Review of the Standard and Advanced Screening, Staging Systems and Treatment Modalities for Cervical Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. From HPV Infection to Precancerous Formation
3. Detection of Premalignancy and Malignancy of the Uterine Cervix
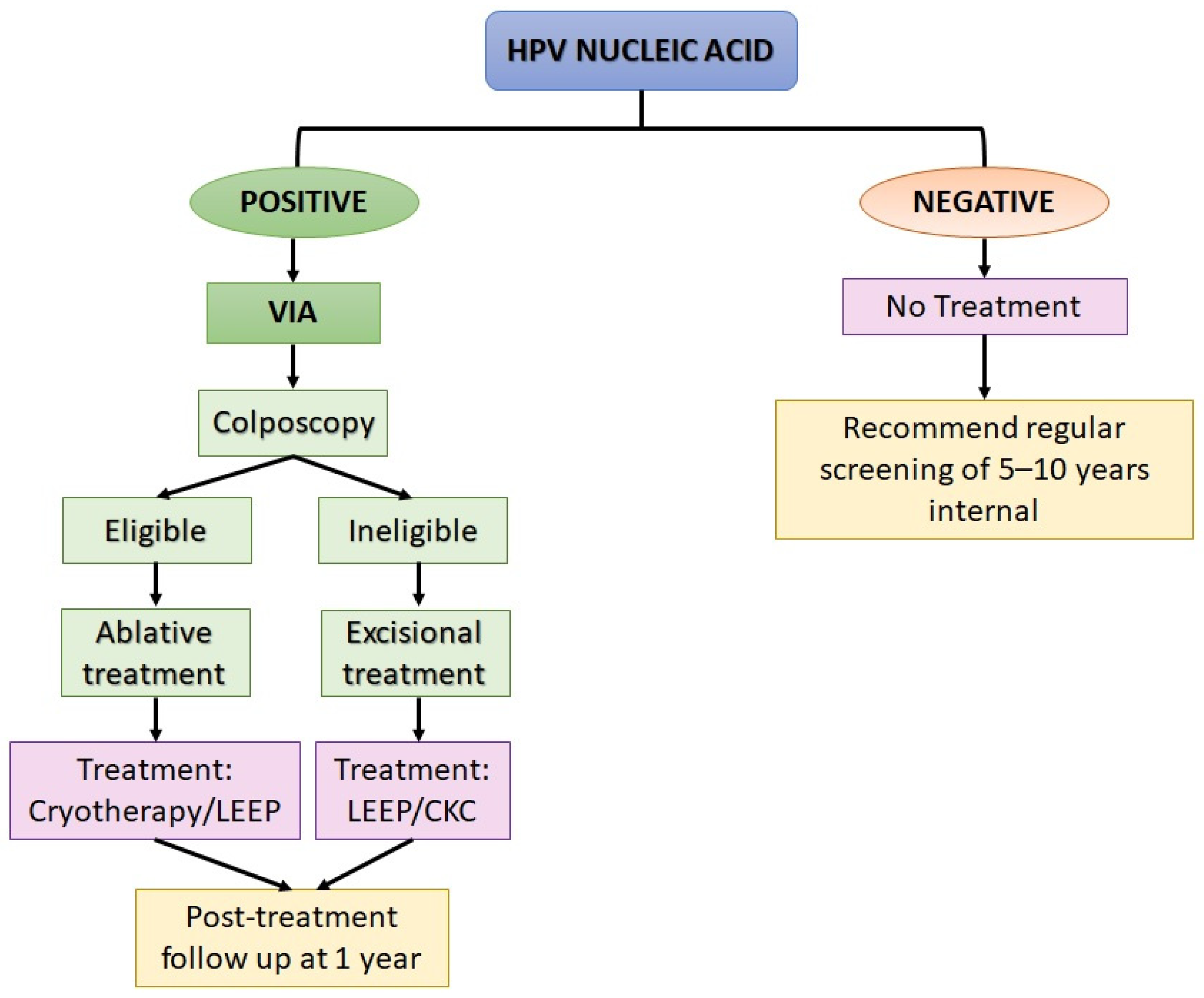
3.1. Standard Detection Methods
3.1.1. Visual Inspection
3.1.2. Cervical Cytology Detection
3.1.3. Human Papillomavirus (HPV) Nucleic Acid Detection and Genotyping
3.1.4. Viral and Cellular Biomarkers
3.2. AI-Assisted Cervical Cancer Detection
4. Staging System for Cervical Intraepithelial Neoplasia and Cancer of Uterine Cervix
4.1. Cervical Intraepithelial Neoplasia (CIN) System
4.2. FIGO System
4.3. TNM System
4.3.1. Tumour (T) Category
4.3.2. Lymph Node (N) Category
4.3.3. Metastasis (M) Category
5. Cervical Cancer and Treatment
5.1. Screen-and-Treat Strategies
5.1.1. Robotic-Assisted Laparoscopy
5.1.2. Radiotherapy and Chemotherapy
5.1.3. Immune Checkpoint Inhibitors
5.1.4. Target-Specific Inhibitors
5.1.5. Anti-Angiogenesis
5.1.6. Drug-Antibody Conjugate
5.1.7. HPV Vaccines
6. Methods
6.1. Literature Search Strategies
6.2. Inclusion and Exclusion Criteria
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses in Human Cancers. Proc. Assoc. Am. Physicians 1999, 111, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Tanton, C.; Soldan, K.; Beddows, S.; Mercer, C.H.; Waller, J.; Field, N.; Clifton, S.; Copas, A.J.; Panwar, K.; Manyenga, P.; et al. High-Risk Human Papillomavirus (HPV) Infection and Cervical Cancer Prevention in Britain: Evidence of Differential Uptake of Interventions from a Probability Survey. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Akram Husain, R.S.; Rajakeerthana, R.; Sreevalsan, A.; Prema Jayaprasad, P.; Ahmed, S.S.S.J.; Ramakrishnan, V. Prevalence of human papilloma virus with risk of cervical cancer among south Indian women: A genotypic study with meta-analysis and molecular dynamics of HPV E6 oncoprotein. Infect. Genet. Evol. 2018, 62, 130–140. [Google Scholar] [CrossRef]
- Thomsen, L.T.; Frederiksen, K.; Munk, C.; Junge, J.; Castle, P.E.; Iftner, T.; Kjaer, S.K. High-risk and low-risk human papillomavirus and the absolute risk of cervical intraepithelial neoplasia or cancer. Obs. Gynecol. 2014, 123, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.-M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Santesso, N.; Mustafa, R.A.; Schünemann, H.J.; Arbyn, M.; Blumenthal, P.D.; Cain, J.; Chirenje, M.; Denny, L.; de Vuyst, H.; Eckert, L.O.N.; et al. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2–3 and screen-and-treat strategies to prevent cervical cancer. Int. J. Gynecol. Obstet. 2016, 132, 252–258. [Google Scholar] [CrossRef]
- WHO Histological Classification of Tumours of the Uterine Cervix. Available online: https://screening.iarc.fr/atlasclassifwho.php (accessed on 1 June 2022).
