Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Basic Principles and Mechanisms of Photodynamic Therapy
3. Technological Approaches to Overcoming PDT Limitations
4. Overview of Main Clinically Approved Chemotherapeutics
4.1. Antibiotics
- -
- DNA intercalation and production of single- and double-strand breaks;
- -
- Oxidative stress caused by the generation of important concentrations of free radicals;
- -
- Inhibition of topoisomerase II and disruption of enzyme-mediated DNA repair mechanisms.
4.2. Antimitotic Agents
- -
- Taxanes are a class of drugs derived from diterpenes produced by plants of the genus Taxus. They stabilize microtubules polymerization by binding to a specific domain found in β-tubulin. Clinically approved taxane agents are paclitaxel (PTX), docetaxel (DTX), and cabazitaxel (CBZ).
- -
- Vinca alkaloids are derived from Catharanthus roseus. They induce the destabilization of the polymerization process of microtubules by binding to the interface between α- and β-tubulin.
4.3. Platinum-Based Chemotherapeutics
4.4. Topoisomerases Inhibitors (TIs)
5. Drug Delivery Systems for PSs and Chemotherapeutics: An Overview of Recent Advances
5.1. Combination of PSs with Antibiotics
5.2. Combination of PSs with Antimitotic Agents
5.3. Combination of PSs with Platinum Compounds
5.4. Combination of PSs with Topoisomerase Inhibitors (TIs)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin Derivative: A Possible Aid in the Diagnosis and Therapy of Carcinoma of the Bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Sidoroff, A.; Braathen, L.R. European Guidelines for Topical Photodynamic Therapy Part 1: Treatment Delivery and Current Indications—Actinic Keratoses, Bowen’s Disease, Basal Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef]
- Fu, C.; Kuang, B.-H.; Qin, L.; Zeng, X.-Y.; Wang, B.-C. Efficacy and Safety of Photodynamic Therapy with Amino-5-Laevulinate Nanoemulsion versus Methyl-5-Aminolaevulinate for Actinic Keratosis: A Meta-Analysis. Photodiagnosis Photodyn. Ther. 2019, 27, 408–414. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially Resolved Cellular Responses to Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.; Tsubone, T.; Pavani, C.; Baptista, M. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Subcellular Targeting as a Determinant of the Efficacy of Photodynamic Therapy. Photochem. Photobiol. 2017, 93, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Moserova, I.; Kralova, J. Role of ER Stress Response in Photodynamic Therapy: ROS Generated in Different Subcellular Compartments Trigger Diverse Cell Death Pathways. PLoS ONE 2012, 7, e32972. [Google Scholar] [CrossRef]
- Fabris, C.; Valduga, G.; Miotto, G.; Borsetto, L.; Jori, G.; Garbisa, S.; Reddi, E. Photosensitization with Zinc (II) Phthalocyanine as a Switch in the Decision between Apoptosis and Necrosis. Cancer Res. 2001, 61, 7495–7500. [Google Scholar]
- Hsieh, Y.-J.; Wu, C.-C.; Chang, C.-J.; Yu, J.-S. Subcellular Localization of Photofrin® Determines the Death Phenotype of Human Epidermoid Carcinoma A431 Cells Triggered by Photodynamic Therapy: When Plasma Membranes Are the Main Targets. J. Cell. Physiol. 2003, 194, 363–375. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef]
- Kessel, D.; Reiners, J.J. Photodynamic Therapy: Autophagy and Mitophagy, Apoptosis and Paraptosis. Autophagy 2020, 16, 2098–2101. [Google Scholar] [CrossRef]
- Miki, Y.; Akimoto, J.; Moritake, K.; Hironaka, C.; Fujiwara, Y. Photodynamic Therapy Using Talaporfin Sodium Induces Concentration-Dependent Programmed Necroptosis in Human Glioblastoma T98G Cells. Lasers Med. Sci. 2015, 30, 1739–1745. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheung, Y.-K.; Ng, D.K.P.; Fong, W.-P. Immunogenic Necroptosis in the Anti-Tumor Photodynamic Action of BAM-SiPc, a Silicon(IV) Phthalocyanine-Based Photosensitizer. Cancer Immunol. Immunother. 2021, 70, 485–495. [Google Scholar] [CrossRef]
- Shui, S.; Zhao, Z.; Wang, H.; Conrad, M.; Liu, G. Non-Enzymatic Lipid Peroxidation Initiated by Photodynamic Therapy Drives a Distinct Ferroptosis-like Cell Death Pathway. Redox Biol. 2021, 45, 102056. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.; Mora-Espí, I.; Alea-Reyes, M.E.; Pérez-García, L.; Barrios, L.; Ibáñez, E.; Nogués, C. Cell Death Mechanisms in Tumoral and Non-Tumoral Human Cell Lines Triggered by Photodynamic Treatments: Apoptosis, Necrosis and Parthanatos. Sci. Rep. 2017, 7, 41340. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell Death in Photodynamic Therapy: From Oxidative Stress to Anti-Tumor Immunity. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chiu, S.; Oleinick, N.L. Photochemical Destruction of the Bcl-2 Oncoprotein during Photodynamic Therapy with the Phthalocyanine Photosensitizer Pc 4. Oncogene 2001, 20, 3420–3427. [Google Scholar] [CrossRef]
- Thong, P.S.-P.; Ong, K.-W.; Goh, N.S.-G.; Kho, K.-W.; Manivasager, V.; Bhuvaneswari, R.; Olivo, M.; Soo, K.-C. Photodynamic-Therapy-Activated Immune Response against Distant Untreated Tumours in Recurrent Angiosarcoma. Lancet Oncol. 2007, 8, 950–952. [Google Scholar] [CrossRef]
