Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies
Abstract
Simple Summary
Abstract
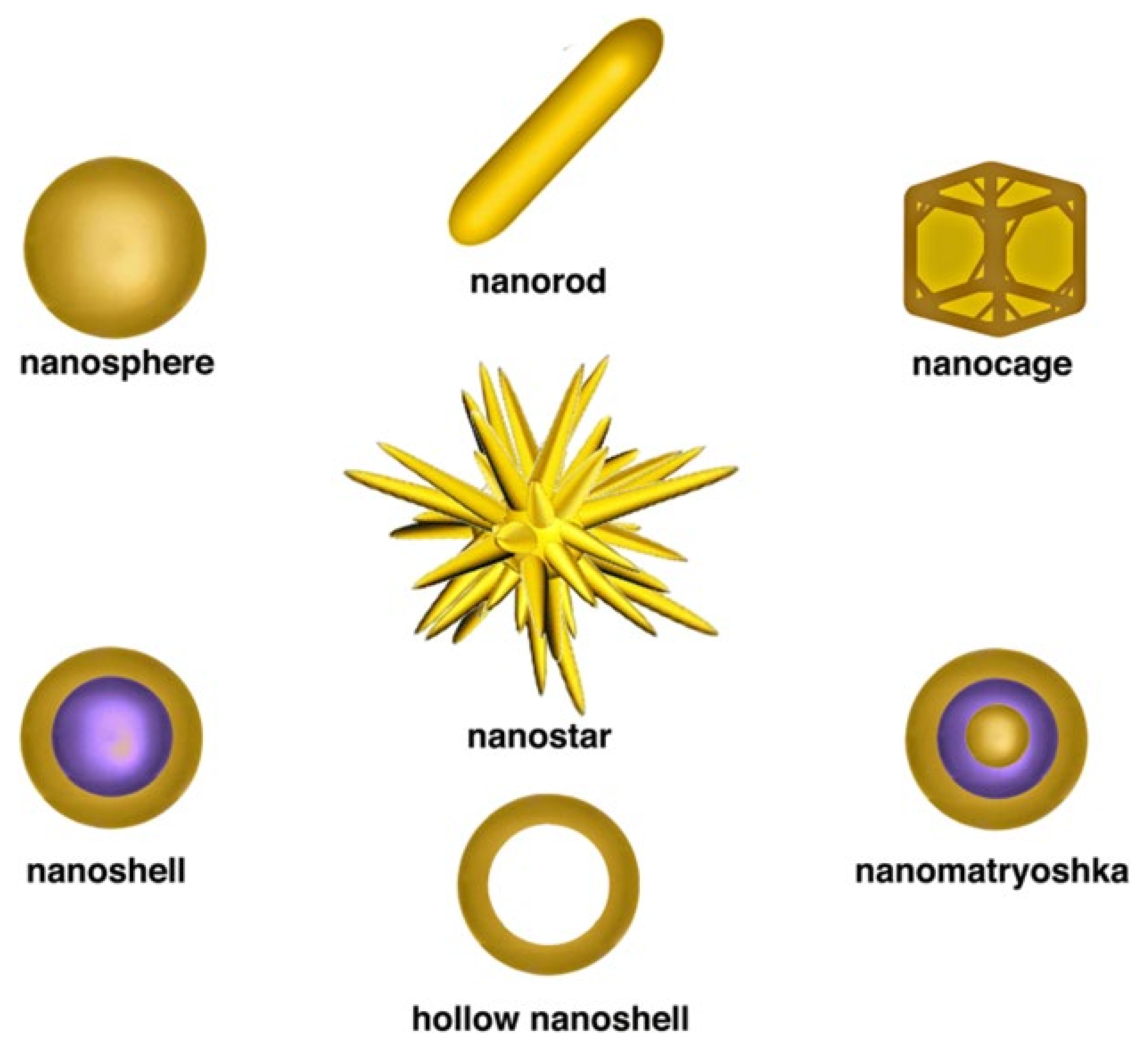
1. Introduction
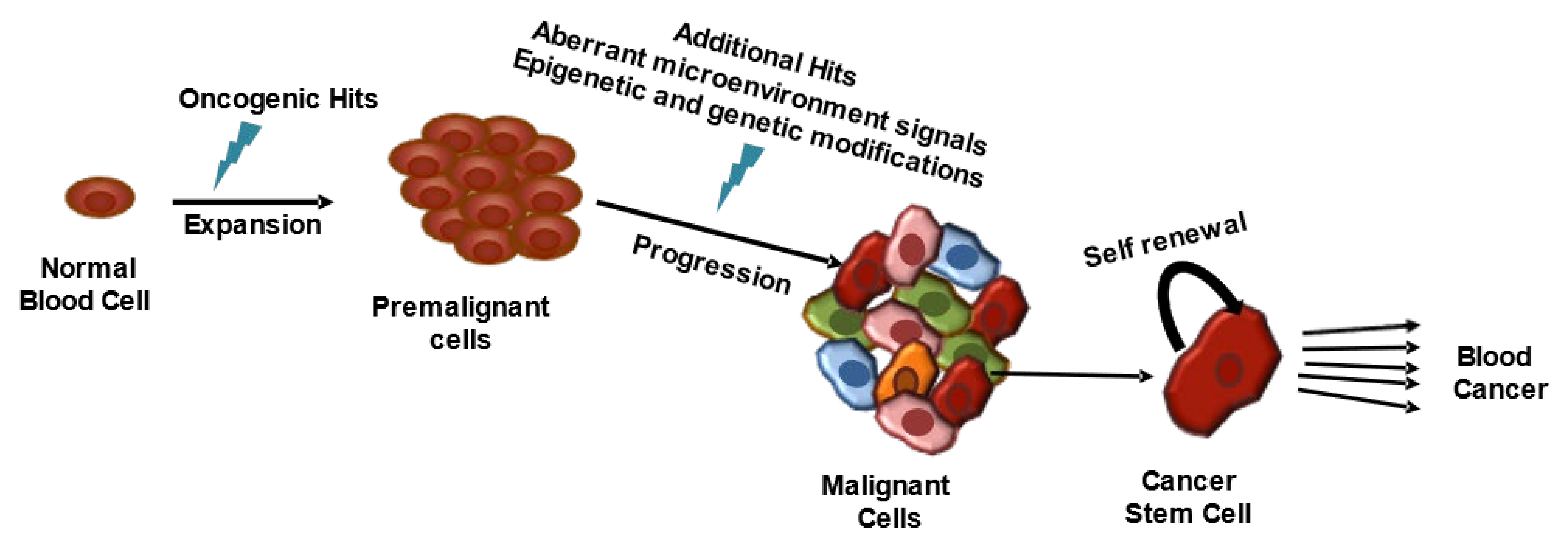
2. Hematological Malignancies
3. Gold-Nanomaterial-Based Diagnosis
3.1. Detection of Cancer Cells
3.2. Detection of Cancer-Associated Biomarkers
3.2.1. Surface-Antigen Detection
3.2.2. Detection of Receptor Overexpression
3.2.3. Detection of Receptor Overexpression
3.2.4. Nucleic-Acids Biomarker Detection
3.2.5. Proteomic Biomarker Detection
4. Gold-Nanomaterial-Based Treatments in Hematological Malignancies
4.1. Photothermal Therapy
4.2. Photodynamic Therapy
4.3. Radiation Therapy
4.4. Gene Therapy
4.5. Improved Delivery of Chemotherapeutic Drugs, Peptides, Antibodies, or Bioactive Compounds
4.5.1. Delivery of Conventional Drugs
4.5.2. Improve Delivery of Antibody Drugs
4.5.3. Peptide-Based Anti-Angiogenic Therapy
4.5.4. Bioactive Compounds
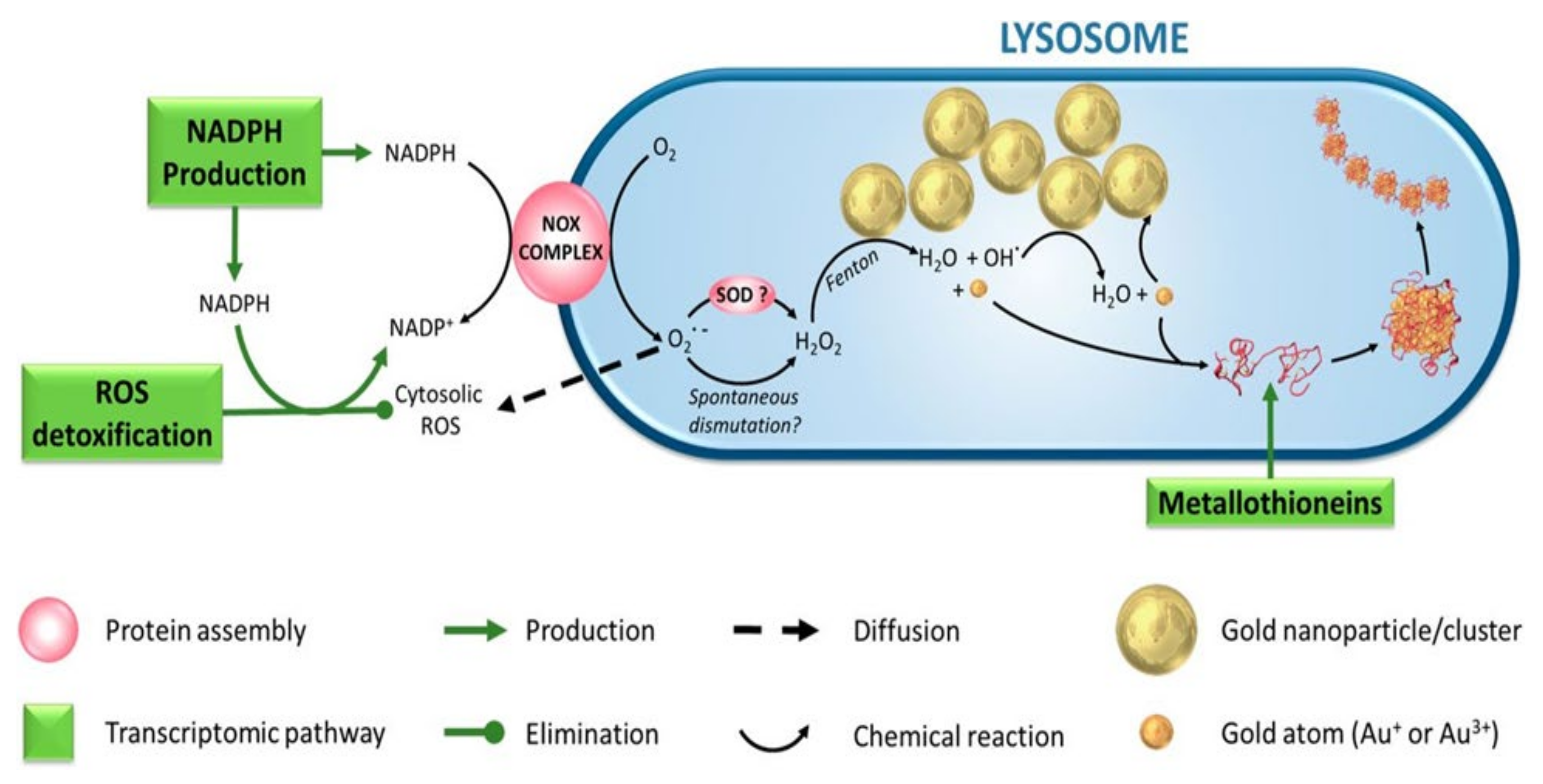
4.6. Reactive Oxygen Species-Mediated Cytotoxicity
4.7. Induction of Apoptosis
5. Challenges of Using GNMs in HMs from Pharmacological and Toxicological Point of View
5.1. Selectivity of Gold Nanomaterials
5.2. Preclinical Experimentation in a Complex System
5.3. Immunomodulatory Effects
5.4. Safety and Biodistribution
5.5. Adverse Effects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Button, E.; Chan, R.J.; Chambers, S.; Butler, J.; Yates, P. A systematic review of prognostic factors at the end of life for people with a hematological malignancy. BMC Cancer 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D.; Lukianova, E.; Shnip, A.; Zheltov, G.; Potapnev, M.; Savitsky, V.; Klimovich, O.; Oraevsky, A. Laser activated nanothermolysis of leukemia cells monitored by photothermal microscopy. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2005: The Sixth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-Optics, San Jose, CA, USA, 25 April 2005; pp. 82–89. [Google Scholar]
- Koutsi, A.; Vervesou, E.C. Diagnostic molecular techniques in haematology: Recent advances. Ann. Transl. Med. 2018, 6, 242. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Pratt, G. Modern molecular diagnostics and the management of haematological malignancies. Clin. Lab. Haematol. 1998, 20, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Ayala, R.; Martínez-López, J. Minimal Residual Disease Monitoring with Next-Generation Sequencing Methodologies in Hematological Malignancies. Int. J. Mol. Sci. 2019, 20, 2832. [Google Scholar] [CrossRef] [PubMed]
- Jółkowska, J.; Derwich, K.; Dawidowska, M. Methods of minimal residual disease (MRD) detection in childhood haematological malignancies. J. Appl. Genet. 2007, 48, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Flanders, A.; Stetler-Stevenson, M.; Landgren, O. Minimal residual disease testing in multiple myeloma by flow cytometry: Major heterogeneity. Blood 2013, 122, 1088–1089. [Google Scholar] [CrossRef]
- Mimmi, S.; Maisano, D.; Nisticò, N.; Vecchio, E.; Chiurazzi, F.; Ferrara, K.; Iannalfo, M.; D’Ambrosio, A.; Fiume, G.; Iaccino, E.; et al. Detection of chronic lymphocytic leukemia subpopulations in peripheral blood by phage ligands of tumor immunoglobulin B cell receptors. Leukemia 2021, 35, 610–614. [Google Scholar] [CrossRef]
- Iaccino, E.; Mimmi, S.; Dattilo, V.; Marino, F.; Candeloro, P.; Di Loria, A.; Marimpietri, D.; Pisano, A.; Albano, F.; Vecchio, E.; et al. Monitoring multiple myeloma by idiotype-specific peptide binders of tumor-derived exosomes. Mol. Cancer 2017, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Klener, P.; Klanova, M. Drug Resistance in Non-Hodgkin Lymphomas. Int. J. Mol. Sci. 2020, 21, 2081. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Introduction by the Guest Editor: Recent advances in biology and treatment of multiple myeloma. Int. J. Hematol. 2020, 111, 494–495. [Google Scholar] [CrossRef]
- Palanca-Wessels, M.C.; Press, O.W. Advances in the treatment of hematologic malignancies using immunoconjugates. Blood 2014, 123, 2293–2301. [Google Scholar] [CrossRef]
- Wildes, T.M.; Stirewalt, D.L.; Medeiros, B.; Hurria, A. Hematopoietic stem cell transplantation for hematologic malignancies in older adults: Geriatric principles in the transplant clinic. J. Natl. Compr. Canc. Netw. 2014, 12, 128–136. [Google Scholar] [CrossRef]
