Cordycepin (3′-Deoxyadenosine) Suppresses Heat Shock Protein 90 Function and Targets Tumor Growth in an Adenosine Deaminase-Dependent Manner
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Plasmids and Reagents
2.2. Cell Growth Assays
2.3. ADA Immunohistochemical Analysis and Activity Assays
2.4. Synergy Experiments
2.5. RNA Extraction and Quantitative Real-Time PCR
2.6. Soft Agar Assays (Anchorage-Independent Colony Formation Assays)
2.7. Anchorage-Dependent Colony Formation Assays
2.8. Migration Assays
2.9. Western Blot Analysis
2.10. Annexin V Assay
2.11. Animal Experiments
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
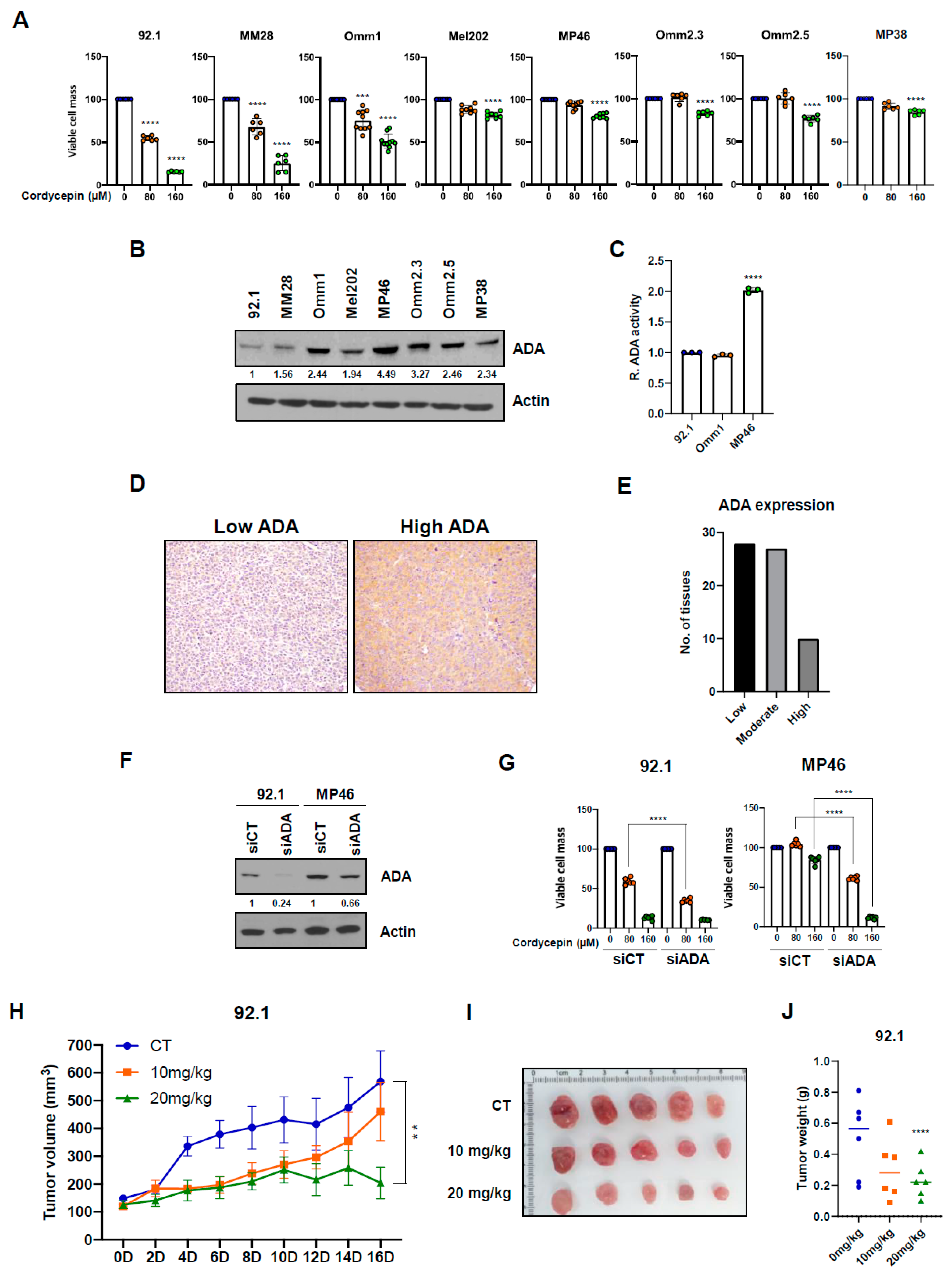
3.1. Cordycepin Slows Growth of Uveal Melanoma Cells with Low Adenosine Deaminase
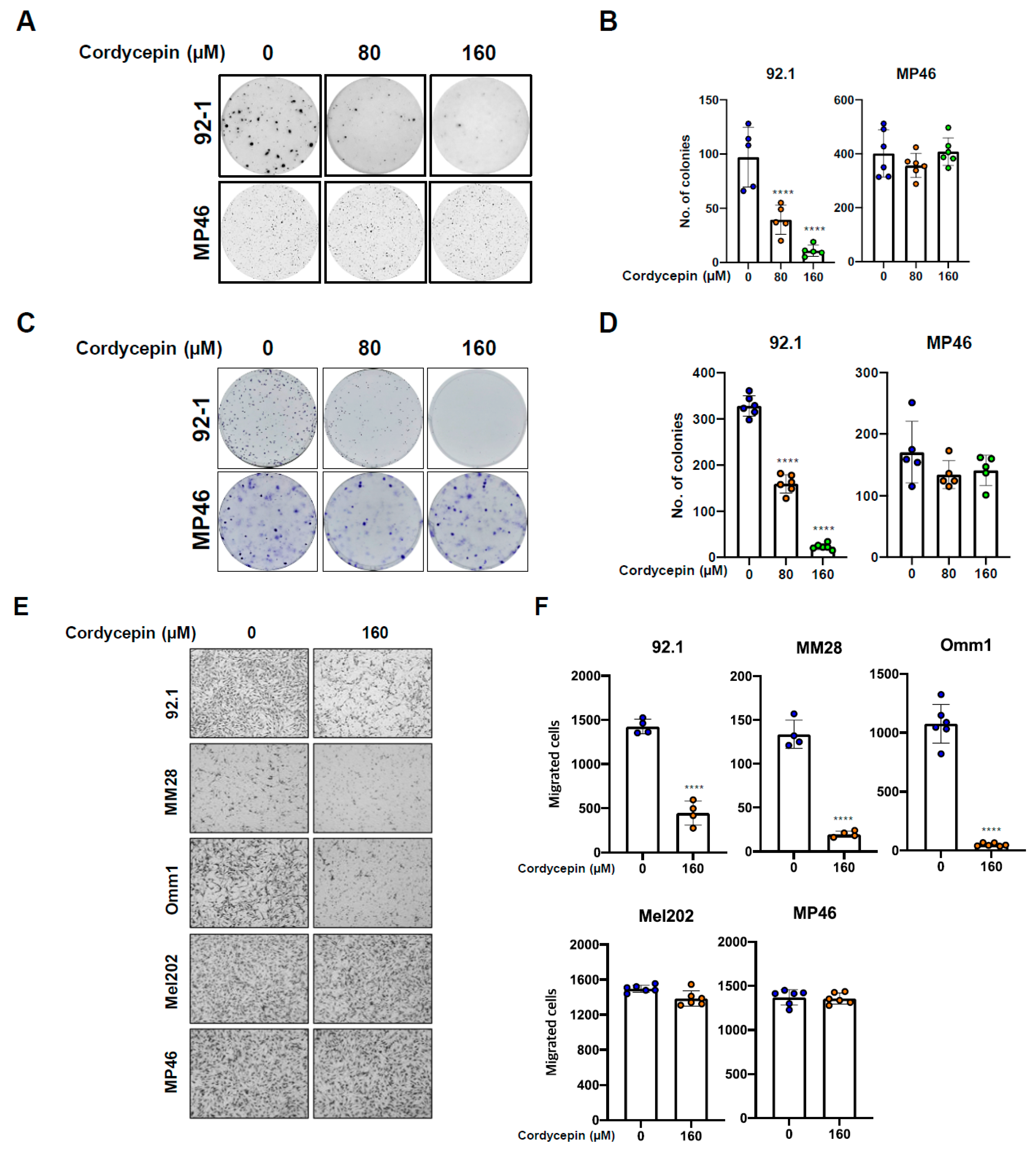
3.2. Cordycepin Decreases Tumor Migration and Colony Formation
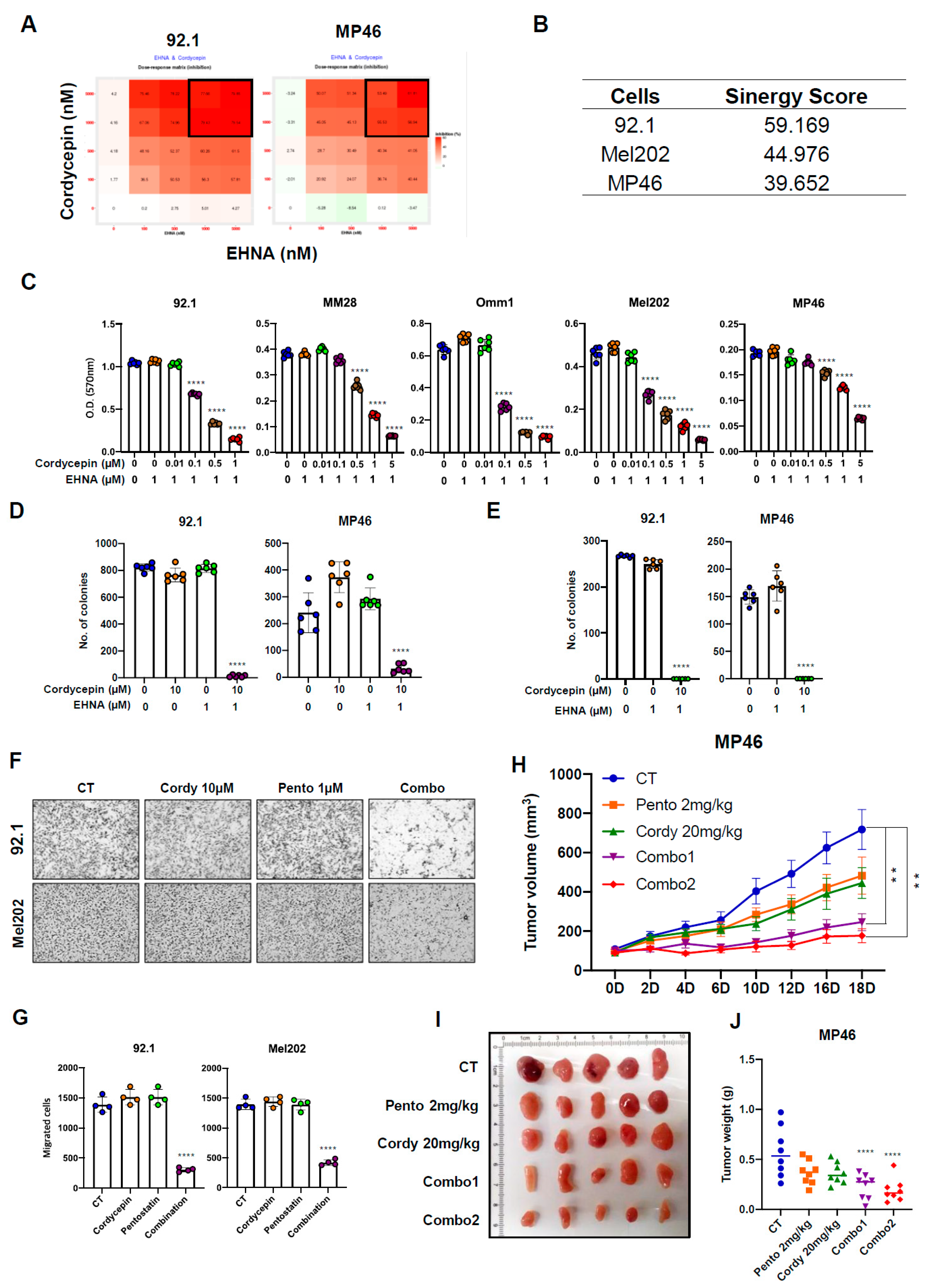
3.3. Targeting ADA Promotes Anticancer Effects of Cordycepin In Vitro and In Vivo
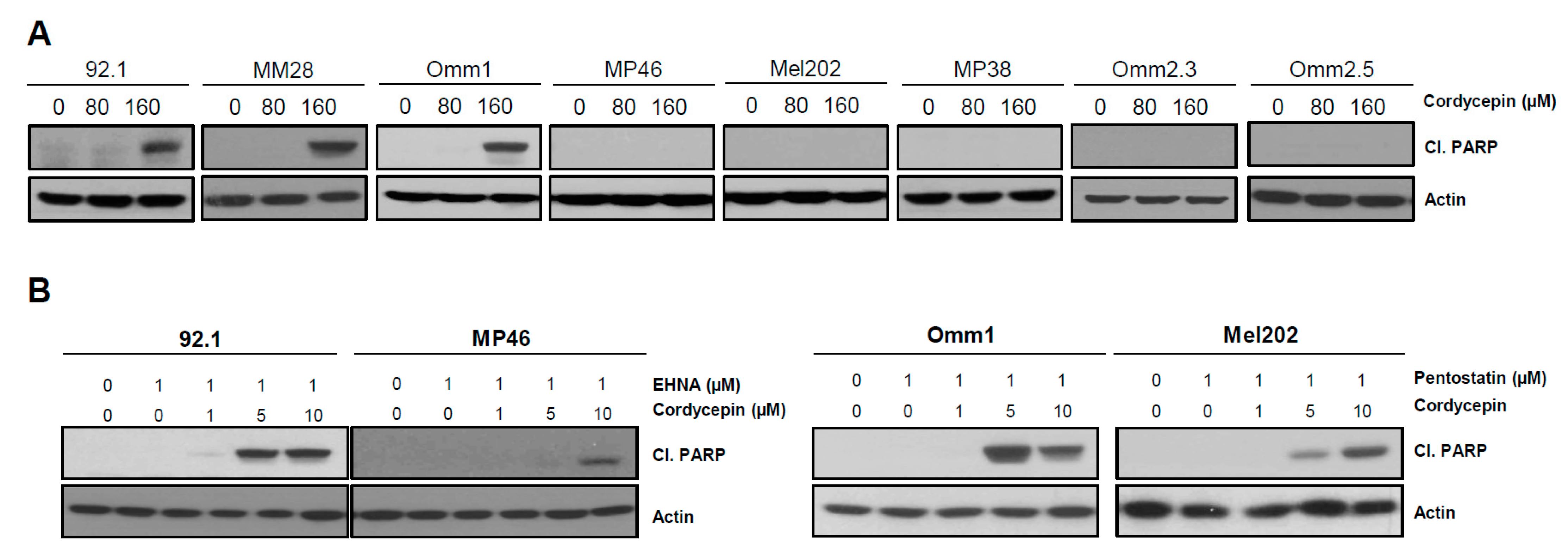
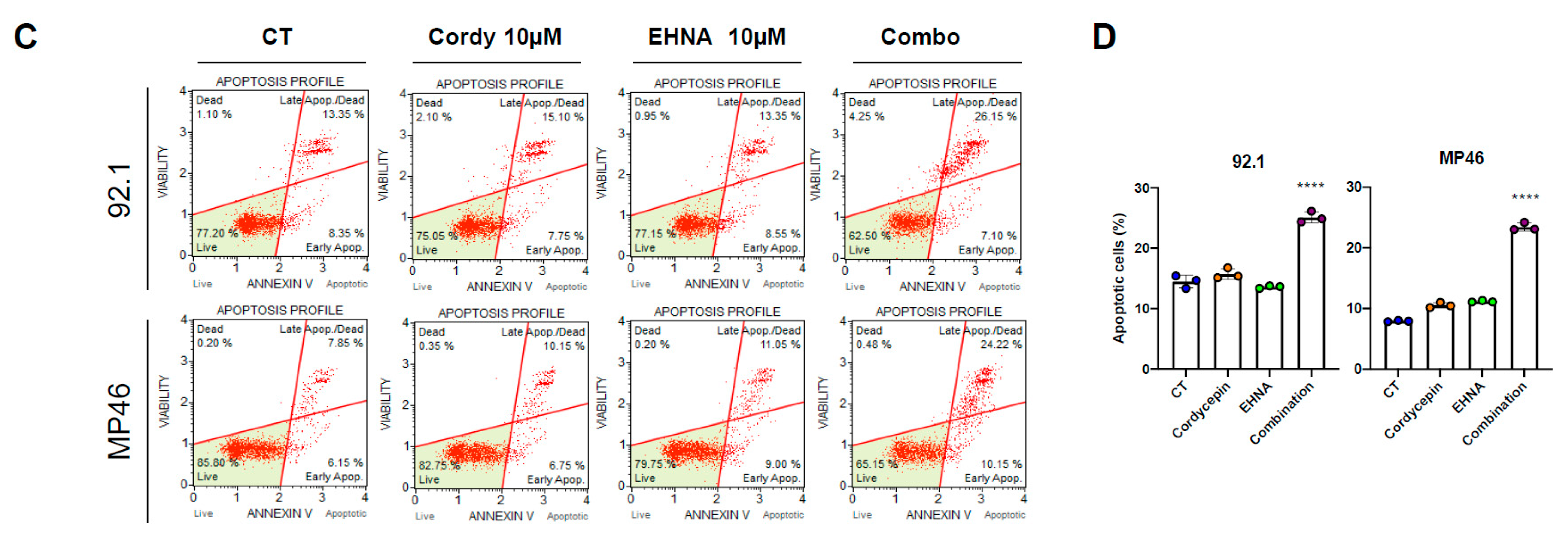
3.4. Cordycepin Induces Apoptotic Cell Death in Uveal Melanoma
3.5. Cordycepin Disrupts the Function of Hsp90 and Induces Degradation of Its Client Proteins
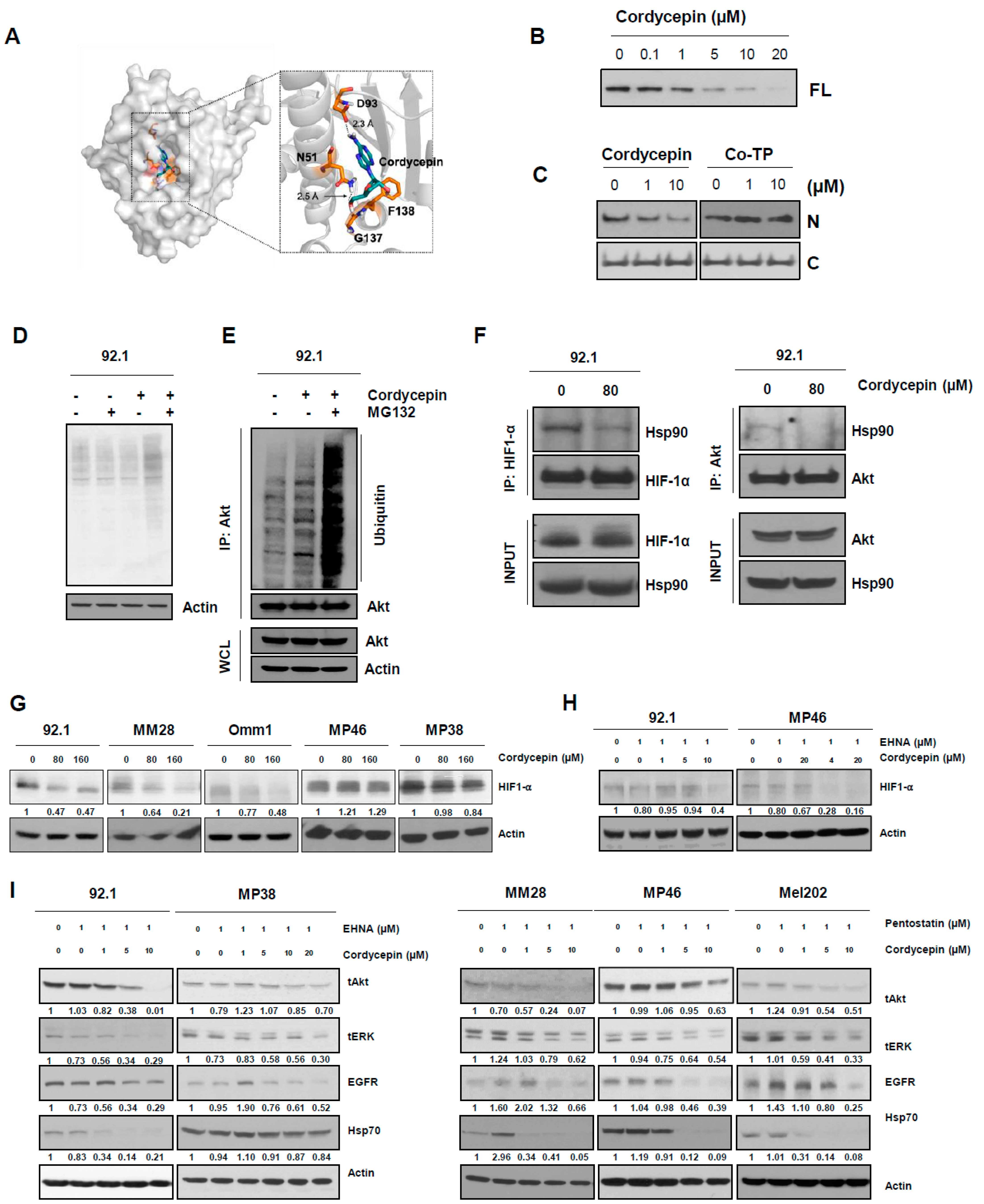
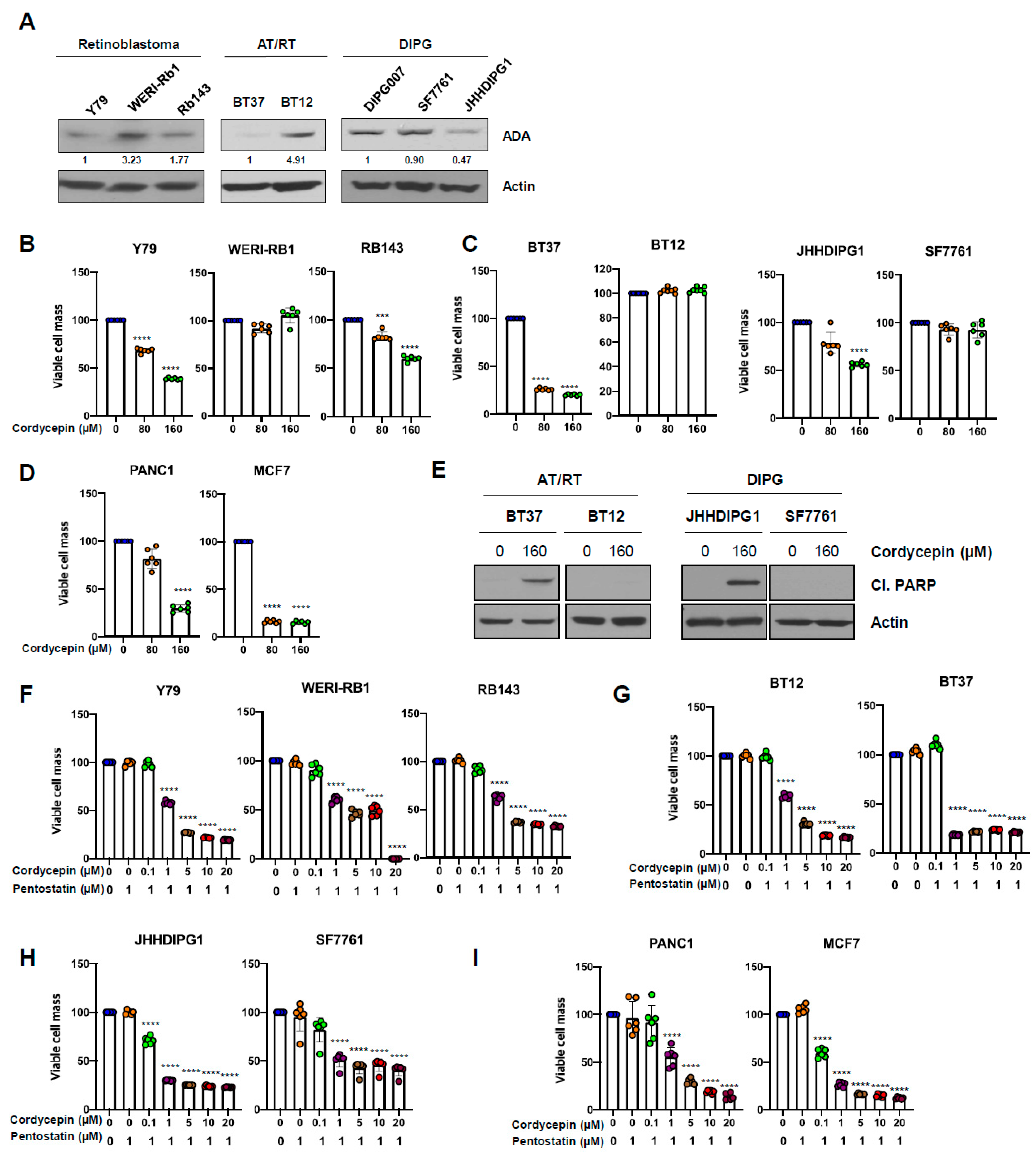
3.6. ADA-Dependent Effects of Cordycepin in Other Cancers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radhi, M.; Ashraf, S.; Lawrence, S.; Tranholm, A.A.; Wellham, P.A.D.; Hafeez, A.; Khamis, A.S.; Thomas, R.; McWilliams, D.; De Moor, C.H. A Systematic Review of the Biological Effects of Cordycepin. Molecules 2021, 26, 5886. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Radhi, M.; Cipolla, E.; Gandhi, R.D.; Sarmad, S.; Zgair, A.; Kim, T.H.; Feng, W.; Qin, C.; Adrower, C. A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin. Sci. Rep. 2019, 9, 15760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J. Pharmacol. Sci. 2015, 127, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.-L.; Ko, B.-S.; Liu, T.-A.; Liang, S.-M.; Liu, C.-C.; Lu, Y.-J.; Tzean, S.-S.; Shen, T.-L.; Liou, J.-Y. Cordycepin suppresses integrin/FAK signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014, 14, 29–34. [Google Scholar] [CrossRef]
- Pao, H.-Y.; Pan, B.-S.; Leu, S.-F.; Huang, B.-M. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C Pathway. J. Agric. Food Chem. 2012, 60, 4905–4913. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.-H.; Hueng, D.-Y.; Syu, J.-P.; Liao, C.-C.; Wu, Y.-C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem.-Biol. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef]
- Cao, H.-L.; Liu, Z.-J.; Chang, Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumor Biol. 2017, 39, 1010428317706915. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 2006, 26, 43–47. [Google Scholar]
- Yoshikawa, N.; Yamada, S.; Takeuchi, C.; Kagota, S.; Shinozuka, K.; Kunitomo, M.; Nakamura, K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A 3 receptor followed by glycogen synthase kinase-3β activation and cyclin D 1 suppression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 591–595. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Liang, Y.-N.; Wang, L.; Song, Z.-X.; Liu, J.-L.; Tang, Z.-S. Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR. Molecules 2016, 21, 1267. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onken, M.D.; Worley, L.A.; Long, M.D.; Duan, S.; Council, M.L.; Bowcock, A.M.; Harbour, J.W. Oncogenic mutations in GNAQ occur early in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5230–5234. [Google Scholar] [CrossRef] [Green Version]