- Morrison, S.A.; Hill, S.L.; Rogers, G.S.; Graham, R.A. Efficacy and Safety of Continuous Low-Irradiance Photodynamic Therapy in the Treatment of Chest Wall Progression of Breast Cancer. J. Surg. Res. 2014, 192, 235–241. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of Photodynamic Therapy (PDT) and Anti-Tumor Immunity in Cancer Therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheung, Y.-K.; Ng, D.K.P.; Fong, W.-P. Enhancement of Innate and Adaptive Anti-Tumor Immunity by Serum Obtained from Vascular Photodynamic Therapy-Cured BALB/c Mouse. Cancer Immunol. Immunother. 2021, 70, 3217–3233. [Google Scholar] [CrossRef]
- Anand, S.; Govande, M.; Yasinchak, A.; Heusinkveld, L.; Shakya, S.; Fairchild, R.L.; Maytin, E.V. Painless Photodynamic Therapy Triggers Innate and Adaptive Immune Responses in a Murine Model of UV-induced Squamous Skin Pre-cancer. Photochem. Photobiol. 2021, 97, 607–617. [Google Scholar] [CrossRef]
- Milla Sanabria, L.; Rodríguez, M.E.; Cogno, I.S.; Rumie Vittar, N.B.; Pansa, M.F.; Lamberti, M.J.; Rivarola, V.A. Direct and Indirect Photodynamic Therapy Effects on the Cellular and Molecular Components of the Tumor Microenvironment. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2013, 1835, 36–45. [Google Scholar] [CrossRef]
- Olivo, M.; Bhuvaneswari, R.; Lucky, S.S.; Dendukuri, N.; Soo-Ping Thong, P. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-Faceted Anti-Tumor Modalities. Pharmaceuticals 2010, 3, 1507–1529. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.; Wiegell, S.R.; Wulf, H.C. Light Protection of the Skin after Photodynamic Therapy Reduces Inflammation: An Unblinded Randomized Controlled Study. Br. J. Dermatol. 2014, 171, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Moret, F.; Reddi, E. Strategies for Optimizing the Delivery to Tumors of Macrocyclic Photosensitizers Used in Photodynamic Therapy (PDT). J. Porphyr. Phthalocyanines 2017, 21, 239–256. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, Y.; Na, K. Photodynamic Therapy with Smart Nanomedicine. Arch. Pharm. Res. 2020, 43, 22–31. [Google Scholar] [CrossRef]
- Maas, A.L.; Carter, S.L.; Wileyto, E.P.; Miller, J.; Yuan, M.; Yu, G.; Durham, A.C.; Busch, T.M. Tumor Vascular Microenvironment Determines Responsiveness to Photodynamic Therapy. Cancer Res. 2012, 72, 2079–2088. [Google Scholar] [CrossRef]
- Korbelik, M.; Krosl, G. Cellular Levels of Photosensitisers in Tumours: The Role of Proximity to the Blood Supply. Br. J. Cancer 1994, 70, 604–610. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor Penetrating Peptides for Improved Drug Delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Park, S. IRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pang, X.; Liu, R.; Xiao, Q.; Wang, P.; Leung, A.W.; Luan, Y.; Xu, C. Design of an Amphiphilic IRGD Peptide and Self-Assembling Nanovesicles for Improving Tumor Accumulation and Penetration and the Photodynamic Efficacy of the Photosensitizer. ACS Appl. Mater. Interfaces 2018, 10, 31674–31685. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wang, Z.; Ngandeu Neubi, G.M.; Cheng, H.; Zhang, C.; Zhang, H.; Wang, R.; Zhou, J.; Ding, Y. Lipoprotein-Inspired Penetrating Nanoparticles for Deep Tumor-Targeted Shuttling of Indocyanine Green and Enhanced Photo-Theranostics. Biomater. Sci. 2019, 7, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, Y.; Sun, B.; Zhao, Z.; Opoku-Damoah, Y.; Cheng, H.; Zhang, H.; Zhou, J.; Ding, Y. Deep Tumor Penetrating Bioparticulates Inspired Burst Intracellular Drug Release for Precision Chemo-Phototherapy. Small 2018, 14, 1703110. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ Heel of Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef]
- Bown, S.G. Photodynamic Therapy for Cancer of the Pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic Therapy with Pd-Bacteriopheophorbide (TOOKAD): Successfulin Vivo Treatment of Human Prostatic Small Cell Carcinoma Xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic Therapy for Cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef]
- Bhawalkar, J.D.; Kumar, N.D.; Zhao, C.F.; Prasad, P.N. Two-Photon Photodynamic Therapy. J. Clin. Laser Med. Surg. 1997, 15, 201–204. [Google Scholar] [CrossRef]
- Karotki, A.; Khurana, M.; Lepock, J.R.; Wilson, B.C. Simultaneous Two-Photon Excitation of Photofrin in Relation to Photodynamic Therapy. Photochem. Photobiol. 2006, 82, 443. [Google Scholar] [CrossRef] [PubMed]
- Starkey, J.R.; Rebane, A.K.; Drobizhev, M.A.; Meng, F.; Gong, A.; Elliott, A.; McInnerney, K.; Spangler, C.W. New Two-Photon Activated Photodynamic Therapy Sensitizers Induce Xenograft Tumor Regressions after Near-IR Laser Treatment through the Body of the Host Mouse. Clin. Cancer Res. 2008, 14, 6564–6573. [Google Scholar] [CrossRef] [PubMed]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular Photosensitisers for Two-Photon Photodynamic Therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef]