- Santos, G.W. Bone marrow transplantation in hematologic malignancies. Current status. Cancer 1990, 65, 786–791. [Google Scholar] [CrossRef]
- Allegra, A.G.; Mannino, F.; Innao, V.; Musolino, C.; Allegra, A. Radioprotective Agents and Enhancers Factors. Preventive and Therapeutic Strategies for Oxidative Induced Radiotherapy Damages in Hematological Malignancies. Antioxidants 2020, 9, 1116. [Google Scholar] [CrossRef]
- Bogaert, D.J.; Laureys, G.; Naesens, L.; Mazure, D.; De Bruyne, M.; Hsu, A.P.; Bordon, V.; Wouters, E.; Tavernier, S.J.; Lambrecht, B.N.; et al. GATA2 deficiency and haematopoietic stem cell transplantation: Challenges for the clinical practitioner. Br. J. Haematol. 2020, 188, 768–773. [Google Scholar] [CrossRef]
- Jurcek, T.; Razga, F.; Jeziskova, I.; Dvorakova, D.; Zackova, D.; Tomasikova, L.; Oltova, A.; Mayer, J. Failure of molecular diagnostics in chronic myeloid leukemia: An aberrant form of e13a2 BCR-ABL transcript causing false-negative results by standard polymerase chain reaction. Leuk. Lymphoma 2010, 51, 558–561. [Google Scholar] [CrossRef]
- Allart-Vorelli, P.; Porro, B.; Baguet, F.; Michel, A.; Cousson-Gélie, F. Haematological cancer and quality of life: A systematic literature review. Blood Cancer J. 2015, 5, e305. [Google Scholar] [CrossRef]
- Kotrova, M.; van der Velden, V.H.J.; van Dongen, J.J.M.; Formankova, R.; Sedlacek, P.; Brüggemann, M.; Zuna, J.; Stary, J.; Trka, J.; Fronkova, E. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transpl. 2017, 52, 962–968. [Google Scholar] [CrossRef]
- Rocha, J.M.C.; Xavier, S.G.; Souza, M.E.L.; Murao, M.; de Oliveira, B.M. Comparison between flow cytometry and standard PCR in the evaluation of MRD in children with acute lymphoblastic leukemia treated with the GBTLI LLA—2009 protocol. Pediatr. Hematol. Oncol. 2019, 36, 287–301. [Google Scholar] [CrossRef]
- Pfeifer, H.; Cazzaniga, G.; van der Velden, V.H.J.; Cayuela, J.M.; Schäfer, B.; Spinelli, O.; Akiki, S.; Avigad, S.; Bendit, I.; Borg, K.; et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leukemia 2019, 33, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Denys, B.; van der Sluijs-Gelling, A.J.; Homburg, C.; van der Schoot, C.E.; de Haas, V.; Philippé, J.; Pieters, R.; van Dongen, J.J.; van der Velden, V.H. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia 2013, 27, 635–641. [Google Scholar] [CrossRef]
- Mehn, D.; Morasso, C.; Vanna, R.; Schiumarini, D.; Bedoni, M.; Ciceri, F.; Gramatica, F. Surface enhanced Raman spectroscopy-based method for leukemia biomarker detection using magnetic core@ gold shell nanoparticles. Bionanoscience 2014, 4, 119–127. [Google Scholar] [CrossRef]
- Hallett, W.H.; Jing, W.; Drobyski, W.R.; Johnson, B.D. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol. Blood Marrow Transpl. 2011, 17, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Yasinska, I.M.; Ceccone, G.; Ojea-Jimenez, I.; Ponti, J.; Hussain, R.; Siligardi, G.; Berger, S.M.; Fasler-Kan, E.; Bardelli, M.; Varani, L.; et al. Highly specific targeting of human acute myeloid leukaemia cells using pharmacologically active nanoconjugates. Nanoscale 2018, 10, 5827–5833. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Sasidharan, A.; Monteiro-Riviere, N.A. Biomedical applications of gold nanomaterials: Opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 779–796. [Google Scholar] [CrossRef]
- Kumar, A.; Mazinder Boruah, B.; Liang, X.-J. Gold Nanoparticles: Promising Nanomaterials for the Diagnosis of Cancer and HIV/AIDS. J. Nanomater. 2011, 2011, 202187. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Niloy, M.S.; Shakil, M.S.; Hossen, M.S.; Alam, M.; Rosengren, R.J. Promise of gold nanomaterials as a lung cancer theranostic agent: A systematic review. Int. Nano Lett. 2021, 11, 93–111. [Google Scholar] [CrossRef]
- Khandker, S.S.; Shakil, M.; Hossen, M. Gold Nanoparticles; Potential Nanotheranostic Agent in Breast Cancer: A Comprehensive Review with Systematic Search Strategy. Curr. Drug Metab. 2020, 21, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mahmudul, H.M.; Li, Z.; Deng, X.; Su, X.; Xiao, Z.; Zhao, L.; Liu, T.; Li, H. Noble metal nanomaterials for the diagnosis and treatment of hematological malignancies. Front. Biosci. 2022, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, B.; Marchetti, M.; Risuglia, A.; Ietto, F.; Fanizza, C.; Superti, F. Exposure to airborne gold nanoparticles: A review of current toxicological data on the respiratory tract. J. Nanoparticle Res. 2021, 22, 235. [Google Scholar] [CrossRef]
