Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. γδ T Cell Subsets and Their Key Features
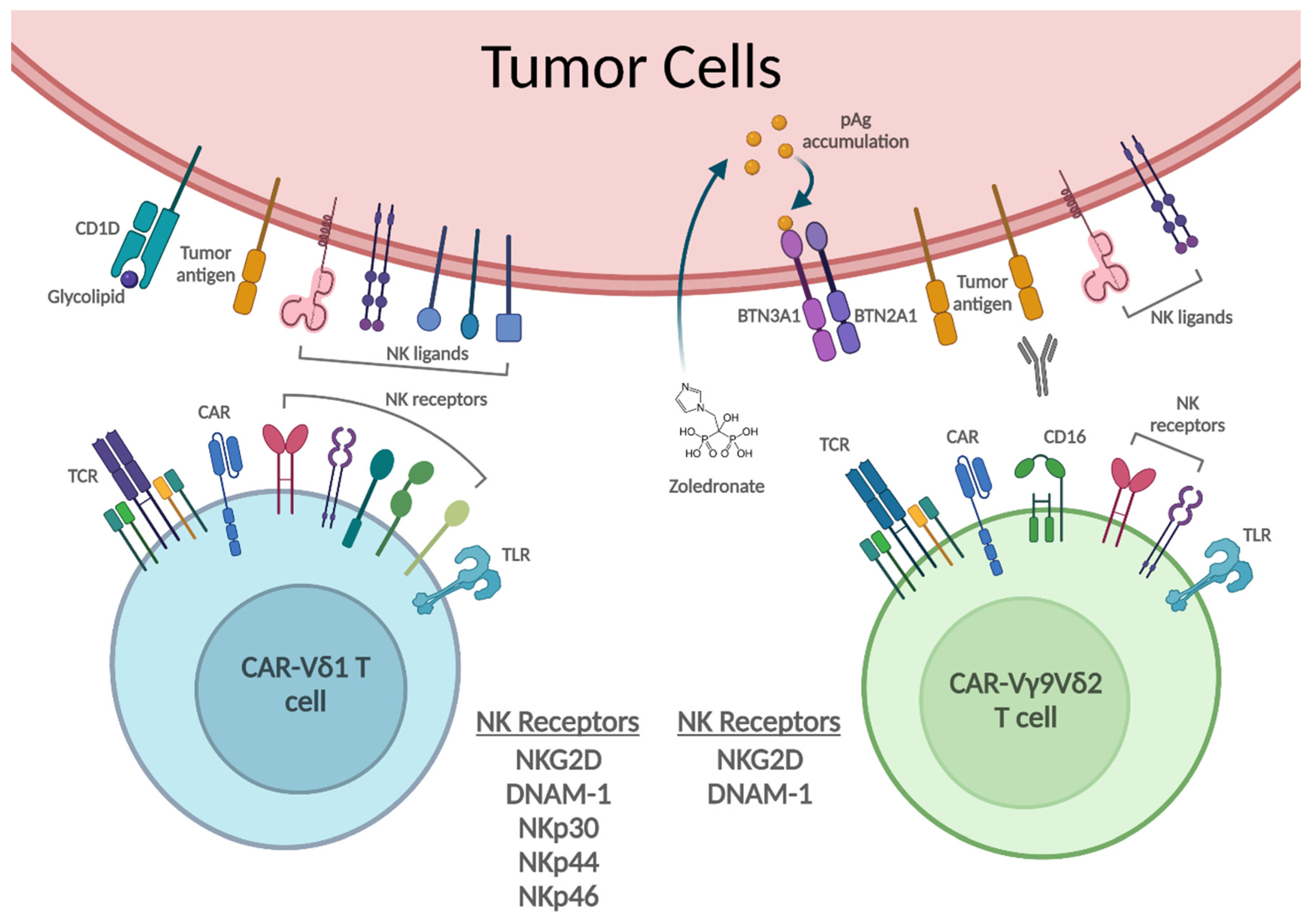
3. γδ T Cell Recognition and Killing of Tumor Cells
4. γδ T Cell Activation via Toll-like Receptors
5. γδ T Cell Modulation of Tumor Microenvironment
6. γδ T Cell Interaction with Other Immune Cells
7. Autologous γδ T Cell Adoptive Transfer Therapy
| Year | Author | Phase | Tumor Type | Treatment | Clinical Outcome |
|---|---|---|---|---|---|
| 2003 | Wilhelm et al. [92] | Pilot clinical study | MM, CLL, MZL | PAM + IL-2 | Cohort A SD: 1/10, PD: 8/10
Cohort B PR: 3/9, SD: 2/9, PD: 4/9
|
| 2007 | Dieli et al. [55] | I | Prostate cancer | ZOL or ZOL + IL-2 | ZOL only PD: 3/9, SD: 1/9, PR: 1/9
ZOL + IL-2. PD: 1/9, SD: 4/9, PR: 2/9
|
| 2010 | Meraviglia et al. [93] | I | Breast cancer | ZOL + IL-2 | PD: 4/10, SD: 2/10, PR: 1/10
|
| 2010 | Bennouna et al. [97] | I | Solid tumor variety | BrHPP + IL-2 | PD: 16/28, SD: 12/28 |
| 2012 | Kunzmann et al. [98] | I/II | RCC, MM, AML | ZOL + IL-2 | PR: 2/21, SD: 6/21, PD: 12/21
|
| 2011 | Lang et al. [99] | Pilot clinical study | RCC | ZOL + IL-2 | SD: 5/12
|
| 2016 | Pressey et al. [100] (NCT01404702) | I | Neuroblastoma | ZOL + IL-2 | PD: 4/4 |
| 2022 | LAVA Therapeutics (NCT04887259) | I/IIa | CLL, MM | Bispecific Ab for Vγ9Vδ2 TCR and CD1d | No patient response has yet been reported.