- Han, R.; Zhao, M.; Wang, Z.; Liu, H.; Zhu, S.; Huang, L.; Wang, Y.; Wang, L.; Hong, Y.; Sha, Y.; et al. Super-Efficient in Vivo Two-Photon Photodynamic Therapy with a Gold Nanocluster as a Type I Photosensitizer. ACS Nano 2020, 14, 9532–9544. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H. Recent Progress in Nanophotosensitizers for Advanced Photodynamic Therapy of Cancer. J. Phys. Mater. 2021, 4, 014003. [Google Scholar] [CrossRef]
- Mahata, M.K.; De, R.; Lee, K.T. Near-Infrared-Triggered Upconverting Nanoparticles for Biomedicine Applications. Biomedicines 2021, 9, 756. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Huang, D.; Chen, G. Recent Advances of Lanthanide-Doped Upconversion Nanoparticles for Biological Applications. Nanotechnology 2020, 31, 072001. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Ju, Q.; Zhang, X.; Chen, X.; Wang, F.; Zhu, G. A Core-Shell-Shell Nanoplatform Upconverting near-Infrared Light at 808 Nm for Luminescence Imaging and Photodynamic Therapy of Cancer. Sci. Rep. 2015, 5, 10785. [Google Scholar] [CrossRef]
- Qiao, X.-F.; Zhou, J.-C.; Xiao, J.-W.; Wang, Y.-F.; Sun, L.-D.; Yan, C.-H. Triple-Functional Core–Shell Structured Upconversion Luminescent Nanoparticles Covalently Grafted with Photosensitizer for Luminescent, Magnetic Resonance Imaging and Photodynamic Therapy in Vitro. Nanoscale 2012, 4, 4611. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Chu, C.-H.; Wang, S.-B.; Chen, K.-Y.; Gao, H.-D.; Lee, M.M.; Sun, S.-S.; Li, J.-P.; Chen, J.-Y.; Chen, J.-K.; et al. Lipid-Wrapped Upconversion Nanoconstruct/Photosensitizer Complex for Near-Infrared Light-Mediated Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 84–95. [Google Scholar] [CrossRef]
- Viana, B.; Sharma, S.K.; Gourier, D.; Maldiney, T.; Teston, E.; Scherman, D.; Richard, C. Long Term in Vivo Imaging with Cr3+ Doped Spinel Nanoparticles Exhibiting Persistent Luminescence. J. Lumin 2016, 170, 879–887. [Google Scholar] [CrossRef]
- Abdurahman, R.; Yang, C.X.; Yan, X.P. Conjugation of a Photosensitizer to near Infrared Light Renewable Persistent Luminescence Nanoparticles for Photodynamic Therapy. Chem. Commun. 2016, 52, 13303–13306. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, L.; Li, J.; Zhang, D.; Lan, S.; Zhang, X.; Lin, X.; Liu, G.; Liu, X.; Liu, J. Self-Luminescing Theranostic Nanoreactors with Intraparticle Relayed Energy Transfer for Tumor Microenvironment Activated Imaging and Photodynamic Therapy. Theranostics 2019, 9, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-Photon Excitation Nanoparticles for Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Rohwer, N.; Cramer, T. Hypoxia-Mediated Drug Resistance: Novel Insights on the Functional Interaction of HIFs and Cell Death Pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting Tumour Hypoxia in Cancer Treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. JNCI J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming Obstacles in the Tumor Microenvironment: Recent Advancements in Nanoparticle Delivery for Cancer Theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Maier, A.; Tomaselli, F.; Anegg, U.; Rehak, P.; Fell, B.; Luznik, S.; Pinter, H.; Smolle-Jüttner, F.M. Combined Photodynamic Therapy and Hyperbaric Oxygenation in Carcinoma of the Esophagus and the Esophago-Gastric Junction. Eur. J. Cardio-Thorac. Surg. 2000, 18, 649–655. [Google Scholar] [CrossRef]
- Tomaselli, F. Photodynamic Therapy Enhanced by Hyperbaric Oxygen in Acute Endoluminal Palliation of Malignant Bronchial Stenosis (Clinical Pilot Study in 40 Patients). Eur. J. Cardio-Thorac. Surg. 2001, 19, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Zheng, M.; Zhao, P.; Chen, Z.; Siu, F.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; et al. Self-Monitoring Artificial Red Cells with Sufficient Oxygen Supply for Enhanced Photodynamic Therapy. Sci. Rep. 2016, 6, 23393. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qu, J.; Zhu, C.; Li, W.; Luo, L.; Yang, J.; Yin, X.; Li, Q.; Du, Y.; Chen, D.; et al. Synchronous Delivery of Oxygen and Photosensitizer for Alleviation of Hypoxia Tumor Microenvironment and Dramatically Enhanced Photodynamic Therapy. Drug Deliv. 2018, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, T.; Zou, M.; Yu, W.; Li, C.; He, Z.; Zhang, M.; Liu, M.; Li, Z.; Feng, J.; et al. Aggressive Man-Made Red Blood Cells for Hypoxia-Resistant Photodynamic Therapy. Adv. Mater. 2018, 30, 1802006. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon Nanoparticles Enhance Reactive Oxygen Levels and Tumour Growth Inhibition in Photodynamic Therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yan, X.; Wang, H.; Tanaka, J.; Wang, M.; You, W.; Li, Z. Perfluorocarbon-Based O2 Nanocarrier for Efficient Photodynamic Therapy. J. Mater. Chem. B 2019, 7, 1116–1123. [Google Scholar] [CrossRef]