- Small, W.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Carstensen, B.; Møller, H.; Zappa, M.; Žakelj, M.P.; Lawrence, G.; Hakama, M.; Weiderpass, E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Bosch, F.X.; Manos, M.M.; Muñoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shan, K.V. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef]
- Cimic, A.; Liu-Jarin, X. Updated Review on Pathology of Endocervical Adenocarcinoma with Emphasis on Clinically Relevant Findings. Acta Med. Acad. 2021, 50, 126–135. [Google Scholar] [CrossRef]
- Stolnicu, S.; Park, K.J.; Kiyokawa, T.; Oliva, E.; McCluggage, W.G.; Soslow, R.A. Tumor Typing of Endocervical Adenocarcinoma: Contemporary Review and Recommendations From the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021, 40, S75. [Google Scholar] [CrossRef]
- Keiffer, T.R.; Soorya, S.; Sapp, M.J. Recent Advances in Our Understanding of the Infectious Entry Pathway of Human Papillomavirus Type 16. Microorganisms 2021, 9, 2076. [Google Scholar] [CrossRef]
- Doorbar, J. The papillomavirus life cycle. J. Clin. Virol. 2005, 32 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Adams, A.K.; Wise-Draper, T.M.; Wells, S.I. Human papillomavirus induced transformation in cervical and head and neck cancers. Cancers 2014, 6, 1793–1820. [Google Scholar] [CrossRef] [Green Version]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Petry, K.U. Management options for cervical intraepithelial neoplasia. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 641–651. [Google Scholar] [CrossRef]
- William, W.; Ware, A.; Basaza-Ejiri, A.H.; Obungoloch, J. A review of image analysis and machine learning techniques for automated cervical cancer screening from pap-smear images. Comput. Methods Programs Biomed. 2018, 164, 15–22. [Google Scholar] [CrossRef]
- Taylor, S.N.; Eckert, K.; Rucki, A.A.; VanSickler, M.; Price, J.A.; Gutierrez, E.; Lizzi, M.; Cammarata, C.L.; von Bredow, B.; Wolfe, D.M.; et al. Evaluation of the Onclarity HPV assay on the high-throughput COR system. Expert Rev. Mol. Diagn. 2021, 21, 333–342. [Google Scholar] [CrossRef]
- Mutyaba, T.; Mmiro, F.A.; Weiderpass, E. Knowledge, attitudes and practices on cervical cancer screening among the medical workers of Mulago Hospital, Uganda. BMC Med. Educ. 2006, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, V.; Krishnamurti, C. George Papanicolaou (1883–1962): Discoverer of the Pap Smear. J. Obstet. Gynaecol. India 2018, 68, 232. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef]
- Qiao, Y.L.; Sellors, J.W.; Eder, P.S.; Bao, Y.-P.; Lim, J.M.; Zhao, F.-H.; Weigl, B.; Zhang, W.-H.; Peck, R.B.; Li, L.; et al. A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008, 9, 929–936. [Google Scholar] [CrossRef]
- Davis-Devine, S.; Day, S.J.; Freund, G.G. Test performance comparison of inform HPV and hybrid capture 2 high-risk HPV DNA tests using the SurePath liquid-based Pap test as the collection method. Am. J. Clin. Pathol. 2005, 124, 24–30. [Google Scholar] [CrossRef]
- Nobbenhuis, M.A.E.; Meijer, C.J.L.M.; van Brule, A.J.C.; Rozendaal, L.; Voorhorst, F.J.; Risse, E.K.J.; Verheijen, R.H.M.; Helmerhorst, T.J.M. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br. J. Cancer 2001, 84, 796. [Google Scholar] [CrossRef] [Green Version]
- Day, S.P.; Hudson, A.; Mast, A.; Sander, T.; Curtis, M.; Olson, S.; Chehak, L.A.; Quigley, N.; Ledford, J.; Yen-Lieberman, B.; et al. Analytical performance of the Investigational Use Only Cervista HPV HR test as determined by a multi-center study. J. Clin. Virol. 2009, 45 (Suppl. S1), S63–S72. [Google Scholar] [CrossRef]
- Guyot, A.; Karim, S.; Kyi, M.S.; Fox, J. Evaluation of adjunctive HPV testing by Hybrid Capture II in women with minor cytological abnormalities for the diagnosis of CIN2/3 and cost comparison with colposcopy. BMC Infect. Dis. 2003, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts Carpenter, A.; Davey, D.D. ThinPrep Pap Test™ Performance and Biopsy Follow-Up in a University Hospital. Cancer 1999, 87, 105–112. [Google Scholar] [CrossRef]
- Wright, T.C., Jr. Chapter 10: Cervical cancer screening using visualization techniques. J. Natl. Cancer Inst. Monogr. 2003, 2003, 66–71. [Google Scholar] [CrossRef]
- Koss, L.G. The new Bethesda System for reporting results of smears of the uterine cervix. J. Natl. Cancer Inst. 1990, 82, 988–991. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomavirus Laboratory Manual, 1st ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Wright, T.C., Jr.; Menton, M.; Myrtle, J.F.; Chow, C.; Singer, A. Visualization techniques (colposcopy, direct visual inspection, and spectroscopic and other visual methods). Summary of task force 7. Acta Cytol. 2002, 46, 793–800. [Google Scholar] [CrossRef]
- Stjernswärd, J.; Eddy, D.M.; Luthra, U.K.; Stanley, K.E. Plotting a new course for cervical cancer screening in developing countries. World Health Forum 1987, 8, 42–45. [Google Scholar]
- Massad, L.S.; Lonky, N.M.; Mutch, D.G.; Mann, W.J.; Blanco, J.S.; Vasilev, S.A.; Finan, M.A.; Scotti, R.J. Use of speculoscopy in the evaluation of women with atypical Papanicolaou smears. Improved cost effectiveness by selective colposcopy. J. Reprod. Med. 1993, 38, 163–169. [Google Scholar]
- Stafl, A. Cervicography: A new method for cervical cancer detection. Am. J. Obstet. Gynecol. 1981, 139, 815–821. [Google Scholar] [CrossRef]
- Nuovo, J.; Melnikow, J.; Hutchison, B.; Paliescheskey, M. Is cervicography a useful diagnostic test? A systematic overview of the literature. J. Am. Board Fam. Pract. 1997, 10, 390–397. [Google Scholar]
- Grant, B.D.; Fregnani, J.H.T.G.; Possati Resende, J.C.; Scapulatempo-Neto, C.; Matsushita, G.M.; Mauad, E.C.; Quang, T.; Stoler, M.H.; Castle, P.E.; Schmeler, K.M. High-resolution microendoscopy: A point-of-care diagnostic for cervical dysplasia in low-resource settings. Eur. J. Cancer Prev. 2017, 26, 63–70. [Google Scholar] [CrossRef]
