Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetic Hyperthermia Device
2.2. In Vivo Magnetic Hyperthermia Therapy
2.3. Blood and Tissue Collection
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. In Vivo Hyperthermia
3.2. Biochemical Changes after Exposure to AMF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hornback, N.B. Historical aspects of hyperthermia in cancer therapy. Radiol. Clin. N. Am. 1989, 27, 481–488. [Google Scholar] [PubMed]
- Seegenschmiedt, M.H.; Vernon, C.C. A Historical Perspective on Hyperthermia in Oncology. In Thermoradiotherapy and Thermochemotherapy: Biology, Physiology, Physics; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–44. ISBN 978-3-642-57858-8. [Google Scholar]
- LeBrun, A.; Zhu, L. Magnetic Nanoparticle Hyperthermia in Cancer Treatment: History, Mechanism, Imaging-Assisted Protocol Design, and Challenges. In Theory and Applications of Heat Transfer in Humans; Shrivastava, D., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 631–667. ISBN 9781119127420. [Google Scholar]
- Jordan, A.; Wust, P.; Fählin, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth. 1993, 9, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Scholz, R.; Faehling, H.; Krause, J.; Felix, R. Magnetic Fluid Hyperthermia (MFH). In Scientific and Clinical Applications of Magnetic Carriers; Häfeli, U., Schütt, W., Teller, J., Zborowski, M., Eds.; Springer: Boston, MA, USA, 1997; pp. 569–595. ISBN 978-1-4757-6482-6. [Google Scholar]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H. Roland Felix Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 41302. [Google Scholar] [CrossRef] [Green Version]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Fellows, B.D.; Rodrigo Arrizabalaga, I.; Yu, E.; Olivo, M.; Conolly, S.M. Magnetic Particle Imaging: An Emerging Modality with Prospects in Diagnosis, Targeting and Therapy of Cancer. Cancers 2021, 13, 5285. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, BME-31, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Brezovich, I.A. Low frequency hyperthermia: Capacitive and ferromagnetic thermoseed methods. Med. Phys. Monogr. 