- Zhao, C.; Tong, Y.; Li, X.; Shao, L.; Chen, L.; Lu, J.; Deng, X.; Wang, X.; Wu, Y. Photosensitive Nanoparticles Combining Vascular-Independent Intratumor Distribution and On-Demand Oxygen-Depot Delivery for Enhanced Cancer Photodynamic Therapy. Small 2018, 14, 1703045. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Zhang, Y.; Yang, B.; He, Y.; Tian, C.; Xu, X.; Gu, Z. An Oxygen Self-Sufficient Fluorinated Nanoplatform for Relieved Tumor Hypoxia and Enhanced Photodynamic Therapy of Cancers. ACS Appl. Mater. Interfaces 2019, 11, 7731–7742. [Google Scholar] [CrossRef]
- Zhou, R.; Ohulchanskyy, T.Y.; Xu, H.; Ziniuk, R.; Qu, J. Catalase Nanocrystals Loaded with Methylene Blue as Oxygen Self-Supplied, Imaging-Guided Platform for Photodynamic Therapy of Hypoxic Tumors. Small 2021, 17, 2103569. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.; Sheng, Z.; Gao, D.; Yan, F.; Ma, T.; Zheng, H.; Hong, M. A Catalase-Loaded Hierarchical Zeolite as an Implantable Nanocapsule for Ultrasound-Guided Oxygen Self-Sufficient Photodynamic Therapy against Pancreatic Cancer. Nanoscale 2018, 10, 17283–17292. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme Decorated Metal–Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, X.; Yang, S.; Lai, X.; Liu, H.; Gao, Y.; Feng, H.; Zhu, M.; Yuan, Y.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Gao, F.; Wei, J.-J.; Qian, C.-G.; Sun, M.-J. Biomimetic Hybrid Nanozymes with Self-Supplied H + and Accelerated O 2 Generation for Enhanced Starvation and Photodynamic Therapy against Hypoxic Tumors. Nano Lett. 2019, 19, 4334–4342. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Cao, Y.; Xue, Y.; Wu, F.; Yu, F.; Wu, M.; Zhu, X. Multifunctional Theranostic Agents Based on Prussian Blue Nanoparticles for Tumor Targeted and MRI—Guided Photodynamic/Photothermal Combined Treatment. Nanotechnology 2020, 31, 135101. [Google Scholar] [CrossRef]
- Song, X.; Feng, L.; Liang, C.; Gao, M.; Song, G.; Liu, Z. Liposomes Co-Loaded with Metformin and Chlorin E6 Modulate Tumor Hypoxia during Enhanced Photodynamic Therapy. Nano Res. 2017, 10, 1200–1212. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Van Vuuren, R.J.; Visagie, M.H.; Theron, A.E.; Joubert, A.M. Antimitotic Drugs in the Treatment of Cancer. Cancer Chemother. Pharm. 2015, 76, 1101–1112. [Google Scholar] [CrossRef]
- Lehmann, F.; Wennerberg, J. Evolution of Nitrogen-Based Alkylating Anticancer Agents. Processes 2021, 9, 377. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and Its Derivatives Are Known to Target Topoisomerase I (Top1) as Their Mechanism of Action: Did We Miss Something in CPT Analogue Molecular Targets for Treating Human Disease Such as Cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar] [PubMed]
- Wang, K.-B.; Elsayed, M.S.A.; Wu, G.; Deng, N.; Cushman, M.; Yang, D. Indenoisoquinoline Topoisomerase Inhibitors Strongly Bind and Stabilize the MYC Promoter G-Quadruplex and Downregulate MYC. J. Am. Chem. Soc. 2019, 141, 11059–11070. [Google Scholar] [CrossRef] [PubMed]
- Hande, K.R. Topoisomerase II Inhibitors. Update Cancer 2008, 3, 13–26. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tian, J.; Chen, C.; Jiang, D.; Xue, Y.; Wang, C.; Gao, Y.; Zhang, W. An Oxygen Self-Sufficient NIR-Responsive Nanosystem for Enhanced PDT and Chemotherapy against Hypoxic Tumors. Chem. Sci. 2019, 10, 5766–5772. [Google Scholar] [CrossRef]
- Tian, J.; Xiao, C.; Huang, B.; Wang, C.; Zhang, W. Janus Macromolecular Brushes for Synergistic Cascade-Amplified Photodynamic Therapy and Enhanced Chemotherapy. Acta Biomater. 2020, 101, 495–506. [Google Scholar] [CrossRef]
- Kim, Y.; Uthaman, S.; Pillarisetti, S.; Noh, K.; Huh, K.M.; Park, I.K. Bioactivatable Reactive Oxygen Species-Sensitive Nanoparticulate System for Chemo-Photodynamic Therapy. Acta Biomater. 2020, 108, 273–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, F.; Ren, C.; Yang, L.; Liu, J.; Cheng, Z.; Chu, L.; Liu, J. Targeted Chemo-Photodynamic Combination Platform Based on the DOX Prodrug Nanoparticles for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 13016–13028. [Google Scholar] [CrossRef]
- He, H.; Zhu, R.; Sun, W.; Cai, K.; Chen, Y.; Yin, L. Selective Cancer Treatment via Photodynamic Sensitization of Hypoxia-Responsive Drug Delivery. Nanoscale 2018, 10, 2856–2865. [Google Scholar] [CrossRef]
- Jin, F.; Qi, J.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Xu, X.; Ying, X.; Ji, J.; et al. Cancer-Cell-Biomimetic Upconversion Nanoparticles Combining Chemo-Photodynamic Therapy and CD73 Blockade for Metastatic Triple-Negative Breast Cancer. J. Control. Release 2021, 337, 90–104. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Liang, C.; Feng, L.; Dong, Z.; Song, X.; Song, G.; Liu, Z. Drug-Induced Co-Assembly of Albumin/Catalase as Smart Nano-Theranostics for Deep Intra-Tumoral Penetration, Hypoxia Relieve, and Synergistic Combination Therapy. J. Control. Release 2017, 263, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Moret, F.; Menilli, L.; Battan, M.; Tedesco, D.; Columbaro, M.; Guerrini, A.; Avancini, G.; Ferroni, C.; Varchi, G. Pheophorbide A and Paclitaxel Bioresponsive Nanoparticles as Double-Punch Platform for Cancer Therapy. Pharmaceutics 2021, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Moret, F.; Menilli, L.; Guerrini, A.; Tedesco, D.; Naldi, M.; Bartolini, M.; Gani, M.; Zorzet, S.; Columbaro, M.; et al. HSA-Binding Prodrugs-Based Nanoparticles Endowed with Chemo and Photo-Toxicity against Breast Cancer. Cancers 2022, 14, 877. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Photodynamic Therapy/Chemotherapy against Breast Cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef]