- Davies, B.R.; Logie, A.; McKay, J.S.; Martin, P.; Steele, S.; Jenkins, R.; Cockerill, M.; Cartlidge, S.; Smith, P.D. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: Mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol. Cancer Ther. 2007, 6, 2209–2219. [Google Scholar] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W. The genetics of uveal melanoma: An emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Landreville, S.; Agapova, O.A.; Matatall, K.A.; Kneass, Z.T.; Onken, M.D.; Lee, R.S.; Bowcock, A.M.; Harbour, J.W. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin. Cancer Res. 2012, 18, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Flinn, A.M.; Gennery, A.R. Adenosine deaminase deficiency: A review. Orphanet J. Rare Dis. 2018, 13, 65. [Google Scholar] [CrossRef]
- Blackburn, M.R.; Kellems, R.E. Adenosine deaminase deficiency: Metabolic basis of immune deficiency and pulmonary inflammation. Adv. Immunol. 2005, 86, 1–41. [Google Scholar]
- Rottenberg, M.E.; Masocha, W.; Ferella, M.; Petitto-Assis, F.; Goto, H.; Kristensson, K.; McCaffrey, R.; Wigzell, H. Treatment of African trypanosomiasis with cordycepin and adenosine deaminase inhibitors in a mouse model. J. Infect. Dis. 2005, 192, 1658–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, Y.; Urbano, A.G.; Sweeney, E.B.; McCaffrey, R. Induction of apoptosis by cordycepin in ADA-inhibited TdT-positive leukemia cells. Leukemia 1996, 10, 1019–1024. [Google Scholar] [PubMed]
- Lee, M.-J.; Lee, J.-C.; Hsieh, J.-H.; Lin, M.-Y.; Shih, I.-A.; You, H.-L.; Wang, K. Cordycepin inhibits the proliferation of malignant peripheral nerve sheath tumor cells through the p53/Sp1/tubulin pathway. Am. J. Cancer Res. 2021, 11, 1247. [Google Scholar] [PubMed]