|
| Year | Author | Phase | Tumor Type | Effector Cells | Clinical Outcome |
|---|---|---|---|---|---|
| 2007 | Kobayashi et al. [94] (NCT00588913) | Pilot clinical study | RCC | Vγ9Vδ2 T + IL-2 | PD: 7/7 5/7 showed an increased γδ T cell population. |
| 2008 | Bennouna et al. [101] | I | RCC | Vγ9Vδ2 T + IL-2 | At one time point, SD: 6/10, PD: 4/10. However, 4 patients were later withdrawn prematurely. |
| 2009 | Abe et al. [102] | Pilot clinical study | MM | Vγ9Vδ T | PD: 6/6
|
| 2010 | Nakajima et al. [103] (C000000336) | I | NSCLC | Vγ9Vδ2 T | At one time point, SD: 3/10, PD: 5/10. However, only 6 patients remained at the end of the study. |
| 2011 | Kobayashi et al. [95] (NCT00588913) | I/II | RCC | Vγ9Vδ2 T + ZOL + IL-2 | PD: 5/11, SD: 5/11, CR: 1/11 |
| 2011 | Sakamoto et al. [104] (C000000336) | I | NSCLC | Vγ9Vδ2 T | PD: 6/15, SD: 6/15
|
| 2011 | Nicol et al. [105] | I | Solid tumors | Vγ9Vδ2 T + ZOL /Vγ9Vδ2 T + ZOL + conventional therapy | Vγ9Vδ2 T+ ZOL only: SD: 3/15, PD: 11/15 Vγ9Vδ2 T+ ZOL + other treatments: CR: 1/3, PR: 2/3
|
| 2013 | Izumi et al. [106] (UMIN000000854) | - | CRC | Vγ9Vδ2 T | PD: 6/6
|
| 2014 | Kakimi et al. [107] (C000000336) | I | NSCLC | Vγ9Vδ2 T | PD: 6/15, SD: 6/15
|
| 2014 | Wada et al. [96] (UMIN000004130) | Pilot clinical study | Gastric cancer | Vγ9Vδ2 T + ZOL | PD: 7/7
|
| 2015 | Cui et al. [108] | Pilot clinical study | Gastric cancer | Chemotherapy + γδ T/NK/Killer cells |
|
| 2017 | Gadeta (NL6357) | I | AML, MM, MDS | Vγ9Vδ2 TCR transduced αβ T cells | - |
8. Allogeneic γδ T Cell Adoptive Transfer Therapy
9. CAR-Engineered γδ T Cell Adoptive Transfer Therapy
10. Other γδ T Cell-Based Therapies
11. γδ T Cell-Based Cancer Immunotherapy: Challenges and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengelov, H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand. J. Immunol. 2015, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Schilbach, K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood 2018, 131, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.M.; Groh, V.; Band, H.; Porcelli, S.A.; Morita, C.; Fabbi, M.; Glass, D.; Strominger, J.L.; Brenner, M.B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med. 1990, 171, 1597–1612. [Google Scholar] [CrossRef] [Green Version]
- Kunzmann, V.; Wilhelm, M. Anti-lymphoma effect of gammadelta T cells. Leuk. Lymphoma 2005, 46, 671–680. [Google Scholar] [CrossRef]
- Di Lorenzo, B.; Simoes, A.E.; Caiado, F.; Tieppo, P.; Correia, D.V.; Carvalho, T.; da Silva, M.G.; Dechanet-Merville, J.; Schumacher, T.N.; Prinz, I.; et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells. Cancer Immunol. Res. 2019, 7, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Kabelitz, D.; He, W. The multifunctionality of human Vgamma9Vdelta2 gammadelta T cells: Clonal plasticity or distinct subsets? Scand. J. Immunol. 2012, 76, 213–222. [Google Scholar] [CrossRef]
- Pistoia, V.; Tumino, N.; Vacca, P.; Veneziani, I.; Moretta, A.; Locatelli, F.; Moretta, L. Human gammadelta T-Cells: From Surface Receptors to the Therapy of High-Risk Leukemias. Front. Immunol. 2018, 9, 984. [Google Scholar] [CrossRef] [Green Version]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. gammadelta T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Morita, C.T.; Parker, C.M.; Brenner, M.B.; Band, H. TCR usage and functional capabilities of human gamma delta T cells at birth. J. Immunol. 1994, 153, 3979–3988. [Google Scholar] [PubMed]
- McVay, L.D.; Carding, S.R.; Bottomly, K.; Hayday, A.C. Regulated expression and structure of T cell receptor gamma/delta transcripts in human thymic ontogeny. EMBO J. 1991, 10, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Casorati, G.; De Libero, G.; Lanzavecchia, A.; Migone, N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J. Exp. Med. 1989, 170, 1521–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.D.; Teunissen, M.B.; Cairo, I.; Krieg, S.R.; Kapsenberg, M.L.; Das, P.K.; Borst, J. T-cell receptor gamma delta bearing cells in normal human skin. J. Investig. Dermatol. 1990, 94, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Deusch, K.; Luling, F.; Reich, K.; Classen, M.; Wagner, H.; Pfeffer, K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur. J. Immunol. 1991, 21, 1053–1059. [Google Scholar] [CrossRef]
- Sandberg, Y.; Almeida, J.; Gonzalez, M.; Lima, M.; Barcena, P.; Szczepanski, T.; van Gastel-Mol, E.J.; Wind, H.; Balanzategui, A.; van Dongen, J.J.; et al. TCRgammadelta+ large granular lymphocyte leukemias reflect the spectrum of normal antigen-selected TCRgammadelta+ T-cells. Leukemia 2006, 20, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Wang, Y.; Li, P.; Zhao, J. Gamma Delta T Cells and Their Pathogenic Role in Psoriasis. Front. Immunol. 2021, 12, 627139. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vdelta2+ T-cell compartment comprises distinct innate-like Vgamma9+ and adaptive Vgamma9− subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef]