1988, 16, 82–111. [Google Scholar]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüß, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and characterization of the novel hyperthermia- and thermoablation-system for clinical magnetic fluid hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, D.; Ghosh, S.; Das, S.; Chatterjee, J. A review of recent advances in magnetic nanoparticle-based theranostics of glioblastoma. Nanomedicine 2022, 17, 107–132. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor magnetic hyperthermia induced by RGD-functionalized Fe3O4 nanoparticles, in an experimental model of colorectal liver metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- de la Parte, B.H.; Irazola, M.; Pérez-Muñoz, J.; Rodrigo, I.; Iturrizaga Correcher, S.; Mar Medina, C.; Castro, K.; Etxebarria, N.; Plazaola, F.; García, J.Á.; et al. Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats. Nanomaterials 2021, 11, 1318. [Google Scholar] [CrossRef]
- Dössel, O.; Bohnert, J. Safety considerations for magnetic fields of 10 mT to 100 mT amplitude in the frequency range of 10 kHz to 100 kHz for magnetic particle imaging. Biomed. Technol. 2013, 58, 611–621. [Google Scholar] [CrossRef]
- Kossatz, S.; Ludwig, R.; Dähring, H.; Ettelt, V.; Rimkus, G.; Marciello, M.; Salas, G.; Patel, V.; Teran, F.J.; Hilger, I. High Therapeutic Efficiency of Magnetic Hyperthermia in Xenograft Models Achieved with Moderate Temperature Dosages in the Tumor Area. Pharm. Res. 2014, 31, 3274–3288. [Google Scholar] [CrossRef] [Green Version]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jang, J.; Choi, J.; Moon, S.H.; Noh, S.; Kim, J.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Mutalik, S.; Rai, S.; Udupa, N.; Rao, B.S.S. PEGylation of superparamagnetic iron oxide nanoparticle for drug delivery applications with decreased toxicity: An in vivo study. J. Nanoparticle Res. 2015, 17, 412. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Xu, Y.; Du, W.; Cai, X.; Wang, F.; Ling, Y.; Chen, H.; Wang, Z.; Hu, B.; et al. A pH and magnetic dual-response hydrogel for synergistic chemo-magnetic hyperthermia tumor therapy. RSC Adv. 2018, 8, 9812–9821. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Liang, B.; Zheng, Y.; Exner, A.; Kolios, M.; Xu, T.; Guo, D.; Cai, X.; Wang, Z.; Ran, H.; et al. PMMA-Fe3O4 for internal mechanical support and magnetic thermal ablation of bone tumors. Theranostics 2019, 9, 4192–4207. [Google Scholar] [CrossRef]