- Taylor, I.C.; Hütt-Cabezas, M.; Brandt, W.D.; Kambhampati, M.; Nazarian, J.; Chang, H.T.; Warren, K.E.; Eberhart, C.G.; Raabe, E.H. Disrupting NOTCH slows diffuse intrinsic pontine glioma growth, enhances radiation sensitivity, and shows combinatorial efficacy with bromodomain inhibition. J. Neuropathol. Exp. Neurol. 2015, 74, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Asnaghi, L.; Handa, J.T.; Merbs, S.L.; Harbour, J.W.; Eberhart, C.G. A role for Jag2 in promoting uveal melanoma dissemination and growth. Investig. Ophthalmol. Vis. Sci. 2013, 54, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Asnaghi, L.; Ebrahimi, K.B.; Schreck, K.C.; Bar, E.E.; Coonfield, M.L.; Bell, W.R.; Handa, J.; Merbs, S.L.; Harbour, J.W.; Eberhart, C.G. Notch signaling promotes growth and invasion in uveal melanoma. Clin. Cancer Res. 2012, 18, 654–665. [Google Scholar] [CrossRef] [Green Version]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020, 48, W488–W493. [Google Scholar] [CrossRef]
- Lewis, H.D.; Leveridge, M.; Strack, P.R.; Haldon, C.D.; O’Neil, J.; Kim, H.; Madin, A.; Hannam, J.C.; Look, A.T.; Kohl, N.; et al. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem. Biol. 2007, 14, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G. Density functional theory of atoms and molecules. In Horizons of Quantum Chemistry; Springer: Dordrecht, The Netherlands, 1980; pp. 5–15. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Dunning Jr, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Edinger, S.R.; Cortis, C.; Shenkin, P.S.; Friesner, R.A. Solvation free energies of peptides: Comparison of approximate continuum solvation models with accurate solution of the Poisson–Boltzmann equation. J. Phys. Chem. B 1997, 101, 1190–1197. [Google Scholar] [CrossRef]