- McVay, L.D.; Carding, S.R. Extrathymic origin of human gamma delta T cells during fetal development. J. Immunol. 1996, 157, 2873–2882. [Google Scholar]
- Papadopoulou, M.; Tieppo, P.; McGovern, N.; Gosselin, F.; Chan, J.K.Y.; Goetgeluk, G.; Dauby, N.; Cogan, A.; Donner, C.; Ginhoux, F.; et al. TCR Sequencing Reveals the Distinct Development of Fetal and Adult Human Vgamma9Vdelta2 T Cells. J. Immunol. 2019, 203, 1468–1479. [Google Scholar] [CrossRef] [Green Version]
- Willcox, C.R.; Davey, M.S.; Willcox, B.E. Development and Selection of the Human Vgamma9Vdelta2+ T-Cell Repertoire. Front. Immunol. 2018, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Kenna, T.; Golden-Mason, L.; Norris, S.; Hegarty, J.E.; O’Farrelly, C.; Doherty, D.G. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 2004, 113, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, M.; Wang, C.; Zhu, L.; Hu, J.; Chen, S.; Wu, X.; Li, B.; Li, Y. The feature of distribution and clonality of TCR gamma/delta subfamilies T cells in patients with B-cell non-Hodgkin lymphoma. J. Immunol. Res. 2014, 2014, 241246. [Google Scholar] [CrossRef] [Green Version]
- Rigau, M.; Uldrich, A.P.; Behren, A. Targeting butyrophilins for cancer immunotherapy. Trends Immunol. 2021, 42, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Recio, C.; Aranda-Tavio, H.; Guerra-Rodriguez, M.; Garcia-Castellano, J.M.; Fernandez-Perez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Mizuno, H.; Zhao, X.; Langerod, A.; Moon, S.H.; Rodriguez-Barrueco, R.; Barsotti, A.; Chicas, A.; Li, W.; Polotskaia, A.; et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012, 148, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gammadelta T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef]
- Malinowska, M.; Tokarz-Deptula, B.; Deptula, W. Butyrophilins: An important new element of resistance. Cent. Eur. J. Immunol. 2017, 42, 399–403. [Google Scholar] [CrossRef]
- Salot, S.; Laplace, C.; Saiagh, S.; Bercegeay, S.; Tenaud, I.; Cassidanius, A.; Romagne, F.; Dreno, B.; Tiollier, J. Large scale expansion of gamma 9 delta 2 T lymphocytes: Innacell gamma delta cell therapy product. J. Immunol. Methods 2007, 326, 63–75. [Google Scholar] [CrossRef]
- Deniger, D.C.; Moyes, J.S.; Cooper, L.J. Clinical applications of gamma delta T cells with multivalent immunity. Front. Immunol. 2014, 5, 636. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Sakuta, K.; Noguchi, A.; Ariyoshi, N.; Sato, K.; Sato, S.; Sato, K.; Hosoi, A.; Nakajima, J.; Yoshida, Y.; et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008, 10, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Izumi, T.; Fujieda, N.; Kondo, A.; Morishita, T.; Matsushita, H.; Kakimi, K. Expansion of human peripheral blood γδ T cells using zoledronate. J. Vis. Exp. 2011, 55, e3182. [Google Scholar] [CrossRef] [Green Version]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Komuczki, J.; Rahm, A.; Thurnher, M. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J. Immunol. 2013, 191, 1346–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef]
- Marcu-Malina, V.; Heijhuurs, S.; van Buuren, M.; Hartkamp, L.; Strand, S.; Sebestyen, Z.; Scholten, K.; Martens, A.; Kuball, J. Redirecting alphabeta T cells against cancer cells by transfer of a broadly tumor-reactive gammadeltaT-cell receptor. Blood 2011, 118, 50–59. [Google Scholar] [CrossRef]
- Luoma, A.M.; Castro, C.D.; Adams, E.J. gammadelta T cell surveillance via CD1 molecules. Trends Immunol. 2014, 35, 613–621. [Google Scholar] [CrossRef]
- Bai, L.; Picard, D.; Anderson, B.; Chaudhary, V.; Luoma, A.; Jabri, B.; Adams, E.J.; Savage, P.B.; Bendelac, A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur. J. Immunol. 2012, 42, 2505–2510. [Google Scholar] [CrossRef]
- Russano, A.M.; Agea, E.; Corazzi, L.; Postle, A.D.; De Libero, G.; Porcelli, S.; de Benedictis, F.M.; Spinozzi, F. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J. Allergy Clin. Immunol. 2006, 117, 1178–1184. [Google Scholar] [CrossRef]