- Sant, M.; Allemani, C.; Tereanu, C.; De Angelis, R.; Capocaccia, R.; Visser, O.; Marcos-Gragera, R.; Maynadié, M.; Simonetti, A.; Lutz, J.M.; et al. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood 2010, 116, 3724–3734. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, D.; Bordoni, A.; Zucca, E. Epidemiology of hematological malignancies. Ann. Oncol. 2007, 18 (Suppl. S1), i3–i8. [Google Scholar] [CrossRef]
- Batista, J.L.; Birmann, B.M.; Epstein, M.M. Epidemiology of Hematologic Malignancies. In Pathology and Epidemiology of Cancer; Loda, M., Mucci, L.A., Mittelstadt, M.L., Van Hemelrijck, M., Cotter, M.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 543–569. [Google Scholar]
- cBioPortal. Leukemia. Available online: https://bit.ly/3uz1IM4 (accessed on 29 January 2022).
- cBioPortal. Lymphoma. Available online: https://bit.ly/3usTDbt (accessed on 29 January 2022).
- Méndez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff , M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef]
- Yang, L.; Rau, R.; Goodell, M.A. DNMT3A in haematological malignancies. Nat. Rev. Cancer 2015, 15, 152–165. [Google Scholar] [CrossRef]
- Szymczyk, A.; Macheta, A.; Podhorecka, M. Abnormal microRNA expression in the course of hematological malignancies. Cancer Manag. Res. 2018, 10, 4267–4277. [Google Scholar] [CrossRef]
- Haas, M.; Lonial, S. Targeted therapy for haematological malignancies: Clinical update from the American Society of Hematology, 2004. Expert. Opin. Investig. Drugs 2005, 14, 1161–1169. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Holmfeldt, L.; Wei, L.; Diaz-Flores, E.; Walsh, M.; Zhang, J.; Ding, L.; Payne-Turner, D.; Churchman, M.; Andersson, A.; Chen, S.C.; et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M.; et al. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- TCGA, Firehose Legacy. Acute Myeloid Leukemia. Available online: https://bit.ly/3vPyg4p (accessed on 29 January 2022).
- TARGET. Pediatric Acute Myeloid Leukemia. Available online: https://bit.ly/3tA7cow (accessed on 29 January 2022).
- Diamond, E.L.; Durham, B.H.; Ulaner, G.A.; Drill, E.; Buthorn, J.; Ki, M.; Bitner, L.; Cho, H.; Young, R.J.; Francis, J.H.; et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 2019, 567, 521–524. [Google Scholar] [CrossRef]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627, quiz 3699. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef]
- Andersson, A.K.; Ma, J.; Wang, J.; Chen, X.; Gedman, A.L.; Dang, J.; Nakitandwe, J.; Holmfeldt, L.; Parker, M.; Easton, J.; et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet. 2015, 47, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McCastlain, K.; Yoshihara, H.; Xu, B.; Chang, Y.; Churchman, M.L.; Wu, G.; Li, Y.; Wei, L.; Iacobucci, I.; et al. Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat. Genet. 2016, 48, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Carter, S.L.; Stojanov, P.; McKenna, A.; Stevenson, K.; Lawrence, M.S.; Sougnez, C.; Stewart, C.; Sivachenko, A.; Wang, L.; et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 2013, 152, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- TARGET. Pediatric Acute Lymphoid Leukemia—Phase II. Available online: https://bit.ly/3h9oZjw (accessed on 29 January 2022).
- Puente, X.S.; Beà, S.; Valdés-Mas, R.; Villamor, N.; Gutiérrez-Abril, J.; Martín-Subero, J.I.; Munar, M.; Rubio-Pérez, C.; Jares, P.; Aymerich, M.; et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef]
- Quesada, V.; Conde, L.; Villamor, N.; Ordóñez, G.R.; Jares, P.; Bassaganyas, L.; Ramsay, A.J.; Beà, S.; Pinyol, M.; Martínez-Trillos, A.; et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat. Genet. 2011, 44, 47–52. [Google Scholar] [CrossRef]
- Da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e415. [Google Scholar] [CrossRef]
- TCGA, PanCancer Atlas. Diffuse Large B-Cell Lymphoma. Available online: https://bit.ly/2REehXx (accessed on 29 January 2022).
- Morin, R.D.; Mungall, K.; Pleasance, E.; Mungall, A.J.; Goya, R.; Huff, R.D.; Scott, D.W.; Ding, J.; Roth, A.; Chiu, R.; et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 2013, 122, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- TCGA, Firehose Legacy. Lymphoid Neoplasm Diffuse Large B-cell Lymphoma. Available online: https://bit.ly/3uvhass (accessed on 29 January 2022).