- Friedrichs, M.; Zhou, R.; Edinger, S.R.; Friesner, R.A. Poisson−Boltzmann analytical gradients for molecular modeling calculations. J. Phys. Chem. B 1999, 103, 3057–3061. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New model for calculation of solvation free energies: Correction of self-consistent reaction field continuum dielectric theory for short-range hydrogen-bonding effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Cristalli, G.; Costanzi, S.; Lambertucci, C.; Lupidi, G.; Vittori, S.; Volpini, R.; Camaioni, E. Adenosine deaminase: Functional implications and different classes of inhibitors. Med. Res. Rev. 2001, 21, 105–128. [Google Scholar] [CrossRef]
- Li, G.; Nakagome, I.; Hirono, S.; Itoh, T.; Fujiwara, R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol. Res. Perspect. 2015, 3, e00121. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. 2010, 24, 1755–1761. [Google Scholar] [CrossRef]
- Kane, B.J.; Kuhn, J.G.; Roush, M.K. Pentostatin: An adenosine deaminase inhibitor for the treatment of hairy cell leukemia. Ann. Pharmacother. 1992, 26, 939–947. [Google Scholar] [CrossRef]
- Lloyd, H.; Fredholm, B. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem. Int. 1995, 26, 387–395. [Google Scholar] [CrossRef]
- Tesch, A.M.; MacDonald, M.H.; Kollias-Baker, C.; Benton, H.P. Chondrocytes respond to adenosine via A2receptors and activity is potentiated by an adenosine deaminase inhibitor and a phosphodiesterase inhibitor. Osteoarthr. Cartil. 2002, 10, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.-Y.; Lin, Y.-H.; Yeh, E.-L.; Lo, H.-C.; Hsu, T.-H.; Su, C.