- Marchevsky, A.M.; Bartels, P.H. Image Analysis: A Primer for Pathologists; Raven Press: New York, NY, USA, 1994; ISBN 0781701708. [Google Scholar]
- Hartikainen, J. The Papanicolaou test: Its utility and efficacy in cancer detection. Contemp. Nurse 2001, 11, 45–49. [Google Scholar] [CrossRef]
- Rebolj, M.; Rask, J.; van Ballegooijen, M.; Kirschner, B.; Rozemeijer, K.; Bonde, J.; Rygaard, C.; Lynge, E. Cervical histology after routine ThinPrep or SurePath liquid-based cytology and computer-assisted reading in Denmark. Br. J. Cancer 2015, 113, 1259–1274. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, W.D.; Gibbons-Fideler, I.; Shen, R.; Li, Z. The reporting rates of high-grade squamous intraepithelial lesion s and their human papillomavirus testing and histologic follow-up results: A comparison between ThinPrep and SurePath preparations. Diagn. Cytopathol. 2021, 49, 959–963. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, Q.; Wang, Y.; Li, L.; Yi, X.; Yan, B.; Zou, M.; Dai, G.; Guo, P.; Ma, Q. The Application of DNA Ploidy Analysis in Large-Scale Population Screening for Cervical Cancer. Acta Cytol. 2021, 65, 385–392. [Google Scholar] [CrossRef]
- Kędzia, H.; Spaczyński, M.; Józefiak, A.; Przybylski, M.; Kędzia, W.; Pruski, D. The assesment of real optoelectronic method in the detection of cervical intraepithelial neoplasia. Ginekol. Pol. 2008, 79, 342–346. [Google Scholar]
- Wei, Y.; Wang, W.; Cheng, M.; Hong, Z.; Gu, L.; Niu, J.; Di, W.; Qiu, L. Clinical evaluation of a real-time optoelectronic device in cervical cancer screening. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 266, 182–186. [Google Scholar] [CrossRef]
- Suchońska, B.; Gajzlerska-Majewska, W.; Wielgoś, M. Evaluation of a real-time optoelectronic method in the diagnostics of CIN over four years of observations. PLoS ONE 2021, 16, e0247702. [Google Scholar] [CrossRef]
- Swid, M.A.; Monaco, S.E. Should screening for cervical cancer go to primary human papillomavirus testing and eliminate cytology? Mod. Pathol. 2022, 1–7, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dürst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.M.; Warke, H.; Chaudhari, H.; Mavani, P.; Katke, R.D.; Kerkar, S.C.; Mania-Pramanik, J. Human Papillomavirus E6/E7 oncogene transcripts as biomarkers for the early detection of cervical cancer. J. Med. Virol. 2022, 94, 3368–3375. [Google Scholar] [CrossRef]
- Galati, L.; Combes, J.-D.; le Calvez-Kelm, F.; McKay-Chopin, S.; Forey, N.; Ratel, M.; McKay, J.; Waterboer, T.; Schroeder, L.; Clifford, G.; et al. Detection of Circulating HPV16 DNA as a Biomarker for Cervical Cancer by a Bead-Based HPV Genotyping Assay. Microbiol. Spectr. 2022, 10, e0148021. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, D.; Keung, M.H.T.; Huang, Y.; McDermott, T.L.; Romano, J.; Saville, M.; Brotherton, J.M.L. Self-collection for cervical screening programs: From research to reality. Cancers 2020, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [Green Version]
- Nutthachote, P.; Oranratanaphan, S.; Termrungruanglert, W.; Triratanachat, S.; Chaiwongkot, A.; Baedyananda, F.; Bhattarakosol, P. Comparison of detection rate of high risk HPV infection between self-collected HPV testing and clinician-collected HPV testing in cervical cancer screening. Taiwan J. Obstet. Gynecol. 2019, 58, 477–481. [Google Scholar] [CrossRef]
- Salazar, K.L.; Duhon, D.J.; Olsen, R.; Thrall, M. A review of the FDA-approved molecular testing platforms for human papillomavirus. J. Am. Soc. Cytopathol. 2019, 8, 284–292. [Google Scholar] [CrossRef]
- Poljak, M.; Oštrbenk Valenčak, A.; Gimpelj Domjanič, G.; Xu, L.; Arbyn, M. Commercially available molecular tests for human papillomaviruses: A global overview. Clin. Microbiol. Infect. 2020, 26, 1144–1150. [Google Scholar] [CrossRef]
- Paboriboune, P.; Phongsavan, K.; Arounlangsy, P.; Flaissier, B.; Aphayarath, O.; Phimmasone, P.; Banchongphanith, K.; Xayaovong, M.; Jourdain, G.; Schott, A.-M.; et al. Efficacy of careHPV™ human papillomavirus screening versus conventional cytology tests for the detection of precancerous and cancerous cervical lesions among women living with HIV-1 in Lao People’s Democratic Republic. Cancer Med. 2022, 11, 1984–1994. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Badiga, S.; Simons, J.L.; Bell, W.C.; Jolly, P.E. HPV E1 qPCR, a Low-Cost Alternative Assay to Roche Diagnostic Linear Array is Effective in Identifying Women at Risk for Developing Cervical Cancer. Int. J. Womens. Health 2022, 14, 257. [Google Scholar] [CrossRef]
- Andersen, K.; Holm, K.; Tranberg, M.; Pedersen, C.L.; Bønløkke, S.; Steiniche, T.; Andersen, B.; Stougaard, M. Targeted Next Generation Sequencing for Human Papillomavirus Genotyping in Cervical Liquid-Based Cytology Samples. Cancers 2022, 14, 652. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cutts, R.J.; White, I.; Augustin, Y.; Garcia-Murillas, I.; Fenwick, K.; Matthews, N.; Turner, N.C.; Harrington, K.; Gilbert, D.C.; et al. Next Generation Sequencing Assay for Detection of Circulating HPV DNA (cHPV-DNA) in Patients Undergoing Radical (Chemo)Radiotherapy in Anal Squamous Cell Carcinoma (ASCC). Front. Oncol. 2020, 10, 505. [Google Scholar] [CrossRef]
- Wentzensen, N.; von Knebel Doeberitz, M. Biomarkers in Cervical Cancer Screening. Dis. Markers 2007, 23, 315. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. E6-AP directs the HPV E6-dependent inactivation of p53 and is representative of a family of structurally and functionally related proteins. Cold Spring Harb. Symp. Quant. Biol. 1994, 59, 237–245. [Google Scholar] [CrossRef]
