for Proton Therapy Based on Normal Tissue Complication Probabilities. Adv. Radiat. Oncol. 2022, 7, 100903. [Google Scholar] [CrossRef] [PubMed]
- Tambas, M.; Steenbakkers, R.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First experience with model-based selection of head and neck cancer patients for proton therapy. Radiother. Oncol. 2020, 151, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Hoebers, F.J.P.; De Jong, M.A.; Doornaert, P.; Terhaard, C.H.J.; Steenbakkers, R.J.H.M.; Hamming-Vrieze, O.; Van De Kamer, J.B.; Verbakel, W.F.A.R.; Keskin-Cambay, F.; et al. National Protocol for Model-Based Selection for Proton Therapy in Head and Neck Cancer. Int. J. Part. Ther. 2021, 8, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Pyyry, J.; Keranen, W. Varian APIs A Handbook for Programming in the Varian Oncology Software Ecosystem; 2018. Available online: https://varianapis.github.io/VarianApiBook.pdf (accessed on 20 April 2022).
- Van Duren-Koopman, M.J.; Tol, J.P.; Dahele, M.; Bucko, E.; Meijnen, P.; Slotman, B.J.; Verbakel, W.F. Personalized automated treatment planning for breast plus locoregional lymph nodes using Hybrid RapidArc. Pract. Radiat. Oncol. 2018, 8, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, S.; Storchi, P.R.M.; Voet, P.W.J.; Heijmen, B.J.M. ICycle: Integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med. Phys. 2012, 39, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.P.; Dahele, M.; Peltola, J.; Nord, J.; Slotman, B.J.; Verbakel, W.F.A.R. Automatic interactive optimization for volumetric modulated arc therapy planning. Radiat. Oncol. 2015, 10, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teruel, J.R.; Malin, M.; Liu, E.K.; Mccarthy, A.; Hu, K.; Bejamin, I.; Cooper, T.; Sulman, E.P.; Silverman, J.S.; Barbee, D. Full automation of spinal stereotactic radiosurgery and stereotactic body radiation therapy treatment planning using Varian Eclipse scripting. J. Appl. Clin. Med. Phys. 2020, 21, 122–131. [Google Scholar] [CrossRef]
- Stieler, F.; Yan, H.; Lohr, F.; Wenz, F.; Yin, F.F. Development of a neuro-fuzzy technique for automated parameter optimization of inverse treatment planning. Radiat. Oncol. 2009, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Yin, F.F.; Willett, C. Evaluation of an artificial intelligence guided inverse planning system: Clinical case study. Radiother. Oncol. 2007, 83, 76–85. [Google Scholar] [CrossRef]
- Huang, C.; Nomura, Y.; Yang, Y.; Xing, L. Meta-optimization for fully automated radiation therapy treatment planning. Phys. Med. Biol. 2022, 67, 055011. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.L.; Steenbakkers, R.J.H.M.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; Van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tol, J.P.; Dahele, M.; Delaney, A.R.; Doornaert, P.; Slotman, B.J.; Verbakel, W.F.A.R. Detailed evaluation of an automated approach to interactive optimization for volumetric modulated arc therapy plans. Med. Phys. 2016, 43, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.P.; Dahele, M.; Doornaert, P.; Slotman, B.J.; Verbakel, W.F.A.R. Different treatment planning protocols can lead to large differences in organ at risk sparing. Radiother. Oncol. 2014, 113, 267–271. [Google Scholar] [CrossRef]
- Landelijk Platform Protonentherapie (LPPT) Landelijk Platform Radiotherapie Hoofd-halstumoren (LPRHHT). Landelijk Indicatie Protocol Protonentherapie (Versie 2.2) (LIPPv2.2) HOOFD-HALSTUMOREN; 2019. Available online: http://www.nvro.nl/publicaties/rapporten (accessed on 20 April 2022).
- Taasti, V.T.; Hong, L.; Shim, J.S.; Deasy, J.O.; Zarepisheh, M. Automating proton treatment planning with beam angle selection using Bayesian optimization. Med. Phys. 2020, 47, 3286. [Google Scholar] [CrossRef] [PubMed]
- Steneker, M.; Lomax, A.; Schneider, U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother. Oncol. 2006, 80, 263–267. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Steenbakkers, R.J.H.M.; ten Haken, B.; van der Laan, H.P.; van ‘t Veld, A.A.; Langendijk, J.A.; Korevaar, E.W. Robust Intensity Modulated Proton Therapy (IMPT) Increases Estimated Clinical Benefit in Head and Neck Cancer Patients. PLoS ONE 2016, 11, e01524772016. [Google Scholar] [CrossRef]
- Kraan, A.C.; Van De Water, S.; Teguh, D.N.; Al-Mamgani, A.; Madden, T.; Kooy, H.M.; Heijmen, B.J.M.; Hoogeman, M.S. Dose uncertainties in IMPT for oropharyngeal cancer in the presence of anatomical, range, and setup errors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 888–896. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hytönen, R.; Vanderstraeten, R.; Dahele, M.; Verbakel, W.F.A.R. Influence of Beam Angle on Normal Tissue Complication Probability of Knowledge-Based Head and Neck Cancer Proton Planning. Cancers 2022, 14, 2849. https://doi.org/10.3390/cancers14122849
Hytönen R, Vanderstraeten R, Dahele M, Verbakel WFAR. Influence of Beam Angle on Normal Tissue Complication Probability of Knowledge-Based Head and Neck Cancer Proton Planning. Cancers. 2022; 14(12):2849. https://doi.org/10.3390/cancers14122849
Chicago/Turabian StyleHytönen, Roni, Reynald Vanderstraeten, Max Dahele, and Wilko F. A. R. Verbakel. 2022. "Influence of Beam Angle on Normal Tissue Complication Probability of Knowledge-Based Head and Neck Cancer Proton Planning" Cancers 14, no. 12: 2849. https://doi.org/10.3390/cancers14122849
APA StyleHytönen, R., Vanderstraeten, R., Dahele, M., & Verbakel, W. F. A. R. (2022). Influence of Beam Angle on Normal Tissue Complication Probability of Knowledge-Based Head and Neck Cancer Proton Planning. Cancers, 14(12), 2849. https://doi.org/10.3390/cancers14122849