- Agea, E.; Russano, A.; Bistoni, O.; Mannucci, R.; Nicoletti, I.; Corazzi, L.; Postle, A.D.; De Libero, G.; Porcelli, S.A.; Spinozzi, F. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 2005, 202, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Mangan, B.A.; Dunne, M.R.; O’Reilly, V.P.; Dunne, P.J.; Exley, M.A.; O’Shea, D.; Scotet, E.; Hogan, A.E.; Doherty, D.G. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J. Immunol. 2013, 191, 30–34. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, C.; Guo, H.; Gao, S.H.; Yang, F.Y.; Zhou, G.B.; Wang, G.Z. Comprehensive analysis of BTN3A1 in cancers: Mining of omics data and validation in patient samples and cellular models. FEBS Open Bio 2021, 11, 2586–2599. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Dhodapkar, M.V. Natural Killer T Cells in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebestyen, Z.; Prinz, I.; Dechanet-Merville, J.; Silva-Santos, B.; Kuball, J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2020, 19, 169–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Simoes, A.E.; Di Lorenzo, B.; Silva-Santos, B. Molecular Determinants of Target Cell Recognition by Human gammadelta T Cells. Front. Immunol. 2018, 9, 929. [Google Scholar] [CrossRef] [Green Version]
- Groh, V.; Rhinehart, R.; Secrist, H.; Bauer, S.; Grabstein, K.H.; Spies, T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 1999, 96, 6879–6884. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Cao, W.; Xi, X.; Ma, C.; Cui, L.; He, W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood 2009, 114, 310–317. [Google Scholar] [CrossRef]
- Lanca, T.; Correia, D.V.; Moita, C.F.; Raquel, H.; Neves-Costa, A.; Ferreira, C.; Ramalho, J.S.; Barata, J.T.; Moita, L.F.; Gomes, A.Q.; et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood 2010, 115, 2407–2411. [Google Scholar] [CrossRef] [Green Version]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with gammadelta T cells: Many paths ahead of us. Cell Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Chan, C.J.; Andrews, D.M.; McLaughlin, N.M.; Yagita, H.; Gilfillan, S.; Colonna, M.; Smyth, M.J. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J. Immunol. 2010, 184, 902–911. [Google Scholar] [CrossRef]
- Pende, D.; Bottino, C.; Castriconi, R.; Cantoni, C.; Marcenaro, S.; Rivera, P.; Spaggiari, G.M.; Dondero, A.; Carnemolla, B.; Reymond, N.; et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: Involvement in tumor cell lysis. Mol. Immunol. 2005, 42, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Silva-Santos, B.; Mavilio, D. Natural cytotoxicity receptors: Broader expression patterns and functions in innate and adaptive immune cells. Front. Immunol. 2013, 4, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (gammadelta) T cells: Friend or foe in cancer development? J. Transl Med. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todaro, M.; D’Asaro, M.; Caccamo, N.; Iovino, F.; Francipane, M.G.; Meraviglia, S.; Orlando, V.; La Mendola, C.; Gulotta, G.; Salerno, A.; et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J. Immunol. 2009, 182, 7287–7296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieli, F.; Vermijlen, D.; Fulfaro, F.; Caccamo, N.; Meraviglia, S.; Cicero, G.; Roberts, A.; Buccheri, S.; D’Asaro, M.; Gebbia, N.; et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7450–7457. [Google Scholar] [CrossRef] [Green Version]
- Couzi, L.; Pitard, V.; Sicard, X.; Garrigue, I.; Hawchar, O.; Merville, P.; Moreau, J.F.; Dechanet-Merville, J. Antibody-dependent anti-cytomegalovirus activity of human gammadelta T cells expressing CD16 (FcgammaRIIIa). Blood 2012, 119, 1418–1427. [Google Scholar] [CrossRef]
- Tokuyama, H.; Hagi, T.; Mattarollo, S.R.; Morley, J.; Wang, Q.; So, H.F.; Moriyasu, F.; Nieda, M.; Nicol, A.J. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab. Int. J. Cancer 2008, 122, 2526–2534. [Google Scholar] [CrossRef]
- Dar, A.A.; Patil, R.S.; Chiplunkar, S.V. Insights into the Relationship between Toll Like Receptors and Gamma Delta T Cell Responses. Front. Immunol. 2014, 5, 366. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The History of Toll-like Receptors—Redefining Innate Immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Liu, H.; Komai-Koma, M.; Xu, D.; Liew, F.Y. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7048–7053. [Google Scholar] [CrossRef] [Green Version]
- Sutmuller, R.P.; den Brok, M.H.; Kramer, M.; Bennink, E.J.; Toonen, L.W.; Kullberg, B.J.; Joosten, L.A.; Akira, S.; Netea, M.G.; Adema, G.J. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Investig. 2006, 116, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.S.; Vincent, M.S.; Spada, F.M.; Das, H.; Sugita, M.; Morita, C.T.; Brenner, M.B. CD1-mediated gamma/delta T cell maturation of dendritic cells. J. Exp. Med. 2002, 196, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Welte, T.; Zheng, X.; Chang, G.J.; Holbrook, M.R.; Soong, L.; Wang, T. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol. Med. Microbiol. 2010, 59, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Casetti, R.; Cardone, M.; Varano, B.; Martino, A.; Belardelli, F.; Poccia, F.; Gessani, S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: Role of CD86 and inflammatory cytokines. J. Immunol. 