- Murphy, N.; Ring, M.; Heffron, C.; King, B.; Killalea, A.G.; Hughes, C.; Martin, C.M.; McGuinness, E.; Sheils, O.; O’leary, J.J. p16INK4A, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J. Clin. Pathol. 2005, 58, 525–534. [Google Scholar] [CrossRef]
- Yu, L.; Fei, L.; Liu, X.; Pi, X.; Wang, L.; Chen, S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J. Cancer 2019, 10, 2654. [Google Scholar] [CrossRef] [Green Version]
- Togami, S.; Fukuda, M.; Yanazume, S.; Kamio, M.; Kobayashi, H. A preliminary study on the detection of lymph node metastasis in cervical cancer using a quantitative RT-PCR assay. Jpn. J. Clin. Oncol. 2022, 52, 475–478. [Google Scholar] [CrossRef]
- Xu, W.; Xu, M.; Wang, L.; Zhou, W.; Xiang, R.; Shi, Y.; Zhang, Y.; Piao, Y. Integrative analysis of DNA methylation and gene expression identified cervical cancer-specific diagnostic biomarkers. Signal Transduct. Target. Ther. 2019, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luttmer, R.; de Strooper, L.M.A.; Berkhof, J.; Snijders, P.J.F.; Dijkstra, M.G.; Uijterwaal, M.H.; Steenbergen, R.D.M.; van Kemenade, F.J.; Rozendaal, L.; Helmerhorst, T.J.M. Comparing the performance of FAM19A4 methylation analysis, cytology and HPV16/18 genotyping for the detection of cervical (pre) cancer in high-risk HPV-positive women of a gynecologic outpatient population (COMETH study). Int. J. Cancer 2016, 138, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Zhang, S.; Jiao, J.; Zhang, T.; Qu, W.; Muloye, G.M.; Kong, B.; Zhang, Q.; Cui, B. Promoter methylation of SEPT9 as a potential biomarker for early detection of cervical cancer and its overexpression predicts radioresistance. Clin. Epigenetics 2019, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.M.; Li, C.; Yao, Y.; Kulwa, F.; Wu, X.; Li, X.; Wang, Q. DeepCervix: A deep learning-based framework for the classification of cervical cells using hybrid deep feature fusion techniques. Comput. Biol. Med. 2021, 136, 104649. [Google Scholar] [CrossRef]
- Nambu, Y.; Mariya, T.; Shinkai, S.; Umemoto, M.; Asanuma, H.; Sato, I.; Hirohashi, Y.; Torigoe, T.; Fujino, Y.; Saito, T. A screening assistance system for cervical cytology of squamous cell atypia based on a two-step combined CNN algorithm with label smoothing. Cancer Med. 2022, 11, 520–529. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Ong, K.; Zhang, W.; Li, W.; Li, L.; Young, D.; Su, Y.; Shang, B.; Peng, L. Hybrid AI-assistive diagnostic model permits rapid TBS classification of cervical liquid-based thin-layer cell smears. Nat. Commun. 2021, 12, 3541. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, S.; Yu, J.; Rao, G.; Xiao, Y.; Han, W.; Zhu, W.; Lv, X.; Li, N.; Cai, J.; et al. Robust Whole Slide Image Analysis for Cervical Cancer Screening Using Deep Learning. Nat. Commun. 2021, 12, 5639. [Google Scholar] [CrossRef]
- Fick, R.H.J.; Tayart, B.; Bertrand, C.; Lang, S.C.; Rey, T.; Ciompi, F.; Tilmant, C.; Farre, I.; Hadj, S.B. A Partial Label-Based Machine Learning Approach For Cervical Whole-Slide Image Classification: The Winning TissueNet Solution. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 2127–2131. [Google Scholar] [CrossRef]
- Mehmood, M.; Rizwan, M.; Abbas, S. Machine Learning Assisted Cervical Cancer Detection. Front. Public Health 2021, 9, 788376. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ahmed, K.; Bui, F.M.; Paul, B.K.; Ibrahim, S.M.; Quinn, J.M.W.; Moni, M.A. Machine learning-based statistical analysis for early stage detection of cervical cancer. Comput. Biol. Med. 2021, 139, 104985. [Google Scholar] [CrossRef] [PubMed]
- Kruczkowski, M.; Drabik-Kruczkowska, A.; Marciniak, A.; Tarczewska, M.; Kosowska, M.; Szczerska, M. Predictions of cervical cancer identification by photonic method combined with machine learning. Sci. Rep. 2022, 12, 3762. [Google Scholar] [CrossRef]
- Xue, P.; Wang, J.; Qin, D.; Yan, H.; Qu, Y.; Seery, S.; Jiang, Y.; Qiao, Y. Deep learning in image-based breast and cervical cancer detection: A systematic review and meta-analysis. NPJ Digit. Med. 2022, 5, 19. [Google Scholar] [CrossRef]
- Tan, M.S.; Chang, S.W.; Cheah, P.L.; Yap, H.J. Integrative Machine Learning Analysis of Multiple Gene Expression Profiles in Cervical Cancer. PeerJ 2018, 6, e5285. [Google Scholar] [CrossRef]
- Wang, C.W.; Liou, Y.A.; Lin, Y.J.; Chang, C.C.; Chu, P.H.; Lee, Y.C.; Wang, C.H.; Chao, T.K. Artificial intelligence-assisted fast screening cervical high grade squamous intraepithelial lesion and squamous cell carcinoma diagnosis and treatment planning. Sci. Reports 2021, 11, 16244. [Google Scholar] [CrossRef]
- Bao, H.; Bi, H.; Zhang, X.; Zhao, Y.; Dong, Y.; Luo, X.; Zhou, D.; You, Z.; Wu, Y.; Liu, Z.; et al. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: A multicenter, clinical-based, observational study. Gynecol. Oncol. 2020, 159, 171–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Xing, L.; Lei, H.; Chen, D.; Tang, C.; Li, X. The Performance of Artificial Intelligence in Cervical Colposcopy: A Retrospective Data Analysis. J. Oncol. 2022, 2022, 4370851. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bentley, H.A.; Eijgenraam, M.N.; IntHout, J.; Bekkers, R.L.M.; Bentley, J.R. The Natural History of Cervical Intraepithelial Neoplasia Grades 1, 2, and 3: A Systematic Review and Meta-analysis. J. Low. Genit. Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef]
- Montz, F.J. Management of high-grade cervical intraepithelial neoplasia and low-grade squamous intraepithelial lesion and potential complications. Clin. Obs. Gynecol. 2000, 43, 394–409. [Google Scholar] [CrossRef]
- Morris, M.; Tortolero-Luna, G.; Malpica, A.; Baker, V.V.; Cook, E.; Johnson, E.; Follen Mitchell, M. Cervical intraepithelial neoplasia and cervical cancer. Obs. Gynecol. Clin. N. Am. 1996, 23, 347–410. [Google Scholar]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Comprehensive Cervical Cancer Control: A Guide to Essential Practice; World Health Organization: Geneva, Swizerland, 2014. [Google Scholar]