- Haghighi, F.H.; Binaymotlagh, R.; Mirahmadi-Zare, S.Z.; Hadadzadeh, H. Aptamer/magnetic nanoparticles decorated with fluorescent gold nanoclusters for selective detection and collection of human promyelocytic leukemia (HL-60) cells from a mixture. Nanotechnology 2020, 31, 025605. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Taei, M.; Rahmani, H.; Khayamian, T. Sensitive DNA impedance biosensor for detection of cancer, chronic lymphocytic leukemia, based on gold nanoparticles/gold modified electrode. Electrochim. Acta 2011, 56, 8176–8183. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef]
- Araújo, J.E.; Lodeiro, C.; Capelo, J.L.; Rodríguez-González, B.; Dos Santos, A.A.; Santos, H.M.; Fernández-Lodeiro, J. Novel nanocomposites based on a strawberry-like gold-coated magnetite (Fe@ Au) for protein separation in multiple myeloma serum samples. Nano Res. 2015, 8, 1189–1198. [Google Scholar] [CrossRef]
- Shan, W.; Pan, Y.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta 2014, 126, 130–135. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, M.; Wang, Z.; Zhang, F.; Xia, J.; Shi, G.; Xia, L.; Li, Y.; Xia, Y.; Xia, L. A label-free immunosensor for detecting common acute lymphoblastic leukemia antigen (CD10) based on gold nanoparticles by quartz crystal microbalance. Sens. Actuators B Chem. 2015, 210, 248–253. [Google Scholar] [CrossRef]
- Ai, J.; Xu, Y.; Li, D.; Liu, Z.; Wang, E. Folic acid as delivery vehicles: Targeting folate conjugated fluorescent nanoparticles to tumors imaging. Talanta 2012, 101, 32–37. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Barazesh, B.; Khoshroo, A.; Moshtaghiun, M.; Sheikhha, M.H. A new composite consisting of electrosynthesized conducting polymers, graphene sheets and biosynthesized gold nanoparticles for biosensing acute lymphoblastic leukemia. Bioelectrochemistry 2018, 121, 38–45. [Google Scholar] [CrossRef]
- MacLaughlin, C.; Parker, E.P.; Walker, G.; Wang, C. Polymer-Coated Surface Enhanced Raman Scattering (SERS) Gold Nanoparticles for Multiplexed Labeling of Chronic Lymphocytic Leukemia Cells; SPIE: San Francisco, CA, USA, 2012; Volume 8212, p. 821203. [Google Scholar]
- Nguyen, C.T.; Nguyen, J.T.; Rutledge, S.; Zhang, J.; Wang, C.; Walker, G.C. Detection of chronic lymphocytic leukemia cell surface markers using surface enhanced Raman scattering gold nanoparticles. Cancer Lett. 2010, 292, 91–97. [Google Scholar] [CrossRef]
- Ge, S.; Liu, F.; Liu, W.; Yan, M.; Song, X.; Yu, J. Colorimetric assay of K-562 cells based on folic acid-conjugated porous bimetallic Pd@Au nanoparticles for point-of-care testing. Chem. Commun. 2014, 50, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Giestas, L.; Lima, J.C.; Baptista, P.M. BioCode gold-nanobeacon for the detection of fusion transcripts causing chronic myeloid leukemia. J. Nanobiotechnol. 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wang, J.; Yao, C.; Zhang, Z. Two-photon imaging of lymphoma cells targeted by gold nanoparticles. Chin. Opt. Lett. 2008, 6, 879–881. [Google Scholar]
- Moghiseh, M.; Lowe, C.; Lewis, J.G.; Kumar, D.; Butler, A.; Anderson, N.; Raja, A. Spectral Photon-Counting Molecular Imaging for Quantification of Monoclonal Antibody-Conjugated Gold Nanoparticles Targeted to Lymphoma and Breast Cancer: An In Vitro Study. Contrast Media Mol. Imaging 2018, 2018, 2136840. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. Chapter 1—From the “Magic Bullet” to Advanced Nanomaterials for Active Targeting in Diagnostics and Therapeutics. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–32. [Google Scholar]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Counteracting the effect of leukemia exosomes by antiangiogenic gold nanoparticles. Int. J. Nanomed. 2019, 14, 6843–6854. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Lin, Q.; Chen, W.; Chen, J.; Yao, H.; Liu, A.; Lin, X.; Chen, Y. Electrochemical biosensor based on nanogold-modified poly-eriochrome black T film for BCR/ABL fusion gene assay by using hairpin LNA probe. Talanta 2010, 80, 2113–2119. [Google Scholar] [CrossRef]
- Svaasand, L.O.; Gomer, C.J.; Morinelli, E. On the physical rationale of laser induced hyperthermia. Lasers Med. Sci. 1990, 5, 121–128. [Google Scholar] [CrossRef]
- Ahmad, R.; Fu, J.; He, N.; Li, S. Advanced Gold Nanomaterials for Photothermal Therapy of Cancer. J. Nanosci. Nanotechnol. 2016, 16, 67–80. [Google Scholar] [CrossRef]
- Shao, J.; Cao, S.; Williams, D.S.; Abdelmohsen, L.; van Hest, J.C.M. Photoactivated Polymersome Nanomotors: Traversing Biological Barriers. Angew. Chem. Int. Ed. Engl. 2020, 59, 16918–16925. [Google Scholar] [CrossRef]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Lapotko, D.O.; Lukianova, E.; Oraevsky, A.A. Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles. Lasers Surg. Med. 2006, 38, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Liopo, A.; Conjusteau, A.; Konopleva, M.; Andreeff, M.; Oraevsky, A. Photothermal therapy of acute leukemia cells in the near-infrared region using gold nanorods CD-33 conjugates. In Optical Interactions with Tissue and Cells XXII; SPIE: San Francisco, CA, USA, 2011; p. 789710. [Google Scholar]
- O’Reilly, M.K.; Paulson, J.C. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol. Sci. 2009, 30, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Meng, H.; Zong, Y.; Qian, J.; Jing, Y.; Tang, J.; Zou, J.; An, G.; Song, H.; Zhang, X. Photothermal Targetting therapy of A20 Mouse Lymphoma model using Anti CD-138 Antibody conjugated Gold Nanospheres. J. Hematol. Thromboembolic Dis. 2014, 2, 137. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.S.; Chang, Y.T.; Cho, K.C.; Chiu, K.C.; Lien, C.H.; Yeh, C.S.; Chen, S.J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef]
- Lu, F.; Doane, T.L.; Zhu, J.-J.; Burda, C. Gold nanoparticles for diagnostic sensing and therapy. Inorg. Chim. Acta 2012, 393, 142–153. [Google Scholar] [CrossRef]
- Lkhagvadulam, B.; Kim, J.H.; Yoon, I.; Shim, Y.K. Size-dependent photodynamic activity of gold nanoparticles conjugate of water soluble purpurin-18-N-methyl-d-glucamine. Biomed. Res. Int. 2013, 2013, 720579. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Spikes, J.D. Chlorins as photosensitizers in biology and medicine. J. Photochem. Photobiol. B 1990, 6, 259–274. [Google Scholar] [CrossRef]
- Wang, J.; You, M.; Zhu, G.; Shukoor, M.I.; Chen, Z.; Zhao, Z.; Altman, M.B.; Yuan, Q.; Zhu, Z.; Chen, Y.; et al. Photosensitizer-gold nanorod composite for targeted multimodal therapy. Small 2013, 9, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Philchenkov, A.A.; Shishko, E.D.; Zavelevich, M.P.; Kuiava, L.M.; Miura, K.; Blokhin, D.Y.; Shton, I.O.; Gamaleia, N.F. Photodynamic responsiveness of human leukemia Jurkat/A4 cells with multidrug resistant phenotype. Exp. Oncol. 2014, 36, 241–245. [Google Scholar] [PubMed]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Xu, H.; Wang, B.; Yao, C. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 2015, 20, 51043. [Google Scholar] [CrossRef]
- Belka, C.; Ottinger, H.; Kreuzfelder, E.; Weinmann, M.; Lindemann, M.; Lepple-Wienhues, A.; Budach, W.; Grosse-Wilde, H.; Bamberg, M. Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother. Oncol. 1999, 50, 199–204. [Google Scholar] [CrossRef]