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.H.; Valic, M.S.; Cheng, M.H.Y.; Rosilio, V.; Chen, J.; Zheng, G. Porphyrin-Lipid Stabilized Paclitaxel Nanoemulsion for Combined Photodynamic Therapy and Chemotherapy. J. Nanobiotechnology 2021, 19, 154. [Google Scholar] [CrossRef]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.; Zhang, F.; Zhou, Z.; et al. A Discrete Organoplatinum(II) Metallacage as a Multimodality Theranostic Platform for Cancer Photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, X.; Zhang, C.; Huang, W.; Zhou, Y.; Yan, D. Oxygen and Pt(II) Self-Generating Conjugate for Synergistic Photo-Chemo Therapy of Hypoxic Tumor. Nat. Commun. 2018, 9, 2053. [Google Scholar] [CrossRef]
- Chu, B.; Qu, Y.; He, X.; Hao, Y.; Yang, C.; Yang, Y.; Hu, D.; Wang, F.; Qian, Z. ROS-Responsive Camptothecin Prodrug Nanoparticles for On-Demand Drug Release and Combination of Chemotherapy and Photodynamic Therapy. Adv. Funct. Mater. 2020, 30, 2005918. [Google Scholar] [CrossRef]
- Obaid, G.; Bano, S.; Thomsen, H.; Callaghan, S.; Shah, N.; Swain, J.W.R.; Jin, W.; Ding, X.; Cameron, C.G.; McFarland, S.A.; et al. Remediating Desmoplasia with EGFR-Targeted Photoactivable Multi-Inhibitor Liposomes Doubles Overall Survival in Pancreatic Cancer. Adv. Sci. 2022, 9, 2104594. [Google Scholar] [CrossRef]
- Ghosh, S.; Sun, B.; Jahagirdar, D.; Luo, D.; Ortega, J.; Straubinger, R.M.; Lovell, J.F. Single-Treatment Tumor Ablation with Photodynamic Liposomal Irinotecan Sucrosulfate. Transl. Oncol. 2022, 19, 101390. [Google Scholar] [CrossRef] [PubMed]
- Turchin, I.; Bano, S.; Kirillin, M.; Orlova, A.; Perekatova, V.; Plekhanov, V.; Sergeeva, E.; Kurakina, D.; Khilov, A.; Kurnikov, A.; et al. Combined Fluorescence and Optoacoustic Imaging for Monitoring Treatments against CT26 Tumors with Photoactivatable Liposomes. Cancers 2021, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Ma, Q.; Zhang, H.; Liu, Y.; Hong, J.; Ding, Z.; Liu, M.; Han, J. Novel Carrier-Free Nanoparticles Composed of 7-Ethyl-10-Hydroxycamptothecin and Chlorin E6: Self-Assembly Mechanism Investigation and in Vitro/in Vivo Evaluation. Colloids Surf B Biointerfaces 2020, 188, 110722. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.T.; Sala, V.; Prever, L.; Hirsch, E.; Ardehali, H.; Ghigo, A. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annu. Rev. Pharm. Toxicol. 2021, 61, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, W.; Li, Y.; Li, J.; Jiang, F.; Xie, J.; Huang, X. Combining Doxorubicin-Conjugated Polymeric Nanoparticles and 5-Aminolevulinic Acid for Enhancing Radiotherapy against Lung Cancer. Bioconjug Chem. 2022, 33, 654–665. [Google Scholar] [CrossRef]
- Cabral, Á.S.; Leonel, E.C.R.; Candido, N.M.; Piva, H.L.; de Melo, M.T.; Taboga, S.R.; Rahal, P.; Tedesco, A.C.; Calmon, M.F. Combined Photodynamic Therapy with Chloroaluminum Phthalocyanine and Doxorubicin Nanoemulsions in Breast Cancer Model. J. Photochem. Photobiol. B 2021, 218, 112181. [Google Scholar] [CrossRef]
- Hameed, S.; Bhattarai, P.; Liang, X.; Zhang, N.; Xu, Y.; Chen, M.; Dai, Z. Self-Assembly of Porphyrin-Grafted Lipid into Nanoparticles Encapsulating Doxorubicin for Synergistic Chemo-Photodynamic Therapy and Fluorescence Imaging. Theranostics 2018, 8, 5501–5518. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Wu, T.; Yuan, C.; Zeng, B.; Xu, Y.; Dai, L. A Simple Dual-PH Responsive Prodrug-Based Polymeric Micelles for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17109–17117. [Google Scholar] [CrossRef] [PubMed]
- Helmich, F.; Lee, C.C.; Nieuwenhuizen, M.M.L.; Gielen, J.C.; Christianen, P.C.M.; Larsen, A.; Fytas, G.; Leclère, P.E.L.G.; Schenning, A.P.H.J.; Meijer, E.W. Dilution-Induced Self-Assembly of Porphyrin Aggregates: A Consequence of Coupled Equilibria. Angew. Chem. Int. Ed. 2010, 49, 3939–3942. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Sun, C.; Tao, W.; Li, J.; Yang, X.; Wang, J. ROS-Sensitive Thioketal-Linked Polyphosphoester-Doxorubicin Conjugate for Precise Phototriggered Locoregional Chemotherapy. Biomaterials 2019, 188, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- He, Z.; Jiang, H.; Zhang, X.; Zhang, H.; Cui, Z.; Sun, L.; Li, H.; Qian, J.; Ma, J.; Huang, J. Nano-Delivery Vehicle Based on Chlorin E6, Photodynamic Therapy, Doxorubicin Chemotherapy Provides Targeted Treatment of HER-2 Negative, Aνβ3-Positive Breast Cancer. Pharm. Res. 2020, 160, 105184. [Google Scholar] [CrossRef]