- Freeman, S.J.; Aly, A.M.; Kataoka, M.Y.; Addley, H.C.; Reinhold, C.; Sala, E. The revised FIGO staging system for uterine malignancies: Implications for MR imaging. Radiographics 2012, 32, 1805–1827. [Google Scholar] [CrossRef]
- Amendola, M.A.; Hricak, H.; Mitchell, D.G.; Snyder, B.; Chi, D.S.; Long, H.J., 3rd; Fiorica, J.V.; Gatsonis, C. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: Results of intergroup protocol ACRIN 6651/GOG 183. J. Clin. Oncol. 2005, 23, 7454–7459. [Google Scholar] [CrossRef]
- Mikami, M.; Ikeda, M.; Sato, H.; Iwase, H.; Enomoto, T.; Kobayashi, Y.; Katabuchi, H. The use of conization to identify and treat severe lesions among prediagnosed CIN1 and 2 patients in Japan. J. Gynecol. Oncol. 2018, 29, e46. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obs. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Lee, S.I.; Atri, M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, M.; Zhong, L.; Xie, X.; Liu, Z.; Yan, M.; Yi, H.; Lin, D. Prognostic significance of solitary lymph node metastasis in patients with stages IA2 to IIA cervical carcinoma. J. Int. Med. Res. 2018, 46, 4082–4091. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Cai, J.; Xu, L.; Min, J.; Shen, Y.; Xiong, Z.; Dong, W.; Bunyamanop, V.; Wang, Z. Laparoscopic procedure compared with open radical hysterectomy with pelvic lymphadenectomy in early cervical cancer: A retrospective study. Onco Targets Ther. 2018, 11, 5903–5908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, R.D.; Sapra, A. TNM Classification. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhou, S.; Peng, F. Patterns of metastases in cervical cancer: A population-based study. Int. J. Clin. Exp. Pathol. 2020, 13, 1615–1623. [Google Scholar] [PubMed]
- Pretzsch, E.; Nieß, H.; Bösch, F.; Westphalen, C.B.; Jacob, S.; Neumann, J.; Werner, J.; Heinemann, V.; Angele, M.K. Age and metastasis—How age influences metastatic spread in cancer. Colorectal cancer as a model. Cancer Epidemiol. 2022, 77, 102112. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, V.; Kausar, M.; Naik, A.; Batra, M. Unusual Metastasis from Carcinoma Cervix. J. Obstet. Gynaecol. India 2016, 66, 358–362. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-003082-4. [Google Scholar]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Lewandowska, A.; Szubert, S.; Koper, K.; Koper, A.; Cwynar, G.; Wicherek, L. Analysis of long-term outcomes in 44 patients following pelvic exenteration due to cervical cancer. World J. Surg. Oncol. 2020, 18, 234. [Google Scholar] [CrossRef]
- Lang, J.E.; Mannava, S.; Floyd, A.J.; Goddard, M.S.; Smith, B.P.; Mofidi, A.; Seyler, T.M.; Jinnah, R.H. Robotic systems in orthopaedic surgery. J. Bone Jt. Surg. Br. 2011, 93, 1296–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channa, R.; Iordachita, I.; Handa, J.T. Robotic Vitreoretinal Surgery. Retina 2017, 37, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Arata, J.; Tada, Y.; Kozuka, H.; Wada, T.; Saito, Y.; Ikedo, N.; Hayashi, Y.; Fujii, M.; Kajita, Y.; Mizuno, M.; et al. Neurosurgical robotic system for brain tumor removal. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 375–385. [Google Scholar] [CrossRef]
- Ho, A.L.; Pendharkar, A.V.; Brewster, R.; Martinez, D.L.; Jaffe, R.A.; Xu, L.W.; Miller, K.J.; Halpern, C.H. Frameless Robot-Assisted Deep Brain Stimulation Surgery: An Initial Experience. Oper. Neurosurg. 2019, 17, 424–431. [Google Scholar] [CrossRef]
- Zirafa, C.C.; Romano, G.; Key, T.H.; Davini, F.; Melfi, F. The evolution of robotic thoracic surgery. Ann. Cardiothorac. Surg. 2019, 8, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Giulianotti, P.C.; Bianco, F.M.; Daskalaki, D.; Gonzalez-Ciccarelli, L.F.; Kim, J.; Benedetti, E. Robotic liver surgery: Technical aspects and review of the literature. Hepatobiliary Surg. Nutr. 2016, 5, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, K.; Kanno, T.; Tadano, K. Robots in laparoscopic surgery: Current and future status. BMC Biomed. Eng. 2019, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Prewitt, R.; Bochkarev, V.; McBride, C.L.; Kinney, S.; Oleynikov, D. The patterns and costs of the Da Vinci robotic surgery system in a large academic institution. J. Robot. Surg. 2008, 2, 17–20. [Google Scholar] [CrossRef]
- Sieber, M.A.; Fellmann-Fischer, B.; Mueller, M. Performance of Kymerax© precision-drive articulating surgical system compared to conventional laparoscopic instruments in a pelvitrainer model. Surg. Endosc. 2017, 31, 4298–4308. [Google Scholar] [CrossRef]
- Bensignor, T.; Morel, G.; Reversat, D.; Fuks, D.; Gayet, B. Evaluation of the effect of a laparoscopic robotized needle holder on ergonomics and skills. Surg. Endosc. 2016, 30, 446–454. [Google Scholar] [CrossRef]
- Pereira, R.; Moreira, A.H.J.; Leite, M.; Rodrigues, P.L.; Queirós, S.; Rodrigues, N.F.; Leão, P.; Vilaça, J.L. Hand-held robotic device for laparoscopic surgery and training. In Proceedings of the 2014 IEEE 3nd International Conference on Serious Games and Applications for Health (SeGAH), Rio de Janeiro, Brazil, 14–16 May 2014; pp. 1–8. [Google Scholar]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Khorgami, Z.; Li, W.T.; Jackson, T.N.; Howard, C.A.; Sclabas, G.M. The cost of robotics: An analysis of the added costs of robotic-assisted versus laparoscopic surgery using the National Inpatient Sample. Surg. Endosc. 2019, 33, 2217–2221. [Google Scholar] [CrossRef] [PubMed]
- FDA Authorizes First Robotically-Assisted Surgical Device for Performing Transvaginal Hysterectomy|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-robotically-assisted-surgical-device-performing-transvaginal-hysterectomy (accessed on 28 March 2022).