2005, 174, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, K.; Beetz, S.; Welte, S.; Martens, I.; Gruen, J.; Oberg, H.H.; Wesch, D.; Kabelitz, D. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand. J. Immunol. 2009, 70, 245–255. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Liu, S.; Zhang, J.; Lu, H.; Somasundaram, R.; Choi, R.; Zhang, G.; Ou, L.; Scholler, J.; et al. Costimulation of gammadeltaTCR and TLR7/8 promotes Vdelta2 T-cell antitumor activity by modulating mTOR pathway and APC function. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Corsale, A.M.; Di Simone, M.; Lo Presti, E.; Picone, C.; Dieli, F.; Meraviglia, S. Metabolic Changes in Tumor Microenvironment: How Could They Affect gammadelta T Cells Functions? Cells 2021, 10, 2896. [Google Scholar] [CrossRef]
- Fowler, D.W.; Copier, J.; Dalgleish, A.G.; Bodman-Smith, M.D. Zoledronic acid renders human M1 and M2 macrophages susceptible to Vdelta2+ gammadelta T cell cytotoxicity in a perforin-dependent manner. Cancer Immunol. Immunother. 2017, 66, 1205–1215. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Foda, H.D.; Zucker, S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov. Today 2001, 6, 478–482. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L.; Wind, N.; Hughes, R.; Nutter, F.; Brown, H.K.; Vasiliadou, I.; Ottewell, P.D.; Holen, I. Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models. Cell Oncol. 2013, 36, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.; Quaglino, E.; Iezzi, M.; Curcio, C.; Pantaleoni, F.; Riganti, C.; Holen, I.; Monkkonen, H.; Boccadoro, M.; Forni, G.; et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J. Cell Mol. Med. 2010, 14, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Melani, C.; Sangaletti, S.; Barazzetta, F.M.; Werb, Z.; Colombo, M.P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007, 67, 11438–11446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandes, M.; Willimann, K.; Moser, B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [Green Version]
- Brandes, M.; Willimann, K.; Bioley, G.; Levy, N.; Eberl, M.; Luo, M.; Tampe, R.; Levy, F.; Romero, P.; Moser, B. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc. Natl. Acad. Sci. USA 2009, 106, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Muto, M.; Baghdadi, M.; Maekawa, R.; Wada, H.; Seino, K. Myeloid molecular characteristics of human gammadelta T cells support their acquisition of tumor antigen-presenting capacity. Cancer Immunol. Immunother. 2015, 64, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Saveanu, L.; Carroll, O.; Weimershaus, M.; Guermonprez, P.; Firat, E.; Lindo, V.; Greer, F.; Davoust, J.; Kratzer, R.; Keller, S.R.; et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science 2009, 325, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Scotet, E.; Nedellec, S.; Devilder, M.C.; Allain, S.; Bonneville, M. Bridging innate and adaptive immunity through gammadelta T-dendritic cell crosstalk. Front. Biosci. 2008, 13, 6872–6885. [Google Scholar] [CrossRef] [Green Version]
- van Beek, J.J.; Wimmers, F.; Hato, S.V.; de Vries, I.J.; Skold, A.E. Dendritic cell cross talk with innate and innate-like effector cells in antitumor immunity: Implications for DC vaccination. Crit. Rev. Immunol. 2014, 34, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Devilder, M.C.; Allain, S.; Dousset, C.; Bonneville, M.; Scotet, E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J. Immunol. 2009, 183, 3625–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzmann, V.; Kretzschmar, E.; Herrmann, T.; Wilhelm, M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology 2004, 112, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.W.; Copier, J.; Wilson, N.; Dalgleish, A.G.; Bodman-Smith, M.D. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: A mechanism of action for cancer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Kubo, S.; Okuda, A.; Yamamoto, H.; Ueda, H.; Tanaka, T.; Nakamura, H.; Yamanishi, H.; Terada, N.; Okamura, H. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J. Immunother. 2010, 33, 287–296. [Google Scholar] [CrossRef]
- Fiore, F.; Castella, B.; Nuschak, B.; Bertieri, R.; Mariani, S.; Bruno, B.; Pantaleoni, F.; Foglietta, M.; Boccadoro, M.; Massaia, M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood 2007, 110, 921–927. [Google Scholar] [CrossRef]
- Su, X.; Zhang, L.; Jin, L.; Ye, J.; Guan, Z.; Chen, R. Coculturing dendritic cells with zoledronate acid efficiently enhance the anti-tumor effects of cytokine-induced killer cells. J. Clin. Immunol. 2010, 30, 766–774. [Google Scholar] [CrossRef]