- Coughlin, B.P.; Lawrence, P.T.; Lui, I.; Luby, C.J.; Spencer, D.J.; Sykes, E.C.H.; Mace, C.R. Evidence for biological effects in the radiosensitization of leukemia cell lines by PEGylated gold nanoparticles. J. Nanoparticle Res. 2020, 22, 53. [Google Scholar] [CrossRef]
- Mendes, R.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticle Approach to the Selective Delivery of Gene Silencing in Cancer-The Case for Combined Delivery? Genes 2017, 8, 94. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Cortes, J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009, 113, 1619–1630. [Google Scholar] [CrossRef]
- Vinhas, R.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticles for BCR-ABL1 Gene Silencing: Improving Tyrosine Kinase Inhibitor Efficacy in Chronic Myeloid Leukemia. Mol. Ther. Nucleic Acids 2017, 7, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Vuelta, E.; García-Tuñón, I.; Hernández-Carabias, P.; Méndez, L.; Sánchez-Martín, M. Future Approaches for Treating Chronic Myeloid Leukemia: CRISPR Therapy. Biology 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Zaimy, M.A.; Jebali, A.; Bazrafshan, B.; Mehrtashfar, S.; Shabani, S.; Tavakoli, A.; Hekmatimoghaddam, S.H.; Sarli, A.; Azizi, H.; Izadi, P.; et al. Coinhibition of overexpressed genes in acute myeloid leukemia subtype M2 by gold nanoparticles functionalized with five antisense oligonucleotides and one anti-CD33(+)/CD34(+) aptamer. Cancer Gene Ther. 2016, 23, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef]
- Gullà, A.; Di Martino, M.T.; Gallo Cantafio, M.E.; Morelli, E.; Amodio, N.; Botta, C.; Pitari, M.R.; Lio, S.G.; Britti, D.; Stamato, M.A.; et al. A 13 mer LNA-i-miR-221 Inhibitor Restores Drug Sensitivity in Melphalan-Refractory Multiple Myeloma Cells. Clin. Cancer Res. 2016, 22, 1222–1233. [Google Scholar] [CrossRef]
- Pineau, P.; Volinia, S.; McJunkin, K.; Marchio, A.; Battiston, C.; Terris, B.; Mazzaferro, V.; Lowe, S.W.; Croce, C.M.; Dejean, A. miR-221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 264–269. [Google Scholar] [CrossRef]
- Deng, R.; Shen, N.; Yang, Y.; Yu, H.; Xu, S.; Yang, Y.W.; Liu, S.; Meguellati, K.; Yan, F. Targeting epigenetic pathway with gold nanoparticles for acute myeloid leukemia therapy. Biomaterials 2018, 167, 80–90. [Google Scholar] [CrossRef]
- Deng, R.; Ji, B.; Yu, H.; Bao, W.; Yang, Z.; Yu, Y.; Cui, Y.; Du, Y.; Song, M.; Liu, S.; et al. Multifunctional Gold Nanoparticles Overcome MicroRNA Regulatory Network Mediated-Multidrug Resistant Leukemia. Sci. Rep. 2019, 9, 5348. [Google Scholar] [CrossRef]
- Liu, Q.G.; Zhao, X.; Xu, N.; Wu, L.H.; Li, S.Z.; Mi, Y.C. Organ toxicity and efficacy of high-dose daunorubicin-based chemotherapy in the treatment of acute leukemia. Zhonghua Xue Ye Xue Za Zhi 2013, 34, 587–590. [Google Scholar]
- Jackson, G.; Taylor, P.; Smith, G.M.; Marcus, R.; Smith, A.; Chu, P.; Littlewood, T.J.; Duncombe, A.; Hutchinson, M.; Mehta, A.B.; et al. A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. Br. J. Haematol. 2001, 112, 127–137. [Google Scholar]
- Janssens, A.; Boogaerts, M.; Verhoef, G. Development of fludarabine formulations in the treatment of chronic lymphocytic leukemia. Drug Des. Devel. Ther. 2009, 3, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hao, Y.; Yang, X.; Patra, P.; Chen, J. Using Gold Nanoparticles as Delivery Vehicles for Targeted Delivery of Chemotherapy Drug Fludarabine Phosphate to Treat Hematological Cancers. J. Nanosci. Nanotechnol. 2016, 16, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wu, C.; Song, W.; Jiang, H.; Wang, X.; Chen, B. Effect of colloidal gold nanoparticles on cell interface and their enhanced intracellular uptake of arsenic trioxide in leukemia cancer cells. J. Nanosci. Nanotechnol. 2009, 9, 4611–4617. [Google Scholar] [CrossRef]
- Patra, C.R.; Verma, R.; Kumar, S.; Greipp, P.R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of gold nanoparticle for potential application in multiple myeloma. J. Biomed. Nanotechnol. 2008, 4, 499–507. [Google Scholar] [CrossRef]
- Aubel-Sadron, G.; Londos-Gagliardi, D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie 1984, 66, 333–352. [Google Scholar] [CrossRef]
- Dizman, H.M.; Eroglu, G.O.; Kuruca, S.E.; Arsu, N. Photochemically prepared monodisperse gold nanoparticles as doxorubicin carrier and its cytotoxicity on leukemia cancer cells. Appl. Nanosci. 2021, 11, 309–320. [Google Scholar] [CrossRef]
- Escherich, G.; Zimmermann, M.; Janka-Schaub, G. Doxorubicin or daunorubicin given upfront in a therapeutic window are equally effective in children with newly diagnosed acute lymphoblastic leukemia. A randomized comparison in trial CoALL 07-03. Pediatr. Blood Cancer 2013, 60, 254–257. [Google Scholar] [CrossRef]
- Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Targeted and controlled release delivery of daunorubicin to T-cell acute lymphoblastic leukemia by aptamer-modified gold nanoparticles. Int. J. Pharm. 2015, 489, 311–317. [Google Scholar] [CrossRef]
- Shi, P.-J.; Xu, L.-H.; Lin, K.-Y.; Weng, W.-j.; Fang, J.-P. Synergism between the mTOR inhibitor rapamycin and FAK down-regulation in the treatment of acute lymphoblastic leukemia. J. Hematol. Oncol. 2016, 9, 12. [Google Scholar] [CrossRef]
- Brown, V.I.; Fang, J.; Alcorn, K.; Barr, R.; Kim, J.M.; Wasserman, R.; Grupp, S.A. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 15113–15118. [Google Scholar] [CrossRef]
- Musa, J.; Orth, M.F.; Dallmayer, M.; Baldauf, M.; Pardo, C.; Rotblat, B.; Kirchner, T.; Leprivier, G.; Grünewald, T.G.P. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 2016, 35, 4675–4688. [Google Scholar] [CrossRef] [PubMed]
- Petrushev, B.; Boca, S.; Simon, T.; Berce, C.; Frinc, I.; Dima, D.; Selicean, S.; Gafencu, G.A.; Tanase, A.; Zdrenghea, M.; et al. Gold nanoparticles enhance the effect of tyrosine kinase inhibitors in acute myeloid leukemia therapy. Int. J. Nanomed. 2016, 11, 641–660. [Google Scholar]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Tomuleasa, C.; Bojan, A.; Berindan-Neagoe, I.; Boca, S.; Astilean, S. Design of FLT3 Inhibitor—Gold Nanoparticle Conjugates as Potential Therapeutic Agents for the Treatment of Acute Myeloid Leukemia. Nanoscale Res. Lett. 2015, 10, 466. [Google Scholar] [CrossRef][Green Version]
- Vinhas, R.; Cordeiro, M.; Pedrosa, P.; Fernandes, A.R.; Baptista, P.V. Current trends in molecular diagnostics of chronic myeloid leukemia. Leuk. Lymphoma 2017, 58, 1791–1804. [Google Scholar] [CrossRef]
- Ganbold, E.O.; Byun, J.; Lee, S.; Joo, S.W. Raman spectroscopy of combinatory anticancer drug release from gold nanoparticles inside a single leukemia cell. J. Raman Spectrosc. 2013, 44, 675–679. [Google Scholar] [CrossRef]
- Elion, G.B. The purine path to chemotherapy. Science 1989, 244, 41–47. [Google Scholar] [CrossRef]