- Okazaki, S.; Murata, K.; Noda, S.E.; Kumazaki, Y.; Hirai, R.; Igari, M.; Abe, T.; Komatsu, S.; Nakano, T.; Kato, S. Dose–volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J. Radiat. Res. 2019, 60, 490. [Google Scholar] [CrossRef] [Green Version]
- Mazeron, R.; Petit, C.; Rivin, E.; Limkin, E.; Dumas, I.; Maroun, P.; Annede, P.; Martinetti, F.; Seisen, T.; Lefkopoulos, D.; et al. 45 or 50 Gy, Which is the Optimal Radiotherapy Pelvic Dose in Locally Advanced Cervical Cancer in the Perspective of Reaching Magnetic Resonance Image-guided Adaptive Brachytherapy Planning Aims? Clin. Oncol. 2016, 28, 171–177. [Google Scholar] [CrossRef]
- Monk, B.J.; Tewari, K.S.; Koh, W.J. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J. Clin. Oncol. 2007, 25, 2952–2965. [Google Scholar] [CrossRef]
- Tan, L.T.; Coles, C.E.; Hart, C.; Tait, E. Clinical impact of computed tomography-based image-guided brachytherapy for cervix cancer using the tandem-ring applicator—The Addenbrooke’s experience. Clin. Oncol. 2009, 21, 175–182. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- De Gonzalez, A.B.; Curtis, R.E.; Kry, S.F.; Gilbert, E.; Lamart, S.; Berg, C.D.; Stovall, M.; Ron, E. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet. Oncol. 2011, 12, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wu, S.G.; Sun, J.Y.; Tang, L.Y.; Lin, H.X.; Li, F.Y.; Chen, Q.H.; Jin, X.; He, Z.Y. Clinicopathological features of small cell carcinoma of the uterine cervix in the surveillance, epidemiology, and end results database. Oncotarget 2017, 8, 40425–40433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Cai, H.; Xiao, Z.X.; Wang, H.; Yang, P. Effect of radiotherapy on the survival of cervical cancer patients: An analysis based on SEER database. Medicine 2019, 98, e16421. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Gupta, S. Integrating Chemotherapy in the Management of Cervical Cancer: A Critical Appraisal. Oncology 2016, 91, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef]
- Dueñas-González, A.; Zarbá, J.J.; Patel, F.; Alcedo, J.C.; Beslija, S.; Casanova, L.; Pattaranutaporn, P.; Hameed, S.; Blair, J.M.; Barraclough, H.; et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J. Clin. Oncol. 2011, 29, 1678–1685. [Google Scholar] [CrossRef]
- Rose, P.G.; Ali, S.; Watkins, E.; Thigpen, J.T.; Deppe, G.; Clarke-Pearson, D.L.; Insalaco, S. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A gynecologic oncology group study. J. Clin. Oncol. 2007, 25, 2804–2810. [Google Scholar] [CrossRef]
- Chino, J.; Annunziata, C.M.; Beriwal, S.; Bradfield, L.; Erickson, B.A.; Fields, E.C.; Fitch, K.J.; Harkenrider, M.M.; Holschneider, C.H.; Kamrava, M.; et al. Radiation Therapy for Cervical Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 220–234. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Omura, G.A.; Blessing, J.A.; Vaccarello, L.; Berman, M.L.; Clarke-Pearson, D.L.; Mutch, D.G.; Anderson, B. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J. Clin. Oncol. 2016, 15, 165–171. [Google Scholar] [CrossRef]
- Buxton, E.J.; Meanwell, C.A.; Hilton, C.; Mould, J.J.; Spooner, D.; Chetiyawardana, A.; Latief, T.; Paterson, M.; Redman, C.W.; Luesley, D.M.; et al. Combination bleomycin, ifosfamide, and cisplatin chemotherapy in cervical cancer. J. Natl. Cancer Inst. 1989, 81, 359–361. [Google Scholar] [CrossRef]
- Moore, D.H.; Blessing, J.A.; McQuellon, R.P.; Thaler, H.T.; Cella, D.; Benda, J.; Miller, D.S.; Olt, G.; King, S.; Boggess, J.F.; et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J. Clin. Oncol. 2004, 22, 3113–3119. [Google Scholar] [CrossRef]
- Katsumata, N.; Yoshikawa, H.; Kobayashi, H.; Saito, T.; Kuzuya, K.; Nakanishi, T.; Yasugi, T.; Yaegashi, N.; Yokota, H.; Kodama, S.; et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: A Japan Clinical Oncology Group trial (JCOG 0102). Br. J. Cancer 2013, 108, 1957. [Google Scholar] [CrossRef] [Green Version]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; del Rincon, S.V.; Papneja, N.; Miller, W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87. [Google Scholar] [CrossRef]
- Odiase, O.; Noah-Vermillion, L.; Simone, B.A.; Aridgides, P.D. The Incorporation of Immunotherapy and Targeted Therapy Into Chemoradiation for Cervical Cancer: A Focused Review. Front. Oncol. 2021, 11, 1656. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Battista, M.J.; Schmidt, M.; Garcia, M.; Siepmann, T.; Hasenburg, A.; Anic, K. Efficacy and Safety of Immunotherapy for Cervical Cancer—A Systematic Review of Clinical Trials. Cancers 2022, 14, 441. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II Evaluation of Nivolumab in the Treatment of Persistent or Recurrent Cervical Cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161. [Google Scholar] [CrossRef]
- Doxorubicin Alone Versus Atezolizumab Alone Versus Doxorubicin and Atezolizumab in Recurrent Cervical Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03340376 (accessed on 13 March 2022).