-C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget 2017, 8, 93712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenzer, H.; De Zan, E.; Elshani, M.; Van Stiphout, R.; Kudsy, M.; Morris, J.; Ferrari, V.; Um, I.H.; Chettle, J.; Kazmi, F. The Novel Nucleoside Analogue ProTide NUC-7738 Overcomes Cancer Resistance Mechanisms In Vitro and in a First-in-Human Phase I Clinical Trial. Clin. Cancer Res. 2021, 27, 6500–6513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.V.; Semenza, G.L. RACK1 vs. HSP90: Competition for HIF-1α degradation vs. stabilization. Cell Cycle 2007, 6, 656–659. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Oh, S.H.; Woo, J.-K.; Hong, W.K.; Lee, H.-Y. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1α. Cancer Res. 2009, 69, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Woo, J.K.; Yazici, Y.D.; Myers, J.N.; Kim, W.-Y.; Jin, Q.; Hong, S.S.; Park, H.-J.; Suh, Y.-G.; Kim, K.-W. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J. Natl. Cancer Inst. 2007, 99, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-C.; Min, H.-Y.; Choi, H.; Kim, H.S.; Kim, K.-C.; Park, S.-J.; Seong, M.A.; Seo, J.H.; Park, H.-J.; Suh, Y.-G. Synthesis and evaluation of a novel deguelin derivative, L80, which disrupts ATP binding to the C-terminal domain of heat shock protein 90. Mol. Pharmacol. 2015, 88, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-C.; Min, H.-Y.; Choi, H.; Bae, S.Y.; Park, K.H.; Hyun, S.Y.; Lee, H.J.; Moon, J.; Park, S.-H.; Kim, J.Y. Deguelin analogue SH-1242 inhibits Hsp90 activity and exerts potent anticancer efficacy with limited neurotoxicity. Cancer Res. 2016, 76, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtsev, V.A.; Khokhlova, A.V.; Mosina, V.A.; Selivanova, E.I.; Kabakov, A.E. Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization. PLoS ONE 2017, 12, e0173640. [Google Scholar] [CrossRef]