- Takahara, M.; Miyai, M.; Tomiyama, M.; Mutou, M.; Nicol, A.J.; Nieda, M. Copulsing tumor antigen-pulsed dendritic cells with zoledronate efficiently enhance the expansion of tumor antigen-specific CD8+ T cells via Vgamma9gammadelta T cell activation. J. Leukoc. Biol. 2008, 83, 742–754. [Google Scholar] [CrossRef]
- Maniar, A.; Zhang, X.; Lin, W.; Gastman, B.R.; Pauza, C.D.; Strome, S.E.; Chapoval, A.I. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 2010, 116, 1726–1733. [Google Scholar] [CrossRef]
- Ladel, C.H.; Blum, C.; Kaufmann, S.H. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect. Immun. 1996, 64, 1744–1749. [Google Scholar] [CrossRef] [Green Version]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Thurnher, M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by gammadelta T lymphocytes. Blood 2011, 118, 2743–2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, M.; Kunzmann, V.; Eckstein, S.; Reimer, P.; Weissinger, F.; Ruediger, T.; Tony, H.P. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003, 102, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; La Mendola, C.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Osaka, Y.; Nakazawa, H.; Uchiyama, T.; Minato, N.; Toma, H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: A pilot study. Cancer Immunol. Immunother. 2007, 56, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Minato, N.; Tanabe, K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1075–1084. [Google Scholar] [CrossRef]
- Wada, I.; Matsushita, H.; Noji, S.; Mori, K.; Yamashita, H.; Nomura, S.; Shimizu, N.; Seto, Y.; Kakimi, K. Intraperitoneal injection of in vitro expanded Vgamma9Vdelta2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014, 3, 362–375. [Google Scholar] [CrossRef]
- Bennouna, J.; Levy, V.; Sicard, H.; Senellart, H.; Audrain, M.; Hiret, S.; Rolland, F.; Bruzzoni-Giovanelli, H.; Rimbert, M.; Galea, C.; et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol. Immunother. 2010, 59, 1521–1530. [Google Scholar] [CrossRef]
- Kunzmann, V.; Smetak, M.; Kimmel, B.; Weigang-Koehler, K.; Goebeler, M.; Birkmann, J.; Becker, J.; Schmidt-Wolf, I.G.; Einsele, H.; Wilhelm, M. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: Results from a prospective phase I/II trial. J. Immunother. 2012, 35, 205–213. [Google Scholar] [CrossRef]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef] [Green Version]
- Pressey, J.G.; Adams, J.; Harkins, L.; Kelly, D.; You, Z.; Lamb, L.S., Jr. In vivo expansion and activation of gammadelta T cells as immunotherapy for refractory neuroblastoma: A phase 1 study. Medicine 2016, 95, e4909. [Google Scholar] [CrossRef]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galea, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Muto, M.; Nieda, M.; Nakagawa, Y.; Nicol, A.; Kaneko, T.; Goto, S.; Yokokawa, K.; Suzuki, K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp. Hematol. 2009, 37, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.; Murakawa, T.; Fukami, T.; Goto, S.; Kaneko, T.; Yoshida, Y.; Takamoto, S.; Kakimi, K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur. J. Cardiothorac. Surg. 2010, 37, 1191–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Nakajima, J.; Murakawa, T.; Fukami, T.; Yoshida, Y.; Murayama, T.; Takamoto, S.; Matsushita, H.; Kakimi, K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadeltaTcells: A phase I clinical study. J. Immunother. 2011, 34, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.R.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Izumi, T.; Kondo, M.; Takahashi, T.; Fujieda, N.; Kondo, A.; Tamura, N.; Murakawa, T.; Nakajima, J.; Matsushita, H.; Kakimi, K. Ex vivo characterization of gammadelta T-cell repertoire in patients after adoptive transfer of Vgamma9Vdelta2 T cells expressing the interleukin-2 receptor beta-chain and the common gamma-chain. Cytotherapy 2013, 15, 481–491. [Google Scholar] [CrossRef]
- Kakimi, K.; Matsushita, H.; Murakawa, T.; Nakajima, J. gammadelta T cell therapy for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res. 2014, 3, 23–33. [Google Scholar] [CrossRef]
- Cui, J.; Li, L.; Wang, C.; Jin, H.; Yao, C.; Wang, Y.; Li, D.; Tian, H.; Niu, C.; Wang, G.; et al. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy 2015, 17, 979–988. [Google Scholar] [CrossRef]
- Wilhelm, M.; Smetak, M.; Schaefer-Eckart, K.; Kimmel, B.; Birkmann, J.; Einsele, H.; Kunzmann, V. Successful adoptive transfer and in vivo expansion of haploidentical gammadelta T cells. J. Transl. Med. 2014, 12, 45. [Google Scholar] [CrossRef]