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Mayadev, J.; Nunes, A.T.; Li, M.; Marcovitz, M.; Lanasa, M.C.; Monk, B.J. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. Int. J. Gynecol. Cancer 2020, 30, 1065–1070. [Google Scholar] [CrossRef]
- Lheureux, S.; Butler, M.O.; Clarke, B.; Cristea, M.C.; Martin, L.P.; Tonkin, K.; Fleming, G.F.; Tinker, A.V.; Hirte, H.W.; Tsoref, D.; et al. Association of Ipilimumab With Safety and Antitumor Activity in Women With Metastatic or Recurrent Human Papillomavirus–Related Cervical Carcinoma. JAMA Oncol. 2018, 4, e173776. [Google Scholar] [CrossRef]
- Naumann, R.W.; Oaknin, A.; Meyer, T.; Lopez-Picazo, J.M.; Lao, C.; Bang, Y.-J.; Boni, V.; Sharfman, W.H.; Park, J.C.; Devriese, L.A.; et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Ann. Oncol. 2019, 30, v898–v899. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Oaknin, A.; Monk, B.J.; Leary, A.; Selle, F.; Alexandre, J.; Randall, L.M.; Rojas, C.; Neffa, M.; Kryzhanivska, A.; et al. LBA34 Single-agent anti-PD-1 balstilimab or in combination with anti-CTLA-4 zalifrelimab for recurrent/metastatic (R/M) cervical cancer (CC): Preliminary results of two independent phase II trials. Ann. Oncol. 2020, 31, S1164–S1165. [Google Scholar] [CrossRef]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.L.; Kannapel, E.; Chen, J.; Chen, M.; Cohen, A.L. Safety and PK results from a phase Ib study of AL3818 (anlotinib) hydrochloride in subjects with ovarian, cervical, and endometrial cancers. J. Clin. Oncol. 2017, 35, e17071. [Google Scholar] [CrossRef]
- Schilder, R.J.; Sill, M.W.; Lee, Y.C.; Mannel, R. A Phase II Trial of Erlotinib in recurrent squamous cell carcinoma of the cervix: A Gynaecologic Oncology Group Study. Int. J. Gynecol. Cancer 2009, 19, 929. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, A.; Fabbro, M.; Lhommé, C.; Gladieff, L.; Extra, J.M.; Floquet, A.; Chaigneau, L.; Carrasco, A.T.; Viens, P. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol. Oncol. 2008, 108, 42–46. [Google Scholar] [CrossRef]
- Nogueira-Rodrigues, A.; Moralez, G.; Grazziotin, R.; Carmo, C.C.; Small, I.A.; Alves, F.V.G.; Mamede, M.; Erlich, F.; Viegas, C.; Triginelli, S.A.; et al. Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer 2014, 120, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Tewari, K.S.; Sill, M.W.; Long, H.J., III; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. N. Engl. J. Med. 2014, 370, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, C.; Shen, J.; Wang, Y.; Li, J.; Liu, Z.; He, M.; Cao, X.; Ling, J.; Huang, J.; Zheng, M.; et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): A multicenter, open-label, single-arm, Phase II trial. J. Clin. Oncol. 2020, 38, 4095. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Z.; Li, A.; Zhang, J.; Wang, Y.; Zhao, H.; Zhu, H. The efficacy and safety of apatinib treatment for patients with metastatic or recurrent cervical cancer: A retrospective study. Drug Des. Devel. Ther. 2019, 13, 3419–3424. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Cheng, H.; Wang, L.; Yu, X. Clinical response and safety of apatinib monotherapy in recurrent, metastatic cervical cancer after failure of chemotherapy: A retrospective study. J. Gynecol. Oncol. 2020, 31, e2. [Google Scholar] [CrossRef]
- Symonds, R.P.; Gourley, C.; Davidson, S.; Carty, K.; McCartney, E.; Rai, D.; Banerjee, S.; Jackson, D.; Lord, R.; McCormack, M.; et al. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Schwarz, T.F.; Galaj, A.; Spaczynski, M.; Wysocki, J.; Kaufmann, A.M.; Poncelet, S.; Suryakiran, P.V.; Folschweiller, N.; Thomas, F.; Lin, L.; et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017, 6, 2723. [Google Scholar] [CrossRef] [Green Version]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Zhang, D.; Zhang, J. Caught in the Crossfire: How Contradictory Information and Norms on Social Media Influence Young Women’s Intentions to Receive HPV Vaccination in the United States and China. Front. Psychol. 2020, 11, 3469. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Kenter, G.G.; Piersma, S.J.; Vloon, A.P.G.; Löwik, M.J.G.; Berends-van Der Meer, D.M.A.; Drijfhout, J.W.; Valentijn, A.R.P.M.; Wafelman, A.R.; Oostendorp, J.; et al. Induction of Tumor-Specific CD4+ and CD8+ T-Cell Immunity in Cervical Cancer Patients by a Human Papillomavirus Type 16 E6 and E7 Long Peptides Vaccine. Clin. Cancer Res. 2008, 14, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, M.; Gupta, S.K.; Coleman, H.N.; Sellers, M.A.; Banken, J.A.; Greenfield, W.W. A favorable clinical trend is associated with CD8 T-cell immune responses to the human papillomavirus type 16 e6 antigens in women being studied for abnormal pap smear results. J. Low. Genit. Tract Dis. 2010, 14, 124–129. [Google Scholar] [CrossRef] [PubMed]
- van Poelgeest, M.I.E.; Welters, M.J.P.; van Esch, E.M.G.; Stynenbosch, L.F.M.; Kerpershoek, G.; van Persijn van Meerten, E.L.; van den Hende, M.; Löwik, M.J.G.; Berends-van der Meer, D.M.A.; Fathers, L.M.; et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human Papillomavirus Type 16 Viral Load is Decreased Following a Therapeutic Vaccination. Cancer Immunol. Immunother. 2016, 65, 563. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, W.W.; Stratton, S.L.; Myrick, R.S.; Vaughn, R.; Donnalley, L.M.; Coleman, H.N.; Mercado, M.; Moerman-Herzog, A.M.; Spencer, H.J.; Andrews-Collins, N.R.; et al. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeutic vaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology 2015, 4, e1031439. [Google Scholar] [CrossRef] [Green Version]
- Solares, A.M.; Baladron, I.; Ramos, T.; Valenzuela, C.; Borbon, Z.; Fanjull, S.; Gonzalez, L.; Castillo, D.; Esmir, J.; Granadillo, M.; et al. Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial. ISRN Obstet. Gynecol. 2011, 2011, 292951. [Google Scholar] [CrossRef] [Green Version]
- Slingerland, M.; Speetjens, F.; Welters, M.; Gelderblom, H.; Roozen, I.; van der Velden, L.-A.; Melief, C.J.; Zandvliet, M.; van der Burg, S.; Ossendorp, F. A phase I study in patients with a human papillomavirus type 16 positive oropharyngeal tumor treated with second generation synthetic long peptide vaccine conjugated to a defined adjuvant. J. Clin. Oncol. 2016, 34, TPS3113. [Google Scholar] [CrossRef]
- Zom, G.G.; Willems, M.M.J.H.P.; Khan, S.; van Der Sluis, T.C.; Kleinovink, J.W.; Camps, M.G.M.; van Der Marel, G.A.; Filippov, D.V.; Melief, C.J.M.; Ossendorp, F. Novel TLR2-binding adjuvant induces enhanced T cell responses and tumor eradication. J. Immunother. Cancer 2018, 6, 146. [Google Scholar] [CrossRef]