- Tassone, G.; Mangani, S.; Botta, M.; Pozzi, C. Probing the role of Arg97 in Heat shock protein 90 N-terminal domain from the parasite Leishmania braziliensis through site-directed mutagenesis on the human counterpart. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2018, 1866, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Obermann, W.M.; Sondermann, H.; Russo, A.A.; Pavletich, N.P.; Hartl, F.U. In Vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 1998, 143, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Martin, C.; Serapian, S.A.; Colombo, G.; Rasola, A. Dynamically shaping chaperones. Allosteric modulators of HSP90 family as regulatory tools of cell metabolism in neoplastic progression. Front. Oncol. 2020, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Tsai, K.K.; Bollin, K.B.; Patel, S.P. Obstacles to improving outcomes in the treatment of uveal melanoma. Cancer 2018, 124, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Diefenbach, R.J.; Joshua, A.M.; Kefford, R.F.; Carlino, M.S.; Rizos, H. Oncogenic signaling in uveal melanoma. Pigment Cell Melanoma Res. 2018, 31, 661–672. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Wolkmer, P.; Nunes, J.T.; Duck, M.R.; Oliveira, C.B.; Gressler, L.T.; Costa, M.M.; Zanette, R.A.; Mazzanti, C.M.; Lopes, S.T.; et al. Susceptibility of Trypanosoma evansi to cordycepin. Biomed. Pharmacother. 2011, 65, 220–223. [Google Scholar] [CrossRef]
- Kalirai, H.; Dodson, A.; Faqir, S.; Damato, B.; Coupland, S. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br. J. Cancer 2014, 111, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Yuno, A.; Lee, M.J.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J.B. Clinical Evaluation and Biomarker Profiling of Hsp90 Inhibitors. Methods Mol. Biol. 2018, 1709, 423–441. [Google Scholar] [CrossRef]