- Cheok, M.H.; Evans, W.E. Acute lymphoblastic leukaemia: A model for the pharmacogenomics of cancer therapy. Nat Rev Cancer 2006, 6, 117–129. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.; Lee, J.; Kotov, N.A. Gold nanoparticles enhance the anti-leukemia action of a 6-mercaptopurine chemotherapeutic agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Mandal, P.; Samaddar, S.; Chandra, J.; Parakh, N.; Goel, M. Adverse effects with intravenous methotrexate in children with acute lymphoblastic leukemia/lymphoma: A retrospective study. Indian J. Hematol. Blood Transfus. 2020, 36, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Egusa, S.; Ebrahem, Q.; Mahfouz, R.Z.; Saunthararajah, Y. Ligand exchange on gold nanoparticles for drug delivery and enhanced therapeutic index evaluated in acute myeloid leukemia models. Exp. Biol. Med. 2014, 239, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Patra, C.R.; Earl, A.; Wang, S.; Katarya, A.; Lu, L.; Kizhakkedathu, J.N.; Yaszemski, M.J.; Greipp, P.R.; Mukhopadhyay, D. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 224–238. [Google Scholar] [CrossRef]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.H.; Ku, S.K.; et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.; Cascino, G.; Kimlinger, T.; Rajkumar, V.; Prendergast, F.G.; Fonseca, R.; Bergsagel, P.L.; Low, P.S.; Greipp, P.R. Plasma Cell Folate Receptor Overexpression Differentiates Multiple Myeloma from Monoclonal Gammopathy of Undetermined Significance and Smoldering Myeloma. Blood 2004, 104, 3649. [Google Scholar] [CrossRef]
- Abdalla, M.; Sharada, H.; Mostafa, K.; Azzam, E.; Ali, E.; Abdel-Ghany, I.; Ayad, E. Therapeutic effect of antimyeloma antibodies conjugated with gold nanoparticles on the growth of myeloma cell line. Int. Multidiscip. Res. J. 2012, 2, 64–70. [Google Scholar]
- Bhattacharya, R.; Patra, C.R.; Verma, R.; Kumar, S.; Greipp, P.R.; Mukherjee, P. Gold nanoparticles inhibit the proliferation of multiple myeloma cells. Adv. Mater. 2007, 19, 711–716. [Google Scholar] [CrossRef]
- Boca, S.; Lucan, C.; Frinc, I.; Petrushev, B.; SIMON, T.; Berce, C.; Gafencu, G.-A.; Dima, D.; Tanase, A.; Selicean, S. Gold nanoparticles conjugated with rituximab for the treatment of chronic lymphocytic leukaemia. Farmacia 2016, 64, 688–698. [Google Scholar]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Plosker, G.L.; Figgitt, D.P. Rituximab: A review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2003, 63, 803–843. [Google Scholar] [CrossRef]
- Ocampo García, B.; Miranda Olvera, R.; Santos Cuevas, C.; García Becerra, R.; Azorín Vega, E.; Ordaz Rosado, D. In vitro decrease of the BCL-2 protein levels in lymphoma cells induced by gold nanoparticles and gold nanoparticles-anti-CD20. Nanosci. Technol. 2014, 1, 1–6. [Google Scholar]
- Weiss, A.; Preston, T.C.; Popov, J.; Li, Q.; Wu, S.; Chou, K.C.; Burt, H.M.; Bally, M.B.; Signorell, R. Selective recognition of rituximab-functionalized gold nanoparticles by lymphoma cells studied with 3D imaging. J. Phys. Chem. C 2009, 113, 20252–20258. [Google Scholar] [CrossRef]
- Korkolopoulou, P.; Viniou, N.; Kavantzas, N.; Patsouris, E.; Thymara, I.; Pavlopoulos, P.M.; Terpos, E.; Stamatopoulos, K.; Plata, E.; Anargyrou, K.; et al. Clinicopathologic correlations of bone marrow angiogenesis in chronic myeloid leukemia: A morphometric study. Leukemia 2003, 17, 89–97. [Google Scholar] [CrossRef]
- Deshantri, A.K.; Varela Moreira, A.; Ecker, V.; Mandhane, S.N.; Schiffelers, R.M.; Buchner, M.; Fens, M. Nanomedicines for the treatment of hematological malignancies. J. Control Release 2018, 287, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Ahmeda, A.; Zangeneh, M.M. Novel green synthesis of Boswellia serrata leaf aqueous extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia property in comparison to mitoxantrone in a leukemic mice model: Introducing a new chemotherapeutic drug. Appl. Organomet. Chem. 2020, 34, e5344. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, M.M.; Zangeneh, A. Green formulation and chemical characterization of Lens culinaris seed aqueous extract conjugated gold nanoparticles for the treatment of acute myeloid leukemia in comparison to mitoxantrone in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5369. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A. Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Hemmati, S.; Joshani, Z.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of Thymus vulgaris leaf aqueous extract conjugated gold nanoparticles for the treatment of acute myeloid leukemia in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5267. [Google Scholar] [CrossRef]
- Burdon, R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Samimi, A.; Khodayar, M.J.; Alidadi, H.; Khodadi, E. The Dual Role of ROS in Hematological Malignancies: Stem Cell Protection and Cancer Cell Metastasis. Stem Cell Rev. Rep. 2020, 16, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Sillar, J.R.; Germon, Z.P.; DeIuliis, G.N.; Dun, M.D. The Role of Reactive Oxygen Species in Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2019, 20, 6003. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Bermejo, R.; Romo-González, M.; Pérez-Fernández, A.; Ijurko, C.; Hernández-Hernández, Á. Reactive oxygen species in haematopoiesis: Leukaemic cells take a walk on the wild side. J. Exp. Clin. Cancer Res. CR 2018, 37, 125. [Google Scholar] [CrossRef]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol. 2008, 82, 273–299. [Google Scholar] [CrossRef]
- Higashi, Y.; Mazumder, J.; Yoshikawa, H.; Saito, M.; Tamiya, E. Chemically Regulated ROS Generation from Gold Nanoparticles for Enzyme-Free Electrochemiluminescent Immunosensing. Anal. Chem. 2018, 90, 5773–5780. [Google Scholar] [CrossRef]
- Choi, B.J.; Jung, K.O.; Graves, E.E.; Pratx, G. A gold nanoparticle system for the enhancement of radiotherapy and simultaneous monitoring of reactive-oxygen-species formation. Nanotechnology 2018, 29, 504001. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High levels of reactive oxygen species in gold nanoparticle-targeted cancer cells following femtosecond pulse irradiation. Sci. Rep. 2013, 3, 2146. [Google Scholar] [CrossRef]
- Wen, T.; Yang, A.; Wang, T.; Jia, M.; Lai, X.; Meng, J.; Liu, J.; Han, B.; Xu, H. Ultra-small platinum nanoparticles on gold nanorods induced intracellular ROS fluctuation to drive megakaryocytic differentiation of leukemia cells. Biomater. Sci. 2020, 8, 6204–6211. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.C.; Yang, K.C.; Shieh, H.R.; Wang, T.Y.; Hwu, Y.; Chen, Y.J. Pegylated gold nanoparticles induce apoptosis in human chronic myeloid leukemia cells. Biomed. Res. Int. 2014, 2014, 182353. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Bhattacharya, R.; Bone, N.; Lee, Y.K.; Patra, C.R.; Wang, S.; Lu, L.; Secreto, C.; Banerjee, P.C.; Yaszemski, M.J.; et al. Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): Enhancing apoptosis. J. Nanobiotechnol. 2007, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Deepak, M.; Handa, S.S. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother. Res. 2000, 14, 463–465. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Jiang, C.; Yu, J.; Wu, J.; Zeng, X. Gold nanoshells with verbascoside induce the apoptosis of drug-resistant leukemia cells through caspases pathway and inhibit tumor growth. J. Nanosci. Nanotechnol. 2016, 16, 7118–7124. [Google Scholar] [CrossRef]