- Maynard, S.K.; Marshall, J.D.; MacGill, R.S.; Yu, L.; Cann, J.A.; Cheng, L.I.; McCarthy, M.P.; Cayatte, C.; Robbins, S.H. Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication. BMC Cancer 2019, 19, 540. [Google Scholar] [CrossRef]
- Galliverti, G.; Tichet, M.; Domingos-Pereira, S.; Hauert, S.; Nardelli-Haefliger, D.; Swartz, M.A.; Hanahan, D.; Wullschleger, S. Nanoparticle Conjugation of Human Papillomavirus 16 E7-long Peptides Enhances Therapeutic Vaccine Efficacy against Solid Tumors in Mice. Cancer Immunol. Res. 2018, 6, 1301–1313. [Google Scholar] [CrossRef]
- Esquerré, M.; Bouillette-Marussig, M.; Goubier, A.; Momot, M.; Gonindard, C.; Keller, H.; Navarro, A.; Bissery, M.C. GTL001, a bivalent therapeutic vaccine against human papillomavirus 16 and 18, induces antigen-specific CD8+ T cell responses leading to tumor regression. PLoS ONE 2017, 12, e0174038. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, D.M.; Skeate, J.G.; Chavez-Juan, E.; Lühen, K.P.; Wu, J.M.; Wu, C.M.; Kast, W.M.; Hwang, K.K. Therapeutic efficacy of a human papillomavirus type 16 E7 bacterial exotoxin fusion protein adjuvanted with CpG or GPI-0100 in a preclinical mouse model for HPV-associated disease. Vaccine 2019, 37, 2915–2924. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Berry, J.M.; Jay, N.; Krogstad, M.; da Costa, M.; Darragh, T.M.; Lee, J.Y. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS 2006, 20, 1151–1155. [Google Scholar] [CrossRef]
- Roman, L.D.; Wilczynski, S.; Muderspach, L.I.; Burnett, A.F.; O’Meara, A.; Brinkman, J.A.; Kast, W.M.; Facio, G.; Felix, J.C.; Aldana, M.; et al. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2007, 106, 558–566. [Google Scholar] [CrossRef]
- Einstein, M.H.; Kadish, A.S.; Burk, R.D.; Kim, M.Y.; Wadler, S.; Streicher, H.; Goldberg, G.L.; Runowicz, C.D. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol. Oncol. 2007, 106, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Brun, J.L.; Dalstein, V.; Leveque, J.; Mathevet, P.; Raulic, P.; Baldauf, J.J.; Scholl, S.; Huynh, B.; Douvier, S.; Riethmuller, D.; et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am. J. Obstet. Gynecol. 2011, 204, 169.e1–169.e8. [Google Scholar] [CrossRef]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Oosterhuis, K.; Wunderlich, K.; Bunnik, E.M.; Bhaggoe, M.; Boedhoe, S.; Karia, S.; Steenbergen, R.D.M.; Bosch, L.; Serroyen, J.; et al. Development of a replication-deficient adenoviral vector-based vaccine candidate for the interception of HPV16- and HPV18-induced infections and disease. Int. J. Cancer 2017, 141, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes–Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 764. [Google Scholar] [CrossRef] [Green Version]
- Huh, W.K.; Brady, W.E.; Fracasso, P.M.; Dizon, D.S.; Powell, M.A.; Monk, B.J.; Leath, C.A.; Landrum, L.M.; Tanner, E.J.; Crane, E.K.; et al. Phase II Study of Axalimogene Filolisbac (ADXS-HPV) for Platinum-Refractory Cervical Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2020, 158, 562. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Shen, X.; Amante, D.; Sakata, L.; Parker, L.; Yan, J.; Boyer, J.; Roh, C.; et al. Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Mol. Ther. Oncolytics 2016, 3, 16025. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.D.; Huh, W.K.; Bae, S.; Lamb, L.S.; Conner, M.G.; Boyer, J.; Wang, C.; Hung, C.F.; Sauter, E.; Paradis, M.; et al. A Pilot Study of pNGVL4a-CRT/E7(detox) for the Treatment of Patients with HPV16+ Cervical Intraepithelial Neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2016, 140, 245. [Google Scholar] [CrossRef] [Green Version]
- Trimble, C.L.; Peng, S.; Kos, F.; Gravitt, P.; Viscidi, R.; Sugar, E.; Pardoll, D.; Wu, T.C. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009, 15, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| TNM | FIGO | Description |
|---|---|---|
| TI | I | Carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| TIA | IA | Invasive carcinoma that can be diagnosed only with microscopy, with maximum depth of invasion < 5 mm |
| TIA1 | IA1 | Stromal invasion < 3 mm in depth |
| TIA2 | IA2 | Stromal invasion ≤ 3 mm and <5 mm in depth |
| TIB | IB | Invasive carcinoma confined to the uterine cervix, with measured deepest invasion ≥ 5 mm |
| TIB1 | IB1 | Tumor measures < 2 cm in greatest dimension |
| TIB2 | IB2 | Tumor measures ≤ 2 cm and < 4 cm in greatest dimension |
| TIB3 | IB3 | Tumor measures ≥ 4 cm in greatest dimension |
| TII | II | Carcinoma invades beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| TIIA | IIA | Limited to the upper two-thirds of the vagina without parametrial involvement |
| TIIA1 | IIA1 | Tumor measures < 4 cm in greatest dimension |
| TIIA2 | IIA2 | Tumor measures ≥ 4 cm in greatest dimension |
| TIIB | IIB | With parametrial involvement but not up to the pelvic wall |
| TIII | III | Carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or nonfunctioning kidney and/or involves pelvic and/or para-aortic lymph nodes |
| TIIIA | IIIA | Involves the lower third of the vagina, with no extension to the pelvic wall |
| TIIIB | IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney from tumor |
| TIIIC | IIIC | Involvement of pelvic and/or para-aortic lymph nodes, irrespective of tumor size and extent |
| TIIIC1 | IIIC1 | Pelvic lymph node metastasis only |
| TIIIC2 | IIIC2 | Para-aortic lymph node metastasis |
| TIV | IV | Carcinoma has extended beyond the true pelvis or has involved (biopsy-proven) the mucosa of the bladder or rectum |
| TIVA | IVA | Spread to adjacent pelvic organs |
| TIVB | IVB | Spread to distant organs |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boon, S.S.; Luk, H.Y.; Xiao, C.; Chen, Z.; Chan, P.K.S. Review of the Standard and Advanced Screening, Staging Systems and Treatment Modalities for Cervical Cancer. Cancers 2022, 14, 2913. https://doi.org/10.3390/cancers14122913
Boon SS, Luk HY, Xiao C, Chen Z, Chan PKS. Review of the Standard and Advanced Screening, Staging Systems and Treatment Modalities for Cervical Cancer. Cancers. 2022; 14(12):2913. https://doi.org/10.3390/cancers14122913
Chicago/Turabian StyleBoon, Siaw Shi, Ho Yin Luk, Chuanyun Xiao, Zigui Chen, and Paul Kay Sheung Chan. 2022. "Review of the Standard and Advanced Screening, Staging Systems and Treatment Modalities for Cervical Cancer" Cancers 14, no. 12: 2913. https://doi.org/10.3390/cancers14122913
APA StyleBoon, S. S., Luk, H. Y., Xiao, C., Chen, Z., & Chan, P. K. S. (2022). Review of the Standard and Advanced Screening, Staging Systems and Treatment Modalities for Cervical Cancer. Cancers, 14(12), 2913. https://doi.org/10.3390/cancers14122913