- Amin, M.; Mansourian, M.; Koning, G.A.; Badiee, A.; Jaafari, M.R.; Ten Hagen, T.L.M. Development of a Novel Cyclic RGD Peptide for Multiple Targeting Approaches of Liposomes to Tumor Region. J. Control. Release 2015, 220, 308–315. [Google Scholar] [CrossRef]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane–Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, 1804105. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, Y.; Zhang, H.; Li, R.; Hu, Y.; Liu, B. The Role of Adhesions between Homologous Cancer Cells in Tumor Progression and Targeted Therapy. Expert Rev. Anticancer Ther. 2017, 17, 517–526. [Google Scholar] [CrossRef]
- Xie, B.R.; Yu, Y.; Liu, X.H.; Zeng, J.Y.; Zou, M.Z.; Li, C.X.; Zeng, X.; Zhang, X.Z. A near Infrared Ratiometric Platform Based π-Extended Porphyrin Metal-Organic Framework for O2 Imaging and Cancer Therapy. Biomaterials 2021, 272, 120782. [Google Scholar] [CrossRef]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-Linked Paclitaxel Prodrug Micellar Nanoparticles as a Versatile and Potent Platform for Cancer Therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Özliseli, E.; Zhang, Y.; Pan, G.; Wang, D.; Zhang, H. Fabrication of Redox-Responsive Doxorubicin and Paclitaxel Prodrug Nanoparticles with Microfluidics for Selective Cancer Therapy. Biomater. Sci. 2019, 7, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhong, H.; Zou, H.; Liu, T.; Yu, N.; Zhang, X.; Xu, Z.; Chen, Z.; Guo, S. Acid-Sensitive PEGylated Paclitaxel Prodrug Nanoparticles for Cancer Therapy: Effect of PEG Length on Antitumor Efficacy. J. Control. Release 2020, 326, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.; Conte, C.; Esposito, D.; Reddi, E.; Quaglia, F.; Moret, F. CD44 Targeting Mediated by Polymeric Nanoparticles and Combination of Chlorine TPCS2a-PDT and Docetaxel-Chemotherapy for Efficient Killing of Breast Differentiated and Stem Cancer Cells In Vitro. Cancers 2020, 12, 278. [Google Scholar] [CrossRef]
- Maiolino, S.; Moret, F.; Conte, C.; Fraix, A.; Tirino, P.; Ungaro, F.; Sortino, S.; Reddi, E.; Quaglia, F. Hyaluronan-Decorated Polymer Nanoparticles Targeting the CD44 Receptor for the Combined Photo/Chemo-Therapy of Cancer. Nanoscale 2015, 7, 5643–5653. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharm. Rev. 2001, 53, 283–318. [Google Scholar]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Shaili, E. Platinum Anticancer Drugs and Photochemotherapeutic Agents: Recent Advances and Future Developments. Sci. Prog. 2014, 97, 20–40. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front Pharm. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, H.; Chen, W.; Wu, J.; Feng, X.; Tong, R.; Yu, H.; Chen, Y.; Lv, Z.; et al. Combinatorial Photochemotherapy on Liver Cancer Stem Cells with Organoplatinum(II) Metallacage-Based Nanoparticles. J. Mater. Chem. B 2019, 7, 6476–6487. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Foran, G.J.; Mei, Z.; Beale, P.J.; Hambley, T.W. XANES Determination of the Platinum Oxidation State Distribution in Cancer Cells Treated with Platinum(IV) Anticancer Agents. J. Am. Chem. Soc. 2003, 125, 7524–7525. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, W.; Qi, R.; Yan, L.; Jing, X.; Zheng, M.; Xiao, H. Delivering a Photosensitive Transplatin Prodrug to Overcome Cisplatin Drug Resistance. Chem. Commun. 2015, 51, 11493–11495. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Sadler, P.J. Photoreaction Pathways for the Anticancer Complex Trans,Trans,Trans-[Pt(N3)2(OH)2(NH3)2]. Dalton Trans. 2010, 40, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.G.; Yang, D.Y.; Wang, S.B.; Chen, A.Z. Ultrasmall Platinum Nanoparticles Enable Deep Tumor Penetration and Synergistic Therapeutic Abilities through Free Radical Species-Assisted Catalysis to Combat Cancer Multidrug Resistance. Chem. Eng. J. 2020, 383, 123138. [Google Scholar] [CrossRef]
- Liu, C.; Xing, J.; Akakuru, O.U.; Luo, L.; Sun, S.; Zou, R.; Yu, Z.; Fang, Q.; Wu, A. Nanozymes-Engineered Metal-Organic Frameworks for Catalytic Cascades-Enhanced Synergistic Cancer Therapy. Nano Lett. 2019, 19, 5674–5682. [Google Scholar] [CrossRef]
- Molinaro, R.; Corbo, C.; Martinez, J.O.; Taraballi, F.; Evangelopoulos, M.; Minardi, S.; Yazdi, I.K.; Zhao, P.; De Rosa, E.; Sherman, M.B.; et al. Biomimetic Proteolipid Vesicles for Targeting Inflamed Tissues. Nat. Mater. 2016, 15, 1037–1046. [Google Scholar] [CrossRef]
- Johansson, A.; Hamzah, J.; Payne, C.J.; Ganss, R. Tumor-Targeted TNFα Stabilizes Tumor Vessels and Enhances Active Immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 7841–7846. [Google Scholar] [CrossRef]