- Soleymani, M.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Kalhori, M.R.; Hadjighassem, M.R.; Shaterabadi, Z.; Alizadeh, A.M. Effects of multiple injections on the efficacy and cytotoxicity of folate-targeted magnetite nanoparticles as theranostic agents for MRI detection and magnetic hyperthermia therapy of tumor cells. Sci. Rep. 2020, 10, 1695. [Google Scholar] [CrossRef]
- Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. Int. J. Mol. Sci. 2022, 23, 4234. [Google Scholar] [CrossRef]
- Epstein, Y.; Yanovich, R. Heatstroke. N. Engl. J. Med. 2019, 380, 2449–2459. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef]
- Mustafa, S.; Elgazzar, A.H.; Essam, H.; Gopinath, S.; Mathew, M. Hyperthermia Alters Kidney Function and Renal Scintigraphy. Am. J. Nephrol. 2007, 27, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Krafft, J.; Fink, R.; Rosalki, S.B. Serum Enzymes and Isoenzymes after Surgery. Ann. Clin. Biochem. 1977, 14, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Graeber, C.G.M.; Clagett, G.P.; Wolf, R.E.; Cafferty, P.J.; Harmon, C.J.W.; Rich, N.M. Alterations in Serum Creatine Kinase and Lactate Dehydrogenase: Association with Abdominal Aortic Surgery, Myocardial Infarction and Bowel Necrosis. Chest 1990, 97, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.A.; Vaida, S.; Somri, M.; Mogilner, J.; Lanir, A.; Tamir, A.; Shaoul, R. Changes in creatine phosphokinase (CK) concentrations after minor and major surgeries in children. BJA Br. J. Anaesth. 2006, 96, 786–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzeer, A.H.; El-Hazmi, M.A.F.; Warsy, A.S.; Ansari, Z.A.; Yrkendi, M.S. Serum enzymes in heat stroke: Prognostic implication. Clin. Chem. 1997, 43, 1182–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.-M.; Wang, L.; Zhang, Y.; Yuan, R.; Zhao, Y.; Hu, J.; Zhou, F.-H.; Kang, H.-J. Establishment and effectiveness evaluation of a scoring system for exertional heat stroke by retrospective analysis. Mil. Med. Res. 2020, 7, 40. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levi, M.; Levy, J.H. Heatstroke-induced coagulopathy: Biomarkers, mechanistic insights, and patient management. eClinicalMedicine 2022, 44, 101276. [Google Scholar] [CrossRef]