- Alnaggar, M.; Xu, Y.; Li, J.; He, J.; Chen, J.; Li, M.; Wu, Q.; Lin, L.; Liang, Y.; Wang, X.; et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: A case study for cholangiocarcinoma. J. Immunother. Cancer 2019, 7, 36. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, X.; Liang, S.; Luo, H.; Alnaggar, M.; Liu, A.; Yin, Z.; Chen, J.; Niu, L.; Jiang, Y. Irreversible electroporation plus allogenic Vgamma9Vdelta2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct Target Ther. 2020, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Deniger, D.C.; Switzer, K.; Mi, T.; Maiti, S.; Hurton, L.; Singh, H.; Huls, H.; Olivares, S.; Lee, D.A.; Champlin, R.E.; et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 2013, 21, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.X.; Ng, Y.Y.; Xiao, L.; Chen, C.; Li, Z.; Chi, Z.; Tay, J.C.; Tan, W.K.; Zeng, J.; Toh, H.C.; et al. Electroporation of NKG2D RNA CAR Improves Vgamma9Vdelta2 T Cell Responses against Human Solid Tumor Xenografts. Mol. Ther. Oncolytics 2020, 17, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.; Meir, A.; Aharony, Y.; Itzhaki, O.; Schachter, J.; Bank, I.; Jacoby, E.; Besser, M.J. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukemia. Front. Immunol. 2020, 11, 1347. [Google Scholar] [CrossRef]
- Johanna, I.; Straetemans, T.; Heijhuurs, S.; Aarts-Riemens, T.; Norell, H.; Bongiovanni, L.; de Bruin, A.; Sebestyen, Z.; Kuball, J. Evaluating in vivo efficacy-toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J. Immunother. Cancer 2019, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.; Abramowski, P.; Wisidagamage Don, N.D.; Flutter, B.; Capsomidis, A.; Cheung, G.W.; Gustafsson, K.; Anderson, J. Avoidance of On-Target Off-Tumor Activation Using a Co-stimulation-Only Chimeric Antigen Receptor. Mol. Ther. 2017, 25, 1234–1247. [Google Scholar] [CrossRef] [Green Version]
- Middelburg, J.; Kemper, K.; Engelberts, P.; Labrijn, A.F.; Schuurman, J.; van Hall, T. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers 2021, 13, 287. [Google Scholar] [CrossRef]
- Goebeler, M.E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, Y.; Zong, H.; Han, L.; Ke, Y.; Pan, Z.; Chen, J.; Lu, J.; Li, J.; Ying, T.; et al. IgG-like Bispecific Antibody CD3xEpCAM Generated by Split Intein Against Colorectal Cancer. Front. Pharmacol. 2022, 13, 803059. [Google Scholar] [CrossRef]
- Oberg, H.H.; Peipp, M.; Kellner, C.; Sebens, S.; Krause, S.; Petrick, D.; Adam-Klages, S.; Rocken, C.; Becker, T.; Vogel, I.; et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014, 74, 1349–1360. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, R.; Chennupati, V.; Ramachandran, B.; Hansen, M.R.; Singh, S.; Grewal, I.S. Selective recruitment of gammadelta T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia 2021, 35, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Koyanagi-Aoi, M.; Taniguchi-Ikeda, M.; Yoshida, Y.; Azuma, T.; Aoi, T. The Generation of Human gammadeltaT Cell-Derived Induced Pluripotent Stem Cells from Whole Peripheral Blood Mononuclear Cell Culture. Stem Cells Transl. Med. 2018, 7, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Tang, S.Y.; Wang, S. Derivation of mimetic gammadelta T cells endowed with cancer recognition receptors from reprogrammed gammadelta T cell. PLoS ONE 2019, 14, e0216815. [Google Scholar] [CrossRef] [PubMed]
- Vermijlen, D.; Gatti, D.; Kouzeli, A.; Rus, T.; Eberl, M. gammadelta T cell responses: How many ligands will it take till we know? Semin. Cell Dev. Biol. 2018, 84, 75–86. [Google Scholar] [CrossRef]
- Vavassori, S.; Kumar, A.; Wan, G.S.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat. Immunol. 2013, 14, 908–916. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Function of γδ T cells in tumor immunology and their application to cancer therapy. Exp. Mol. Med. 2021, 53, 318–327. [Google Scholar] [CrossRef]
- Chabab, G.; Barjon, C.; Bonnefoy, N.; Lafont, V. Pro-tumor gammadelta T Cells in Human Cancer: Polarization, Mechanisms of Action, and Implications for Therapy. Front. Immunol. 2020, 11, 2186. [Google Scholar] [CrossRef]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Rong, L.; Li, K.; Li, R.; Liu, H.M.; Sun, R.; Liu, X.Y. Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J. Gastroenterol. 2016, 22, 3573–3580. [Google Scholar] [CrossRef]
- Patil, R.S.; Shah, S.U.; Shrikhande, S.V.; Goel, M.; Dikshit, R.P.; Chiplunkar, S.V. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 2016, 139, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, W.; Xu, R.; Wu, M.; Zhang, X.; Huang, P.; Wang, F.; Pan, S. Distribution and functions of gammadelta T cells infiltrated in the ovarian cancer microenvironment. J. Transl. Med. 2019, 17, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, D.; Zambirinis, C.P.; Seifert, L.; Akkad, N.; Mohan, N.; Werba, G.; Barilla, R.; Torres-Hernandez, A.; Hundeyin, M.; Mani, V.R.K.; et al. gammadelta T Cells Support Pancreatic Oncogenesis by Restraining alphabeta T Cell Activation. Cell 2016, 166, 1485–1499.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Presti, E.; Pizzolato, G.; Corsale, A.M.; Caccamo, N.; Sireci, G.; Dieli, F.; Meraviglia, S. gammadelta T Cells and Tumor Microenvironment: From Immunosurveillance to Tumor Evasion. Front. Immunol. 2018, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Kang, D.; Yang, D.; Tang, Y. Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8+ T cells in patients with lung cancer. Oncol. Lett. 2018, 15, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Menetrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10, 925. [Google Scholar] [CrossRef] [Green Version]