- Lin, A.Y.; Rink, J.S.; Karmali, R.; Xu, J.; Kocherginsky, M.; Thaxton, C.S.; Gordon, L.I. Tri-ethylene glycol modified class B and class C CpG conjugated gold nanoparticles for the treatment of lymphoma. Nanomedicine 2020, 30, 102290. [Google Scholar] [CrossRef]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Rasedee, A.; Yeap, S.K.; Chartrand, M.S.; Azizi, S.; Tahir, P.M. Apoptosis induction in human leukemia cell lines by gold nanoparticles synthesized using the green biosynthetic approach. J. Nanomater. 2016, 16, 330. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Tomar, M.; Singh, R.; Acharya, A. Biologically Synthesized Gold Nanoparticles using Ocimum sanctum (Tulsi Leaf Extract) Induced Anti-Tumor Response in a T Cell Daltons Lymphoma. J. Cell Sci. Ther. 2017, 8, 2. [Google Scholar] [CrossRef]
- Shahriari, S.; Bakhshi, M.; Shahverdi, A.R.; Berahmeh, A.; Safavifar, F.; Khorramizadeh, M.R. Targeted Intracellular Heat Transfer in Cancer Therapy: Assessment of Asparagine-laminated Gold Nanoparticles in Cell Model of T cell Leukemia. Iran J. Public Health 2017, 46, 357–367. [Google Scholar]
- Amin Asnafi, A.; Bagheri, M.; Zibara, K.; Maleki Behzad, M.; Shahrabi, S. Expression and Activity of Matrix Metalloproteinases in Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 87–95. [Google Scholar] [CrossRef]
- Eslahi, N.; Shakeri-Zadeh, A.; Ashtari, K.; Pirhajati-Mahabadi, V.; Tohidi Moghadam, T.; Shabani, R.; Kamrava, K.; Madjd, Z.; Maki, C.; Asgari, H.R.; et al. In Vitro Cytotoxicity of Folate-Silica-Gold Nanorods on Mouse Acute Lymphoblastic Leukemia and Spermatogonial Cells. Cell J. 2019, 21, 14–26. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.S.; Parveen, S.; Rana, Z.; Walsh, F.; Movassaghi, S.; Söhnel, T.; Azam, M.; Shaheen, M.A.; Jamieson, S.M.F.; Hanif, M.; et al. High Antiproliferative Activity of Hydroxythiopyridones over Hydroxypyridones and Their Organoruthenium Complexes. Biomedicines 2021, 9, 123. [Google Scholar] [CrossRef]
- Shakil, M.S.; Hasan, M.A.; Uddin, M.F.; Islam, A.; Nahar, A.; Das, H.; Khan, M.N.I.; Dey, B.P.; Rokeya, B.; Hoque, S.M. In Vivo Toxicity Studies of Chitosan-Coated Cobalt Ferrite Nanocomplex for Its Application as MRI Contrast Dye. ACS Appl. Bio Mater. 2020, 3, 7952–7964. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [PubMed]
- Riches, J.C.; Ramsay, A.G.; Gribben, J.G. Immune reconstitution in chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 2012, 7, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Kosaka, Y.; Laderas, T.; Maffit, A.; Kaempf, A.; Brady, L.K.; Wang, W.; Long, N.; Saultz, J.N.; Mori, M.; et al. Reversible suppression of T cell function in the bone marrow microenvironment of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2020, 117, 14331–14341. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, P.; Kamili, Q.U.; Menter, A.; Cooper, B. Lymphoma and immunosuppression: A report of a case associated with efalizumab therapy. Clin. Lymphoma Myeloma Leuk. 2010, 10, E14–E16. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.R.; Zolla-Pazner, S. Immunosuppression and infection in multiple myeloma. Semin. Oncol. 1986, 13, 282–290. [Google Scholar] [PubMed]
- Lazarovits, J.; Chen, Y.Y.; Sykes, E.A.; Chan, W.C. Nanoparticle-blood interactions: The implications on solid tumour targeting. Chem. Commun. 2015, 51, 2756–2767. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, L.; Mu, Q.; Zhang, Q. Immunomodulation of nanoparticles in nanomedicine applications. Biomed. Res. Int. 2014, 2014, 426028. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.; Lysaght, J.; Liptrott, N.J.; Prina-Mello, A. Immunotoxicity Considerations for Next Generation Cancer Nanomedicines. Adv. Sci. 2019, 6, 1900133. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Huffman, B.J.; Gerdon, A.E.; Cliffel, D.E. Unexpected toxicity of monolayer protected gold clusters eliminated by PEG-thiol place exchange reactions. Chem. Res. Toxicol. 2010, 23, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K. Uptake of Gold Nanoparticles in Several Rat Organs after Intraperitoneal Administration In Vivo: A Fluorescence Study. BioMed Res. Int. 2013, 2013, 353695. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef]
- Rambanapasi, C.; Zeevaart, J.R.; Buntting, H.; Bester, C.; Kotze, D.; Hayeshi, R.; Grobler, A. Bioaccumulation and Subchronic Toxicity of 14 nm Gold Nanoparticles in Rats. Molecules 2016, 21, 763. [Google Scholar] [CrossRef]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Comparative cytotoxicity evaluation of different size gold nanoparticles in human dermal fibroblasts. J. Exp. Nanosci. 2015, 10, 1401–1417. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M. Myeloma as a model for the process of metastasis: Implications for therapy. Blood 2012, 120, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Trendowski, M. The inherent metastasis of leukaemia and its exploitation by sonodynamic therapy. Crit. Rev. Oncol. Hematol. 2015, 94, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Aoudjit, F.; Potworowski, E.F.; St-Pierre, Y. The metastatic characteristics of murine lymphoma cell lines in vivo are manifested after target organ invasion. Blood 1998, 91, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Malinova, T.S.; Huveneers, S. Opening the vascular gate. Nat. Nanotechnol. 2019, 14, 195–196. [Google Scholar] [CrossRef]
- Auger, C.; Said, A.; Nguyen, P.N.; Chabert, P.; Idris-Khodja, N.; Schini-Kerth, V.B. Potential of Food and Natural Products to Promote Endothelial and Vascular Health. J. Cardiovasc. Pharmacol. 2016, 68, 11–18. [Google Scholar] [CrossRef]
- Lindstrom, A.L.; Erlandsen, S.L.; Kersey, J.H.; Pennell, C.A. An in vitro model for toxin-mediated vascular leak syndrome: Ricin toxin A chain increases the permeability of human endothelial cell monolayers. Blood 1997, 90, 2323–2334. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Wang, F.; Liao, M.; Jiang, G. Bimetallic nanoparticles decorated hollow nanoporous carbon framework as nanozyme biosensor for highly sensitive electrochemical sensing of uric acid. Biosens. Bioelectron. 2020, 150, 111869. [Google Scholar] [CrossRef]
- Conte, N.; Mason, J.C.; Halmagyi, C.; Neuhauser, S.; Mosaku, A.; Yordanova, G.; Chatzipli, A.; Begley, D.A.; Krupke, D.M.; Parkinson, H.; et al. PDX Finder: A portal for patient-derived tumor xenograft model discovery. Nucleic Acids Res. 2019, 47, D1073–D1079. [Google Scholar] [CrossRef]
- MMHCdb. Patient Derived Xenograft Search Results. Available online: https://bit.ly/2RKS1vT (accessed on 29 January 2022).
- PDX-Finder. Hematopoietic and Lymphoid System Cancer. Available online: https://bit.ly/3oYTH0N (accessed on 29 January 2022).









| Cancer Class | Cancer Sub-Type | GNMs | Size (nm) | Conjugated Materials | Cell Line/Test Sample | Diagnosed Cells/Biomarker | Detection Techniques | Detection Range | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia | HPL | GNCs | * 26 | Fe3O4, # KH1C12 | HL-60 | HL-60 cells | MRI, FLI | 10 to 200 cells/μL | [71] |
| ALL | AuNPs | NR | Fe3O4, # sgc8c | CCRF-CEM | CCRF-CEM cells | EIS | 10 to 1 × 106 cells/mL | [73] | |
| ALL | AuNPs | NR | APBA, # sgc8c | CCRF-CEM | CCRF-CEM cells | QCM, FLI | 2 × 103–1 × 105 cells/mL | [75] | |
| ALL | AuNPs | 15–18 | Ab2 | Antigen | CD10 | QCM | 1.0 × 10−8 to 1.0 × 10−11 M | [76] | |
| ALL | AuNPs | * 15 | FA, FITC | CCRF-CEM | FAR | FLI | NR | [77] | |
| ALL | AuNPs | NR | shDNA, GPS, PCT | cDNA | BCR-ABL fusion | EIS, CHR, DPV, CV | 100.0 μM to 10.0 pM | [78] | |
| CLL | AuNPs | 60 | PEG, Ab3 | Antigen | CD20 | SERS, DFM | NR | [79] | |
| CLL | AuNPs | 20 | PEG, Ab4 | CCLP | CD19 | SERS, DFM | NR | [80] | |