- Luo, X.; Chi, X.; Lin, Y.; Yang, Z.; Lin, H.; Gao, J. A Camptothecin Prodrug Induces Mitochondria-Mediated Apoptosis in Cancer Cells with Cascade Activations. Chem. Commun. 2021, 57, 11033–11036. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Lin, H.-H.; Su, D.; Yang, D.-C.; Liu, J.-Y. Enzyme-Activated Multifunctional Prodrug Combining Site-Specific Chemotherapy with Light-Triggered Photodynamic Therapy. Mol. Pharm. 2022, 19, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Huang, R.; Cang, H.; Cai, Z.; Sun, B. PH-Sensitive Prodrug Conjugated Polydopamine for NIR-Triggered Synergistic Chemo-Photothermal Therapy. Eur. J. Pharm. Biopharm. 2018, 128, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Shi, J.; Pei, Y.; Pei, Z. A Carrier-Free Supramolecular Nanoprodrug Based on Lactose-Functionalized Dimeric Camptothecin via Self-Assembly in Water for Targeted and Fluorescence Imaging-Guided Chemo-Photodynamic Therapy. J. Colloid Interface Sci. 2022, 609, 353–363. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem Biol 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical Activity of Nanoliposomal Irinotecan Is Governed by Tumor Deposition and Intratumor Prodrug Conversion. Cancer Res. 2014, 74, 7003–7013. [Google Scholar] [CrossRef]
- Chibaudel, B.; Maindrault-Gœbel, F.; Bachet, J.; Louvet, C.; Khalil, A.; Dupuis, O.; Hammel, P.; Garcia, M.; Bennamoun, M.; Brusquant, D.; et al. PEPCOL: A GERCOR Randomized Phase Study of Nanoliposomal Irinotecan PEP02 (MM-398) or Irinotecan with Leucovorin/5-fluorouracil as Second-line Therapy in Metastatic Colorectal Cancer. Cancer Med. 2016, 5, 676–683. [Google Scholar] [CrossRef]
- Ko, A.H.; Tempero, M.A.; Shan, Y.-S.; Su, W.-C.; Lin, Y.-L.; Dito, E.; Ong, A.; Wang, Y.-W.; Yeh, C.G.; Chen, L.-T. A Multinational Phase 2 Study of Nanoliposomal Irinotecan Sucrosofate (PEP02, MM-398) for Patients with Gemcitabine-Refractory Metastatic Pancreatic Cancer. Br. J. Cancer 2013, 109, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Mallidi, S.; Liu, J.; Chiang, C.-T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef]
- Huang, H.-C.; Rizvi, I.; Liu, J.; Anbil, S.; Kalra, A.; Lee, H.; Baglo, Y.; Paz, N.; Hayden, D.; Pereira, S.; et al. Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res. 2018, 78, 558–571. [Google Scholar] [CrossRef]
- Anbil, S.; Pigula, M.; Huang, H.-C.; Mallidi, S.; Broekgaarden, M.; Baglo, Y.; de Silva, P.; Simeone, D.M.; Mino-Kenudson, M.; Maytin, E.V.; et al. Vitamin D Receptor Activation and Photodynamic Priming Enables Durable Low-Dose Chemotherapy. Mol. Cancer 2020, 19, 1308–1319. [Google Scholar] [CrossRef]
- Palakurthi, S. Challenges in SN38 Drug Delivery: Current Success and Future Directions. Expert Opin. Drug Deliv. 2015, 12, 1911–1921. [Google Scholar] [CrossRef] [PubMed]

| Chemo Drug | PS | DDS Composition | DDS Features | Cancer Model | Outcome | Reference |
|---|---|---|---|---|---|---|
| Doxorubicin (DOX) | IR780 | Self-assembled micelles composed of a conjugate (oxygen carrier (F), IR780, and hydrophilic PEG chain) loaded with DOX | Hypoxia relief | MCF7 human breast cancer | Enhanced in vivo tumor regression in mice treated with O2-loaded micelles | [95] |
| Doxorubicin (DOX) | Tetraphenylporphyrin (TPP) | Polymeric nanoparticles (PDPA-TPP-DOX) | pH responsiveness | 4T1 murine breast cancer | Improved uptake and retention in an in vivo tumor site (lower pH) | [96] |
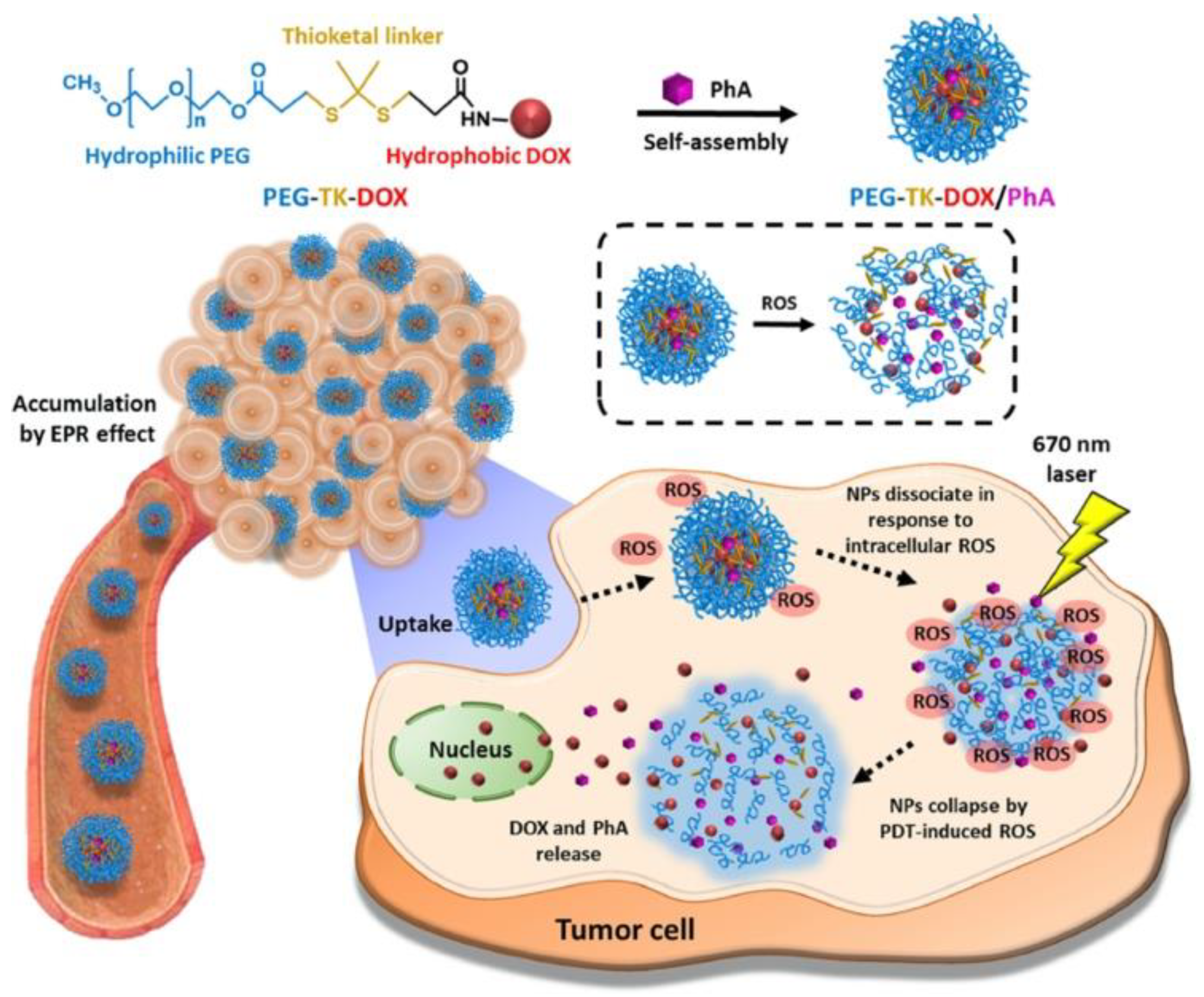
| Doxorubicin (DOX) | Pheophorbide A (PhA) | Self-assembled prodrug nanoparticles (PEG-TK-DOX) loaded with PhA | ROS responsiveness | CT26 murine colon cancer | Higher accumulation of DOX in an in vivo tumor site | [97] |
| Doxorubicin (DOX) | Chlorin e6 (Ce6) | Self-assembled prodrug nanoparticles (CRGDK-PEG-DOX) loaded with Ce6 | pH responsiveness, active targeting through neuropilin-1 (NRP-1) receptor-mediated internalization | MDA-MB-231 and MCF7 human breast cancer | Increased uptake in MDA-MB-231 (NRP-1+), compared to MCF7 (NRP-1−), and longer in vivo blood circulation and intratumoral accumulation | [98] |
| Doxorubicin (DOX) | Chlorin e6 (Ce6) | Polymeric nanoparticles (PEI-NI and HA-Ce6) loaded with DOX | Targeting of CD44+ cells and hypoxia responsiveness | LLC murine lung carcinoma | Recognition of CD44+ cells and consequent endocytosis, leading to higher uptake of targeted NPs, and strong in vivo anticancer effect in hypoxia | [99] |
| Doxorubicin (DOX) | Rose Bengal (RB) | Biomimetic upconverting nanoparticles (PEG-TK-DOX and RB co-loaded upconverting NP core, coated with cancer cell membranes) | ROS responsiveness and tumor infiltration | 4T1 murine breast cancer | Preferential in vitro accumulation in homologous cancer cells and immunogenic cell death induction and stronger in vivo tumor growth inhibition | [100] |
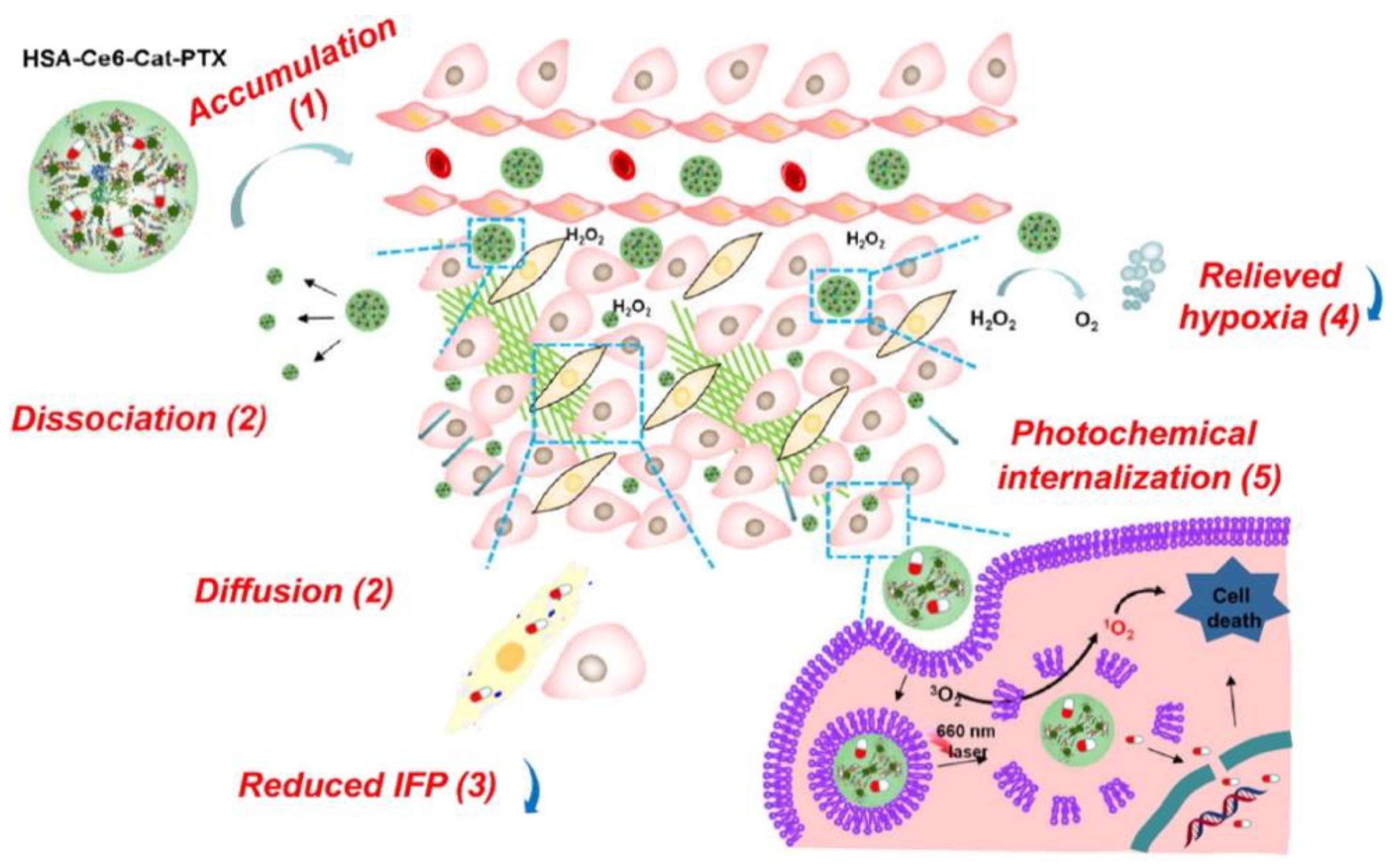
| Paclitaxel (PTX) | Chlorin e6 (Ce6) | Human serum albumin (HSA) nanoparticles loaded with catalase (Cat), PTX, and Ce6 | Hypoxia relief | 4T1 murine breast cancer | Prolonged blood circulation (stealth properties due to HSA), increased in vivo intratumoral oxyhemoglobin levels, and low expression of hypoxia markers | [101] |
| Paclitaxel (PTX) | Pheophorbide A (PheoA) | Carrier-free self-assembled prodrug (PTX2S) micelles loaded with PheoA | Redox and ROS responsiveness | MDA-MB-231 human breast cancer and SKOV-3 human ovarian cancer | Micelles disassembly in simulated reductive TME | [102] |