- Hashim, I.A. Clinical biochemistry of hyperthermia. Ann. Clin. Biochem. 2010, 47, 516–523. [Google Scholar] [CrossRef]
- Nie, J.; Tong, T.K.; George, K.; Fu, F.H.; Lin, H.; Shi, Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports 2011, 21, 625–629. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum Enzyme Monitoring in Sports Medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Weibrecht, K.; Dayno, M.; Darling, C.; Bird, S.B. Liver Aminotransferases Are Elevated with Rhabdomyolysis in the Absence of Significant Liver Injury. J. Med. Toxicol. 2010, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K.H.; Arumugananthan, C.; Lau Hing Yim, C.; Jellie, L.J.; Wong, E.W.W.; Junckerstorff, R.K. A Cross-Sectional Study of the Relationship between Serum Creatine Kinase and Liver Biochemistry in Patients with Rhabdomyolysis. J. Clin. Med. 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.K. Abnormal liver function tests associated with severe rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Tang, L.; Zhang, Y.; Duan, P.; Su, L.; Tong, H. The liver sinusoidal endothelial cell damage in rats caused by heatstroke. Eur. J. Inflamm. 2018, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, X.; Liu, B.; Liu, Y.; Zeng, Z.; Lu, L.; Zheng, Z.; Li, B.; Zheng, Z. Exercises in Hot and Humid Environment Caused Liver Injury in a Rat Model. PLoS ONE 2015, 9, e111741. [Google Scholar] [CrossRef]
- Ward, M.D.; King, M.A.; Gabrial, C.; Kenefick, R.W.; Leon, L.R. Biochemical recovery from exertional heat stroke follows a 16-day time course. PLoS ONE 2020, 15, e0229616. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.M. Tissue/field interactions, MRI safety, and field-related image artifacts. In Electromagnetics in Magnetic Resonance Imaging: Physical Principles, Related Applications, and Ongoing Developments; Morgan & Claypool Publishers: San Rafael, CA, USA, 2016; pp. 4–14. [Google Scholar]