| CLL | AuNPs | 60–70 | shDNA, AED | cDNA | PBGD | EIS | 7.0 × 10−12–2.0 × 10−7 mol/L | [72] | |
| AML | AuNPs | * 40–80 | γ-Fe2O3, ssDNA | cDNA | WT1 | SERS | NR | [24] | |
| CML | Pd@AuNPs | 51 | FA | K652 | FAR | CLR | 104 cells/mL | [81] | |
| CML | AuNPs | 14.6 ± 1.7 | PEG, TFH | cDNA | BCR-ABL fusion | FRETS | NR | [82] | |
| Lymphoma | NR | AuNPs | 30 | Ab5 | K299 | CD25 | MPM | NR | [83] |
| NR | AuNPs | 40 | R-Ab | Raji | CD20 | SPCT | 102 to 1010 cells | [84] | |
| Myeloma | MM | AuNPs | 15 | Magnetite | Myeloma Patients | PPC, HSP75 | MAS | NR | [74] |
| Gene | AO Sequence (5′–3′) | Efficacy Score |
|---|---|---|
| BAG1 | UUGAAGCAGAAGAAACACU | 0.99 |
| MDM2 | UUACAGCACCAUCAGUAGG | 0.99 |
| BCL2 | UCAAUCUUCAGCACUCUCC | 0.98 |
| Survivin | UUCAAGACAAAACAAGAGC | 0.97 |
| XIAP | UAAGAACAACAUAACAUGC | 0.97 |
| # of Pulses | Damage Mechanism | Effect(s) |
|---|---|---|
| 1–2 | ROS | Apoptosis |
| 3–6 | ROS + cell fusion | Apoptosis, necrosis, multi-nucleic cells |
| 7– | ROS + cell fusion + membrane rupture | Necrosis |
| Treatment | HMs Type | GNMs | Size (nm) | Conjugated Materials | Tested Cell Line(s) | IC50 | In Vivo | Upregulated Protein/ Nucleic Acid | Downregulated Protein/Nucleic Acid | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PTT | LKM | AuNPs | 30 | IgG | K562 | NR | NR | NR | NR | [3] |
| LKM | AuNPs | 30 | MAB1, MAB2 | + LKM | NR | NR | NR | NR | [93] | |
| LKM | GNR | NR | CD33, PEG | HL-60, K-562 | NR | NR | NR | NR | [94] | |
| LYP | AuNPs | NR | anti-CD138-Ab | A20 | NR | NR | NR | IgG2a | [96] | |
| PDT | LKM | GNR | NR | Ce6, sgc8c aptamer | CCRF-CEM, Ramos | NR | NR | NR | NR | [103] |
| LKM | AuNPs | 45 | Ce6 | Jurkat, Jurkat/A4 | NR | NR | NR | NR | [104] | |
| LKM | AuNPs | 16 | 5-ALA | K562 | NR | NR | NR | NR | [106] | |
| RDT | LKM | # AuNPs | * 22 ± 2 | PEG | HL-60 II, Jurkat D1.1 | NR | NR | NR | NR | [108] |
| GNT | LKM | AuNPs | 14 | PEG, e14a2 | K562 | IM (22 mM), IMA (17 mM) | NR | Bax, Caspase-3 | BCR-ABL1, Bcl2 | [113] |
| LKM | AuNPs | * <50 | AOs, anti-Apt, Dox | AML-M2 | >150 μg/mL | NR | NR | BCL-2, BAG1, MDM2, BIRC5, XIAP | [115] | |
| LKM | AuNPs | 13 | NLS, AS1411, anti-221 | Jurkat, Kasumi-1, K562, HL60, NB4, Thp1, Molt4, 293 T, U937, C1498 | NR | C57BL/6 mice | p15INK4B, p27kip1 | miR-221, DNMT1 | [119] | |
| LKM | AuNPs | 40 | FA, AS1411, anti-221, Dox | Drug resistant K562, AML RP1, AML RP1, AML RP3 | 0.56 μM (DR K562), 0.31 μM (AML RP1), 0.53 μM (AML RP2), 0.08 μM (AML RP3) | NR | p15INK4B, p27kip1 | miR-221, DNMT1, P-gp | [120] | |
| DCT | LKM | AuNPs | 5 | Anti-Tim-3-ScAb, RAP | THP-1 | NR | NR | NR | p-eIF4E-BP | [26] |
| LKM | AuNPs | 20 | FLP, FA | KG1 | <2 mM | NR | NR | NR | [124] | |
| LKM | AuNPs | 5 | MPA, As2O3 | K562, KA | ~2.2 × 10−2 mg/L (K562), ~1.4 × 10−2 mg/L (KA) | NR | NR | NR | [125] | |
| MM | AuNPs | ∼5 | VEL, FA | RPMI, U226 | NR | NR | NR | NR | [126] | |
| LKM | m-AuNPs | 30–40 | Dox | HL-60 and K562 | NR | NR | NR | NR | [128] | |
| LKM | AuNPs | 15.2 ± 0.7 | Dau, sgc8c aptamer | Molt-4, U266 | ~5 μM (Molt-4), >5 μM (U266) | NR | NR | NR | [130] | |
| LKM | AuNPs | ~12 | PLU, GEL, SOR, LES, QUI | THP1, OCI-AML3 | NR | NR | NR | FLT3 | [134] | |
| LKM | AuNPs | 17 ± 2 | PLU, MDS | THP1, OCI-AML3 | NR | NR | NR | NR | [136] | |
| LKM | AuNPs | ~17 | IM, Topo, CBT | K562 | NR | NR | NR | NR | [138] | |
| LKM | AuNPs | 4–5 | 6-MTP | K-562 | NR | NR | NR | NR | [141] | |
| LKM | AuNPs | ~2.5 | MTX | TPH-1 | NR | NGS mice | NR | NR | [144] | |
| ABT | MM | AuNPs | 26 ± 7 | AbMM | SP2OR | NR | Mice | p21, p27 | NR | [148] |
| LKM | AuNPs | NR | R-Ab | HS 505.T, CLL-AAT | NR | NR | NR | MS4A1, CD20 | [150] | |
| LYP | AuNPs | 20 | R-Ab | Raji | NR | NR | NR | BCL-2 | [153] | |
| LYP | AuNPs | 30 | PEG, R-Ab | Z138 | NR | NR | NR | NR | [154] | |
| PPT | LKM | AuNPs | 3 ± 2 | AP, OEG | K562 | NR | NR | NR | VEGFR1 | [86] |
| BCT | LKM | AuNPs | 15–30 | B. serrata LE | HL-60/vcr, 32D-FLT3-ITD, Murine C1498 | 329 μg/mL (HL-60/vcr) 320 μg/mL (32D-FLT3-ITD), 219 μg/mL (Murine C1498) | DMBA mice | IFNα, IL4, IL5, IL10, IL13, IFNα, S1PR1 and S1PR5 mRNA | IFNY, TNFα, IL1, IL6, IL12, and IL18 | [157] |
| LKM | AuNPs | 10–40 | L. culinaris SE | HL-60/vcr, 32D-FLT3-ITD, Murine C1498 | 246 μg/mL (HL-60/vcr) 367 μg/mL (32D-FLT3-ITD), 212 μg/mL (Murine C1498) | DMBA mice | IFNα, IL4, IL5, IL10, IL13, IFNα, S1PR1, S1PR5 mRNA | IFNY, TNFα, IL1, IL6, IL12, and IL18 | [158] | |
| LKM | AuNPs | 20–30 | C. sinesis LE | HL-60/vcr, 32D-FLT3-ITD, Murine C1498 | 224 μg/mL (HL-60/vcr) 258 μg/mL (32D-FLT3-ITD), 158 μg/mL (Murine C1498) | DMBA mice | IFNα, IL4, IL5, IL10, IL13, IFNα | IFNY, TNFα, IL1, IL6, IL12, and IL18 | [159] | |
| LKM | AuNPs | 10–30 | T. vulgaris LE | HL-60/vcr, 32D-FLT3-ITD, Murine C1498 | 218 μg/mL (HL-60/vcr) 336 μg/mL (32D-FLT3-ITD), 186 μg/mL (Murine C1498) | DMBA mice | IFNα, IL4, IL5, IL10, IL13, IFNα, S1PR1, S1PR5 mRNA | IFNY, TNFα, IL1, IL6, IL12, IL18 | [161] | |
| LKM | AuNPs | 15–45 | H. sabdariffa FE | HL-60/vcr, 32D-FLT3-ITD, Murine C1498 | 189 μg/mL (HL-60/vcr) 309μg/mL (32D-FLT3-ITD), 185 μg/mL (Murine C1498) | DMBA mice | IFNα, IL4, IL5, IL10, IL13, IFNα, S1PR1, S1PR5 mRNA | IFNY, TNFα, IL1, IL6, IL12, IL18 | [160] | |
| RST | LYP | GNHs | 20 | R-Ab | BJAB, K562 | NR | NR | NR | NR | [171] |
| LKM | GNR | * 122 ± 1 | USPN | K562 | NR | NR | Beclin-1 | BCR-ABL, p-PI3K, p-AKT | [172] | |
| APT | LKM | AuNPs | * 10 | PEG | K562 | <10 mM | NR | NR | NR | [173] |
| LKM | AuNPs | 4 | VF-Ab | + CLL B | NR | NR | Cleaved PARP | Mcl-1, BcL-2 and caspase3 | [174] | |
| LKM | GNSs | 2–5 | VER, PNM | KA | NR | Nude mice | GAPDH; Cleaved Caspase-3, 8, and 9 | NR | [176] | |
| LYP | AuNPs | 15 | tmCpG | A20, Ramos, JeKo-1, Mino, RC, REC-1, DLBCL (SUDHL4) | NR | BALB/c mice | IL-6, TNFα, CD19, CD20, CD47 | NR | [177] | |
| LKM | AuNPs | <10 | S. muticum | K562, Jurkat, HL-60, CEM-ss cells | 4.22 ± 1.12 (K562), 5.71 ± 1.4 (HL-60), 6.55 ± 0.9 (Jurkat), 7.29 ± 1.7 μg/mL (CEM-ss) | NR | Caspase-3, caspase-9 | NR | [178] | |
| LYP | AuNPs | 16 | O. sanctum LE | DL | >50 μg/mL | NR | NR | NR | [179] | |
| LKM | AuNPs | 3 | Asn | CCRF-CEM | NR | NR | NR | MMP-2 | [180] | |
| LKM | GNR | 5.55 ± 1.56 | SI, FA | EL4s | <75 μM | NMRI mice | NR | NR | [182] | |
| Others | LKM | AuNPs | 5 | NR | OPM-1, RPMI-8266, U-266 | < 20 μg (OPM-1, RPMI-8266), >20 μg (U-266) | NR | p21, p27 | NR | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakil, M.S.; Niloy, M.S.; Mahmud, K.M.; Kamal, M.A.; Islam, M.A. Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies. Cancers 2022, 14, 3047. https://doi.org/10.3390/cancers14133047
Shakil MS, Niloy MS, Mahmud KM, Kamal MA, Islam MA. Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies. Cancers. 2022; 14(13):3047. https://doi.org/10.3390/cancers14133047
Chicago/Turabian StyleShakil, Md Salman, Mahruba Sultana Niloy, Kazi Mustafa Mahmud, Mohammad Amjad Kamal, and Md Asiful Islam. 2022. "Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies" Cancers 14, no. 13: 3047. https://doi.org/10.3390/cancers14133047
APA StyleShakil, M. S., Niloy, M. S., Mahmud, K. M., Kamal, M. A., & Islam, M. A. (2022). Theranostic Potentials of Gold Nanomaterials in Hematological Malignancies. Cancers, 14(13), 3047. https://doi.org/10.3390/cancers14133047