| Paclitaxel (PTX) | Pheophorbide A (PheoA) | Carrier-free self-assembled prodrug (PTX2S/PTX-SS-PEG-MAL) micelles loaded with PheoA | Redox and ROS responsiveness and HSA-mediated uptake | MDA-MB-231, MCF7 human breast cancer, and 4T1 murine breast cancer | Higher in vitro intracellular uptake of HSA-binding micelles | [103] |
| Paclitaxel (PTX) | Tetraphenylchlorin (TPC) | Biomimetic polymeric (PEG-b-PDLLA) nanoparticles loaded with PTX prodrug (PTX2-TK) and TPC, coated with red blood cell (RBC) membranes | Redox responsiveness and stealth properties | HeLa human cervix adenocarcinoma and RAW264.7 murine macrophages | Reduced macrophage capture of RBC-coated NPs, longer in vivo blood circulation, and reduced hepatic clearance | [104] |
| Docetaxel (DTX) | Chlorin e6 (Ce6) | Self-assembled nanoparticles (core composed of modified hyaluronic acid and Ce6) loaded with DTX | Targeting of CD44+ cells and redox responsiveness | MCF7 human breast cancer (2D cell cultures) and 4T1 murine breast cancer (tumor-bearing mice) | NPs disassembly in reductive environment, higher uptake of Ce6 NPs in MCF7 CD44+ cells, and persistent intratumoral retention in vivo | [105] |
| Paclitaxel (PTX) | Porphyrin-lipid | Porphyrin-lipid nanoemulsion loaded with PTX, stabilized DSPE-PEG2000 | Stealth properties | KB human epithelial carcinoma | Prolonged blood circulation and enhanced intratumoral retention | [106] |
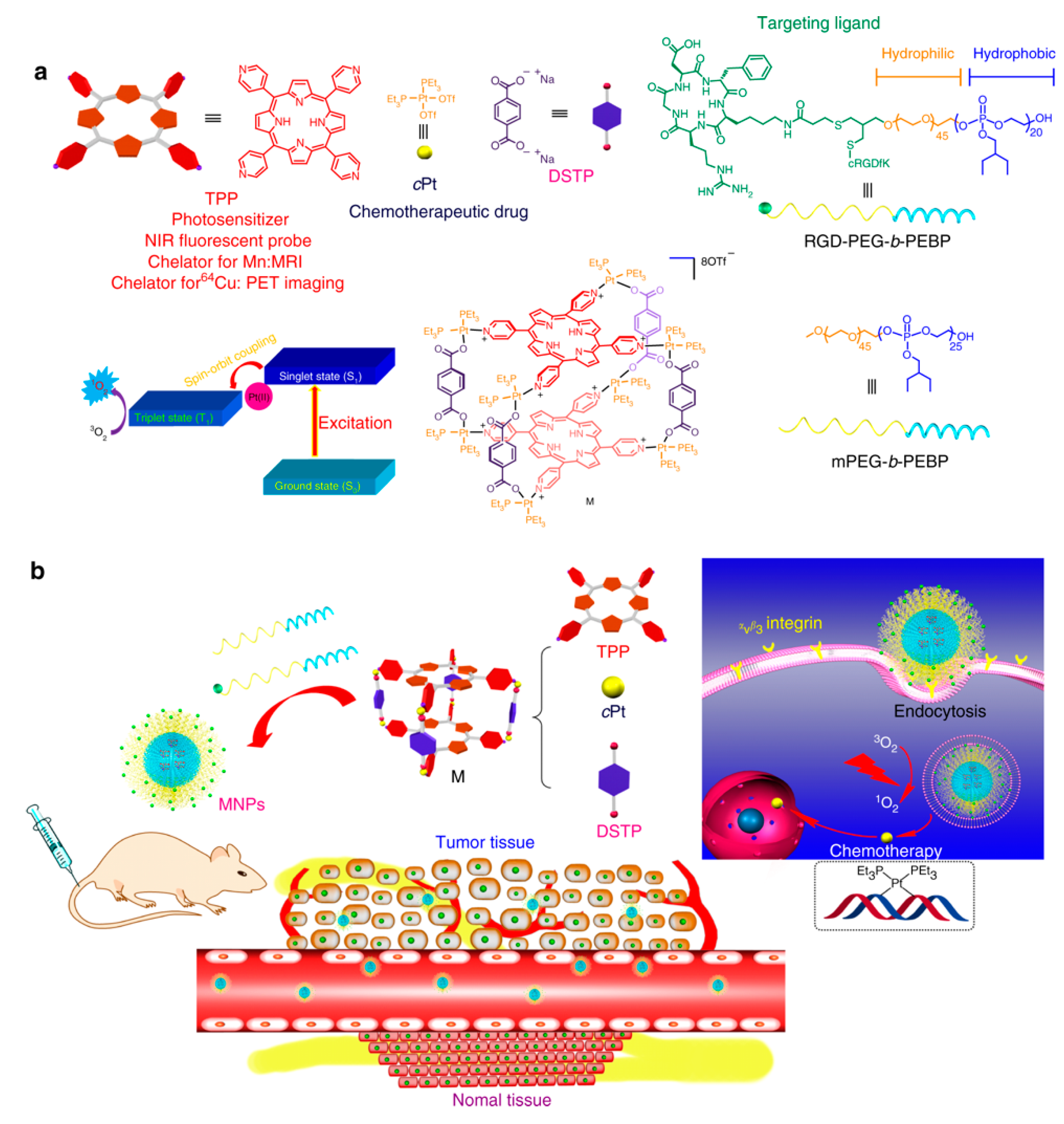
| cis-Platin (cPt) | Tetrapyridylporphyrin (TPP) | Self-assembled coordination complex between cPt, TPP, and DSTP loaded in NPs composed of mPEG-b-PEBP and RGD-PEG-b-PEBP | Active targeting through ανβ3 receptor-mediated uptake | U87MG human glioblastoma | In vitro and in vivo studies on U87MG cells overexpressing αvβ3 integrins showed a higher intracellular accumulation of MNPs | [107] |
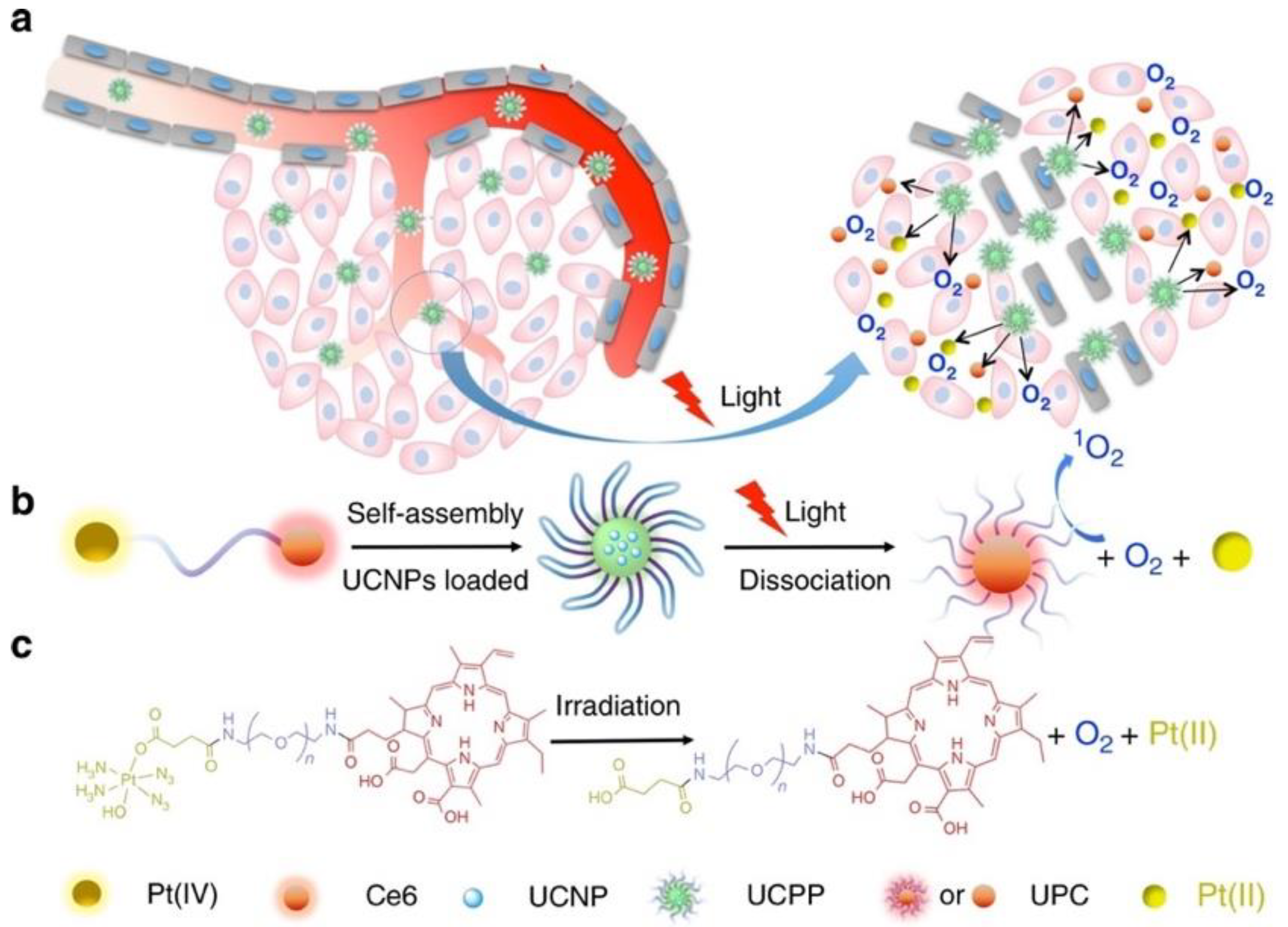
| Platinum (IV) complex | Chlorin e6 (Ce6) | Upconverting nanoparticles loaded with Ce6-PEG-Pt (IV) conjugate | Hypoxia relief | HeLa human cervix adenocarcinoma (2D cell cultures and xenografted mice), HCT116 human colorectal cancer, B16 murine melanoma, and MDA-MB-231 human breast cancer | In vivo suppression of HIF-1α and CD31 hypoxia markers | [108] |
| Nano-platinum (nanoPt) | Verteporfin (VP) | Biomimetic liposomes (lipid bilayer containing VP) loaded with nanoPt and coated with RAW264.7 macrophage membranes | Hypoxia relief and tumor infiltration | 4T1 murine breast cancer (2D and 3D cell cultures; tumor-bearing mice) | Efficient tumor site targeting, with increased efficacy of VP-PDT due to hypoxia reversion by nanoPt catalase-like feature | [83] |
| Camptothecin (CPT) | Pheophorbide A (PPa) | Self-assembled nanoparticles (composed of a conjugate MPEG-(TK-CPT)-PPa) | ROS responsiveness | HCT116 human colorectal cancer | Reduced premature drug release and delivery of the two drugs to the tumor site, where their release was enhanced by ROS production following irradiation | [109] |
| Irinotecan (IRI) | Lipidated benzoporphyrin derivative (BPD-PC) | Liposomes with lipidated BPD in the bilayer, functionalized with cetuximab and loaded with IRI | Active targeting of EGFR+ cells | MIA PaCa-2 human pancreatic cancer, i.e., MIA PaCa-2 + PCAF (xenografted mice) | Improved efficacy in EGFR+ tumors and desmoplasia remediation | [110] |
| Irinotecan (IRI) | Porphyrin-phospholipid derivative (PoP) | Liposomes with PoP bilayer loaded with IRI | Light-controlled release of the chemotherapeutic | MiaPaCa2 human pancreatic cancer (xenografted mice) | Light exposure resulted in increased intratumoral level of active metabolite SN-38 | [111] |
| Irinotecan (IRI) | Lipidated benzoporphyrin derivative (BPD) | Liposomes with lipidated BPD in the bilayer and loaded with IRI | Light-controlled release of the chemotherapeutic | CT26 murine colorectal cancer (tumor-bearing mice) | Light exposure resulted in increased intratumoral level of active metabolite SN-38 | [112] |
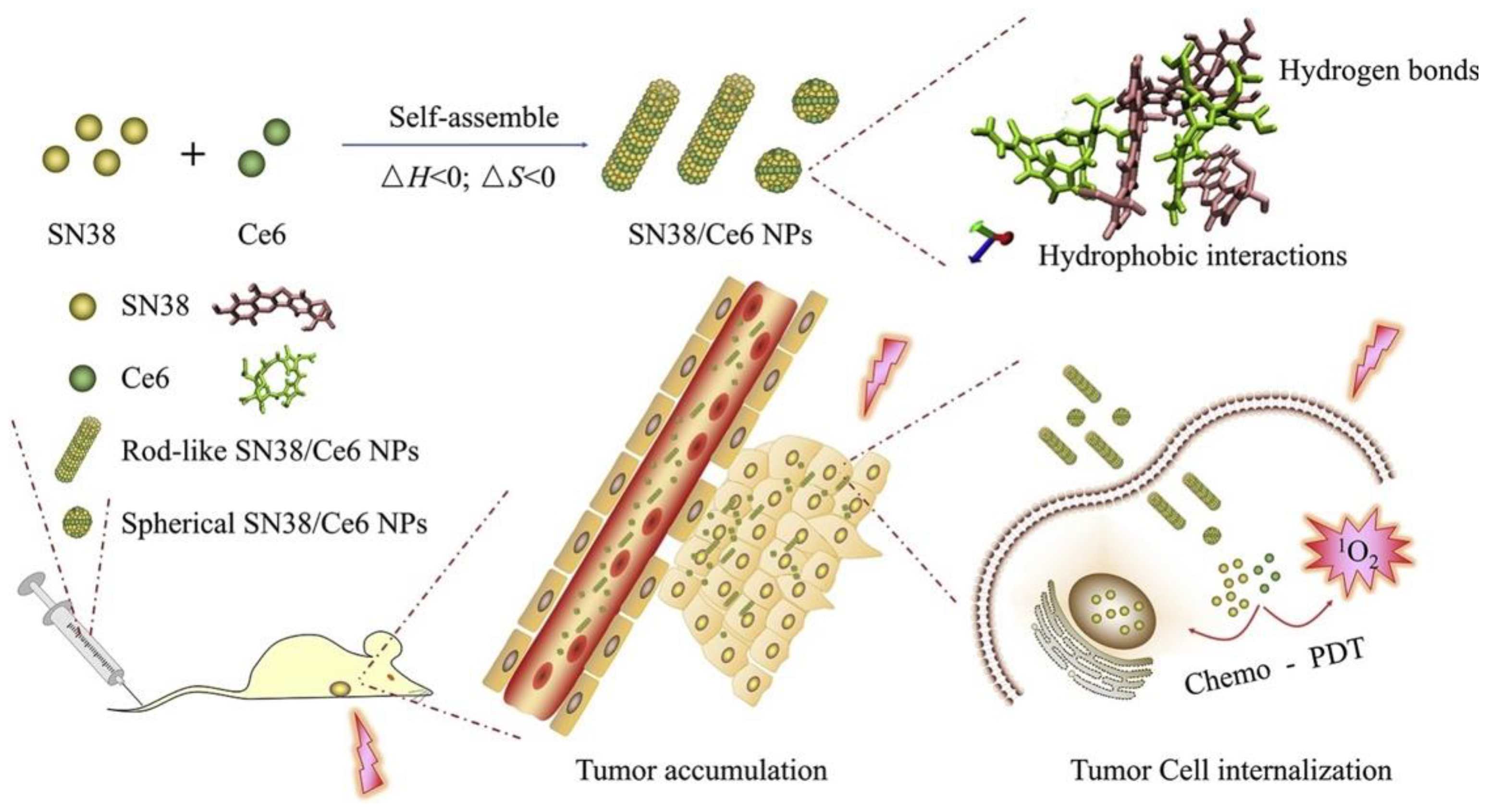
| Ethyl-10-hydroxycamptothecin (SN-38) | Chlorin e6 (Ce6) | Antisolvent-precipitated nanoparticles | Increase drug solubility | 4T1 murine breast cancer (2D cell cultures; tumor-bearing mice) | High drug loading, excellent stability in aqueous solutions, and enhanced intracellular and intratumor uptake and retention | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menilli, L.; Milani, C.; Reddi, E.; Moret, F. Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage. Cancers 2022, 14, 4462. https://doi.org/10.3390/cancers14184462
Menilli L, Milani C, Reddi E, Moret F. Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage. Cancers. 2022; 14(18):4462. https://doi.org/10.3390/cancers14184462
Chicago/Turabian StyleMenilli, Luca, Celeste Milani, Elena Reddi, and Francesca Moret. 2022. "Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage" Cancers 14, no. 18: 4462. https://doi.org/10.3390/cancers14184462
APA StyleMenilli, L., Milani, C., Reddi, E., & Moret, F. (2022). Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage. Cancers, 14(18), 4462. https://doi.org/10.3390/cancers14184462