- Ma, X.; Hibbert, B.; McNulty, M.; Hu, T.; Zhao, X.; Ramirez, F.D.; Simard, T.; de Belleroche, J.S.; O’Brien, E.R. Heat shock protein 27 attenuates neointima formation and accelerates reendothelialization after arterial injury and stent implantation: Importance of vascular endothelial growth factor up-regulation. FASEB J. 2014, 28, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Erlichman, C. Tanespimycin: The opportunities and challenges of targeting heat shock protein 90. Expert Opin. Investig. Drugs 2009, 18, 861–868. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-C.; Alaali, L.; Kwon, H.; Rigi, M.; Eberhart, C.G. Cordycepin (3′-Deoxyadenosine) Suppresses Heat Shock Protein 90 Function and Targets Tumor Growth in an Adenosine Deaminase-Dependent Manner. Cancers 2022, 14, 3122. https://doi.org/10.3390/cancers14133122
Lee S-C, Alaali L, Kwon H, Rigi M, Eberhart CG. Cordycepin (3′-Deoxyadenosine) Suppresses Heat Shock Protein 90 Function and Targets Tumor Growth in an Adenosine Deaminase-Dependent Manner. Cancers. 2022; 14(13):3122. https://doi.org/10.3390/cancers14133122
Chicago/Turabian StyleLee, Su-Chan, Lujain Alaali, HyukJean Kwon, Mohammed Rigi, and Charles G. Eberhart. 2022. "Cordycepin (3′-Deoxyadenosine) Suppresses Heat Shock Protein 90 Function and Targets Tumor Growth in an Adenosine Deaminase-Dependent Manner" Cancers 14, no. 13: 3122. https://doi.org/10.3390/cancers14133122
APA StyleLee, S.-C., Alaali, L., Kwon, H., Rigi, M., & Eberhart, C. G. (2022). Cordycepin (3′-Deoxyadenosine) Suppresses Heat Shock Protein 90 Function and Targets Tumor Growth in an Adenosine Deaminase-Dependent Manner. Cancers, 14(13), 3122. https://doi.org/10.3390/cancers14133122