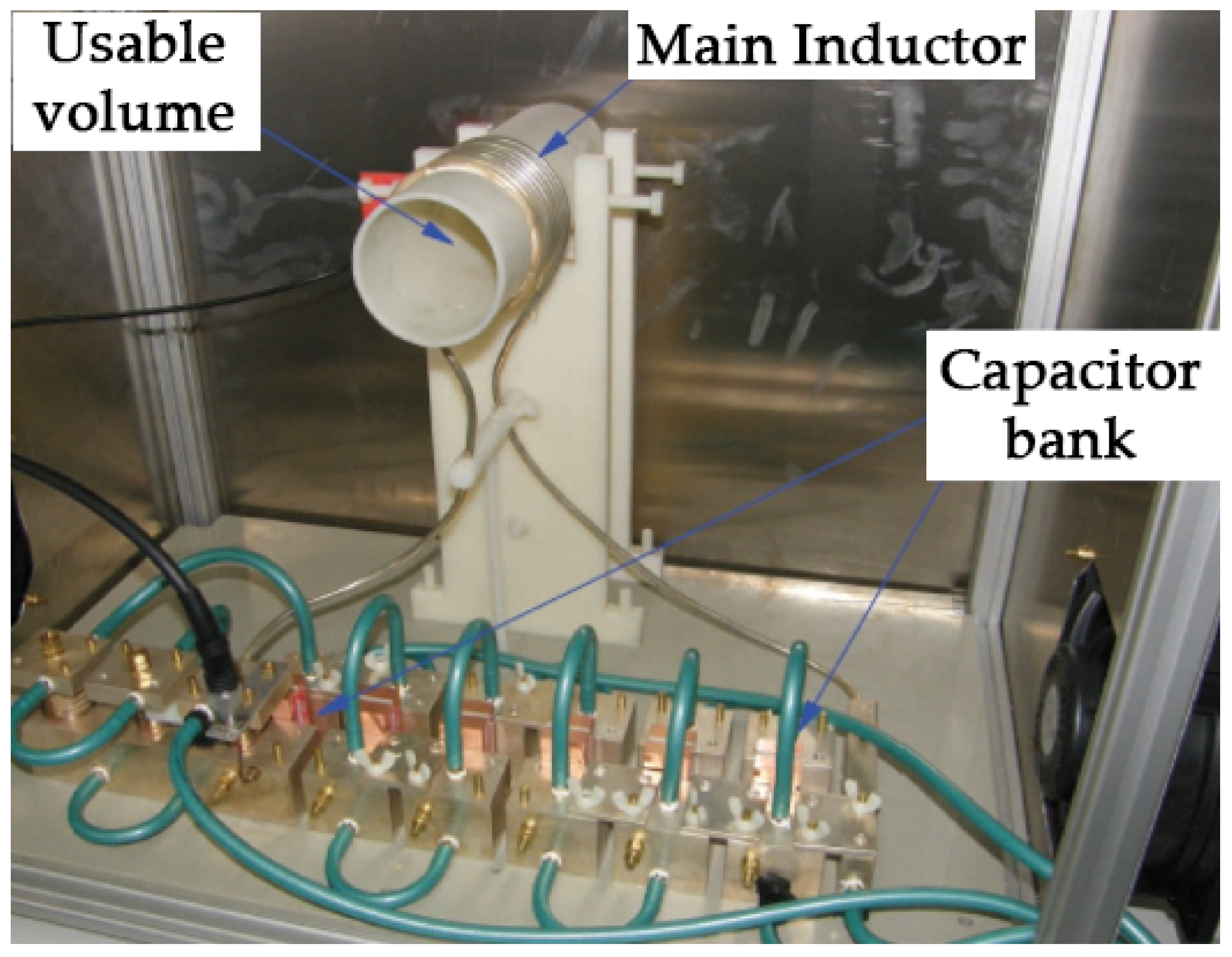




| Group Name | f (kHz) | H (kAm−1) | H × f (109 Am−1s−1) | Survival Rate (% and Number) |
|---|---|---|---|---|
| G1 | 591 | 14 | 8.27 | 100% (12/12) |
| G2 | 591 | 18 | 10.64 | 33% (4/12) |
| G3 | 591 | 16 | 9.46 | 100% (12/12) |
| G4 | 700 | 13.7 | 9.59 | 100% (12/12) |
| G5 | 774 | 12.4 | 9.59 | 92% (11/12) |
| G6 | 856 | 11.2 | 9.59 | 83% (10/12) |
| G7 | 928 | 10.3 | 9.56 | 0% (0/12) |
| Group | T0 Hepatic Temperature | T21 Hepatic Temperature | Hepatic Thermal Increase | T0 Rectal Temperature | T21 Rectal Temperature | Rectal Thermal Increase |
|---|---|---|---|---|---|---|
| G1 | 35.2 ± 1.2 °C | 42.0 ± 1.1 °C | 6.8 ± 0.7 °C | 33.1± 0.6 °C | 35.1 ± 1.9 °C | 2.0 ± 1.5 °C |
| G2 | 35.8 ± 0.3 °C | 42.9 ± 0.1 °C | 7.1 ± 0.3 °C | 34.4 ± 0.6 °C | 35.4 ± 0.8 °C | 0.9 ± 0.9 °C |
| G3 | 37.3 ± 1.1 °C | 42.8 ± 0.4 °C | 5.5 ± 1.4 °C | 35.8 ± 1.3 °C | 36.1 ± 1.8 °C | 0.3 ± 0.8 °C |
| G4 | 36.8 ± 0.9 °C | 42.6 ± 0.6 °C | 5.8 ± 1.0 °C | 36.1 ± 0.9 °C | 36.6 ± 1.2 °C | 0.4 ± 0.8 °C |
| G5 | 35.0 ± 1.2 °C | 43.0 ± 0.1 °C | 7.9 ± 1.2 °C | 33.4 ± 1.1 °C | 34.4 ± 1.2 °C | 1.0 ± 0.6 °C |
| G6 | 36.1 ± 0.5 °C | 43.0 ± 0.1 °C | 6.7 ± 1.0 °C | 33.8 ± 0.9 °C | 35.6 ± 0.8 °C | 1.1 ± 1.1 °C |
| G7 | 35.0 ± 1.2 °C | 43.0 ± 0.1 °C | 6.5 ± 1.3 °C | 35.3 ± 0.9 °C | 35.8 ± 0.9 °C | 0.5 ± 1.1 °C |
| Control | G1 | G3 | G4 | G5 | G6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 10 Days | 12 h | 10 Days | 12 h | 10 Days | 12 h | 10 Days | 12 h | 10 Days | ||
| AST (IU/L) | 51 ± 9.3 | 241 ± 6.2 | 67 ± 14 | 174 ± 23 | 58 ± 10 | 217 ± 32 | 66 ± 2.1 | 545 ± 24 | 56 ± 8.8 | 238 ± 24 | 45 ± 5 |
| p value | <0.001 | ns | <0.001 | ns | <0.001 | ns | <0.001 | ns | <0.001 | ns | |
| ALT (IU/L) | 36 ± 6.1 | 33 ± 3.7 | 30 ± 8.2 | 35 ± 8.7 | 28 ± 6.3 | 78 ± 5.9 | 31 ± 3.7 | 128 ± 10 | 31 ± 4.6 | 71 ± 3.5 | 28 ± 3.6 |
| p value | ns | ns | ns | ns | <0.001 | ns | <0.001 | ns | <0.001 | ns | |
| AST/ALT ratio | 1.4 ± 0.2 | 7 ± 0.9 | 2 ± 0.4 | 4.8 ± 1.2 | 1.9 ± 0.3 | 2.9 ± 0.7 | 2.2 ± 0.3 | 4.4 ± 0.7 | 1.8 ± 0.4 | 3.5 ± 0.2 | 1.7 ± 0.1 |
| p value | <0.001 | ns | <0.001 | ns | <0.001 | ns | <0.001 | ns | <0.001 | ns | |
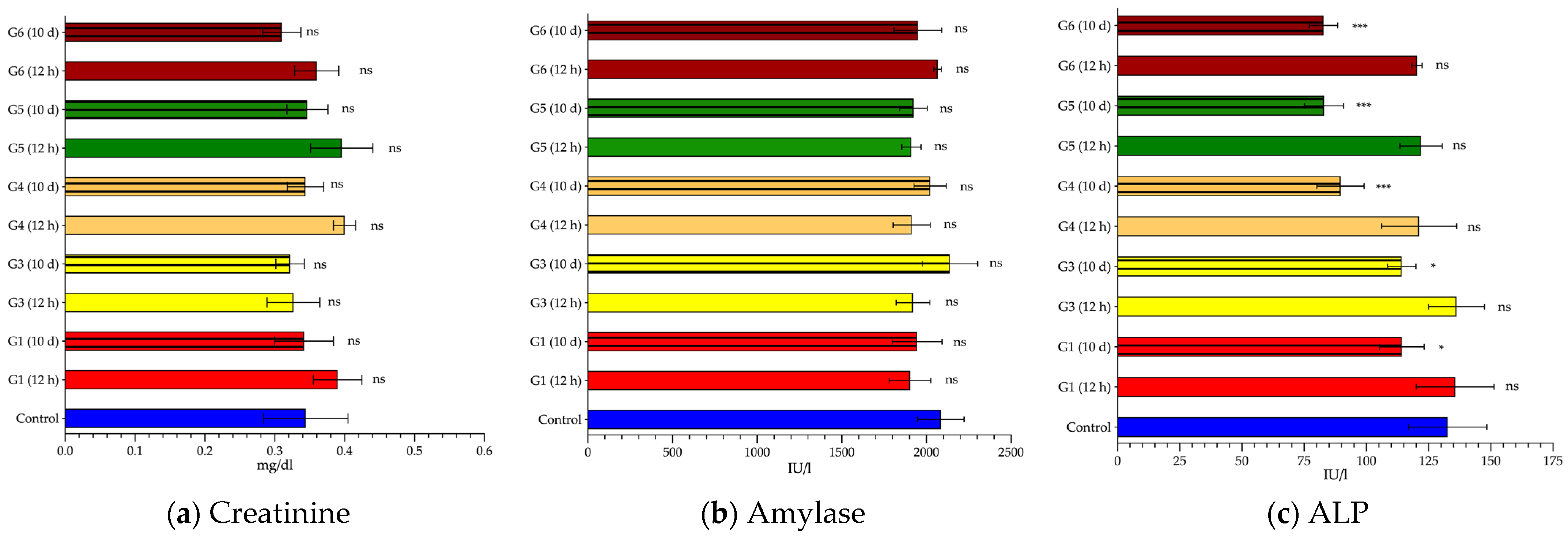