- Marten, A.; von Lilienfeld-Toal, M.; Buchler, M.W.; Schmidt, J. Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing gammadelta T cell cytotoxicity. Int. J. Cancer 2006, 119, 2359–2365. [Google Scholar] [CrossRef]
- Gonnermann, D.; Oberg, H.H.; Kellner, C.; Peipp, M.; Sebens, S.; Kabelitz, D.; Wesch, D. Resistance of cyclooxygenase-2 expressing pancreatic ductal adenocarcinoma cells against gammadelta T cell cytotoxicity. Oncoimmunology 2015, 4, e988460. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chung, Y.S.; Kim, T.J. Heterogeneity of Human gammadelta T Cells and Their Role in Cancer Immunity. Immune Netw. 2020, 20, e5. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.; Vitale, C.; Peola, S.; Foglietta, M.; Rigoni, M.; Griggio, V.; Castella, B.; Angelini, D.; Chiaretti, S.; Riganti, C.; et al. Dysfunctional Vgamma9Vdelta2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012, 120, 3271–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castella, B.; Foglietta, M.; Sciancalepore, P.; Rigoni, M.; Coscia, M.; Griggio, V.; Vitale, C.; Ferracini, R.; Saraci, E.; Omede, P.; et al. Anergic bone marrow Vgamma9Vdelta2 T cells as early and long-lasting markers of PD-1-targetable microenvironment-induced immune suppression in human myeloma. Oncoimmunology 2015, 4, e1047580. [Google Scholar] [CrossRef] [PubMed]
- Hoeres, T.; Holzmann, E.; Smetak, M.; Birkmann, J.; Wilhelm, M. PD-1 signaling modulates interferon-gamma production by Gamma Delta (gammadelta) T-Cells in response to leukemia. Oncoimmunology 2019, 8, 1550618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieli, F.; Poccia, F.; Lipp, M.; Sireci, G.; Caccamo, N.; Di Sano, C.; Salerno, A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003, 198, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Battistini, L.; Caccamo, N.; Borsellino, G.; Meraviglia, S.; Angelini, D.F.; Dieli, F.; Cencioni, M.T.; Salerno, A. Homing and memory patterns of human gammadelta T cells in physiopathological situations. Microbes Infect. 2005, 7, 510–517. [Google Scholar] [CrossRef]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yao, D.; Tan, J.; He, Z.; Yu, Z.; Chen, J.; Luo, G.; Wang, C.; Zhou, F.; Zha, X.; et al. Memory T cells skew toward terminal differentiation in the CD8+ T cell population in patients with acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 93. [Google Scholar] [CrossRef]
- Cui, W.; Kaech, S.M. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol. Rev. 2010, 236, 151–166. [Google Scholar] [CrossRef] [Green Version]

| Year | Author | Phase | Tumor Type | Effector Cells | Clinical Outcome |
|---|---|---|---|---|---|
| 2014 | Wilhelm et al. [109] | Pilot clinical study | NHL, MM, AML, PCL | Vγ9Vδ2 T, with some CD4-/8- αβT + ZOL + IL-2 | CR: 3/4
|
| 2019 | Alnaggar et al. [110] | Case Study | Cholangiocarcinoma | Vγ9Vδ2 T, 8 infusions | CR: 1/1
|
| 2020 | Lin et al. [111] (NCT03180437) | I/II | Pancreatic cancer | IRE + Vγ9Vδ2 T |
|
| Year | Author | Phase | Tumor Type | Effector Cells | Clinical Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Beijing Doing Biomedical (NCT02656147) | I | B-Cell Lymphoma, ALL, CLL | CD19 CAR-γδ T | - | ||||||
| 2019 | CytoMed Therapeutics (NCT04107142) | I | Solid tumors | NKG2DL CAR-γδ T | - | ||||||
| 2021 | Adicet Bio (NCT04735471) | I | B-cell malignancies | CD20 CAR-Vδ1 T + IL-2 | Interim result–CR: 2/4, PR: 1/4, PD: 1/4 | ||||||
| 2021 | PersonGen BioTherapeutics (NCT04702841) | I | CD7+ T lymphoma (ALL) | CD7 CAR-γδ T | - | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Rosenthal, C.J.; Penn, N.E.; Dunn, Z.S.; Zhou, Y.; Yang, L. Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers 2022, 14, 3005. https://doi.org/10.3390/cancers14123005
Lee D, Rosenthal CJ, Penn NE, Dunn ZS, Zhou Y, Yang L. Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers. 2022; 14(12):3005. https://doi.org/10.3390/cancers14123005
Chicago/Turabian StyleLee, Derek, Carl J. Rosenthal, Natalie E. Penn, Zachary Spencer Dunn, Yang Zhou, and Lili Yang. 2022. "Human γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy" Cancers 14, no. 12: 3005. https://doi.org/10.3390/cancers14123005