| Creatinine (mg/dL) | 0.34 ± 0.06 | 0.39 ± 0.04 | 0.34 ± 0.04 | 0.33 ± 0.04 | 0.32 ± 0.02 | 0.40 ± 0.02 | 0.34 ± 0.03 | 0.40 ± 0.03 | 0.35 ± 0.03 | 0.36 ± 0.03 | 0.31 ± 0.03 |
| p value | ns | ns | ns | ns | <0.01 | ns | <0.05 | ns | ns | ns | |
| Amylase | 2085 ± 138 | 1903 ± 123 | 1945 ± 148 | 1921 ± 99 | 2140 ± 163 | 1914 ± 110 | 2023 ± 95 | 1911 ± 57 | 1924 ± 57 | 2067 ± 23 | 1950 ± 143 |
| p value | <0.001 | ns | ns | ns | <0.001 | ns | ns | ns | ns | ns | |
| CK (IU/L) | 85 ± 15 | 159 ± 31 | 118 ± 28 | 267 ± 22 | 149 ± 27 | 229 ± 20 | 153 ± 24 | 243 ± 37 | 75 ± 13 | 185 ± 20 | 67 ± 14 |
| p value | <0.001 | <0.05 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ns | <0.001 | ns | |
| LDH (IU/L) | 60 ± 20 | 326 ± 63 | 167 ± 43 | 636 ± 56 | 361 ± 24 | 537 ± 73 | 326 ± 54 | 575 ± 73 | 88 ±+ 47 | 372 ± 68 | 63 ± 35 |
| p value | <0.001 | <0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ns | <0.001 | Ns | |
| ALP (IU/L) | 133 ± 16 | 136 ± 16 | 114 ± 9 | 136 ± 11 | 114 ± 5.7 | 121 ± 15 | 90 ± 9.5 | 122 ± 8.5 | 83 ± 7.8 | 120 ± 2.1 | 83 ± 5.7 |
| p value | ns | <0.05 | ns | <0.05 | ns | <0.001 | ns | <0.001 | ns | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084. https://doi.org/10.3390/cancers14133084
Herrero de la Parte B, Rodrigo I, Gutiérrez-Basoa J, Iturrizaga Correcher S, Mar Medina C, Echevarría-Uraga JJ, Garcia JA, Plazaola F, García-Alonso I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers. 2022; 14(13):3084. https://doi.org/10.3390/cancers14133084
Chicago/Turabian StyleHerrero de la Parte, Borja, Irati Rodrigo, Jon Gutiérrez-Basoa, Sira Iturrizaga Correcher, Carmen Mar Medina, Jose Javier Echevarría-Uraga, Jose Angel Garcia, Fernando Plazaola, and Ignacio García-Alonso. 2022. "Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy" Cancers 14, no. 13: 3084. https://doi.org/10.3390/cancers14133084
APA StyleHerrero de la Parte, B., Rodrigo, I., Gutiérrez-Basoa, J., Iturrizaga Correcher, S., Mar Medina, C., Echevarría-Uraga, J. J., Garcia, J. A., Plazaola, F., & García-Alonso, I. (2022). Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers, 14(13), 3084. https://doi.org/10.3390/cancers14133084








