Pragmatic Expectancy on Microbiota and Non-Small Cell Lung Cancer: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Microbiota Interplay with Oncogenes Activation and Tumor Onset
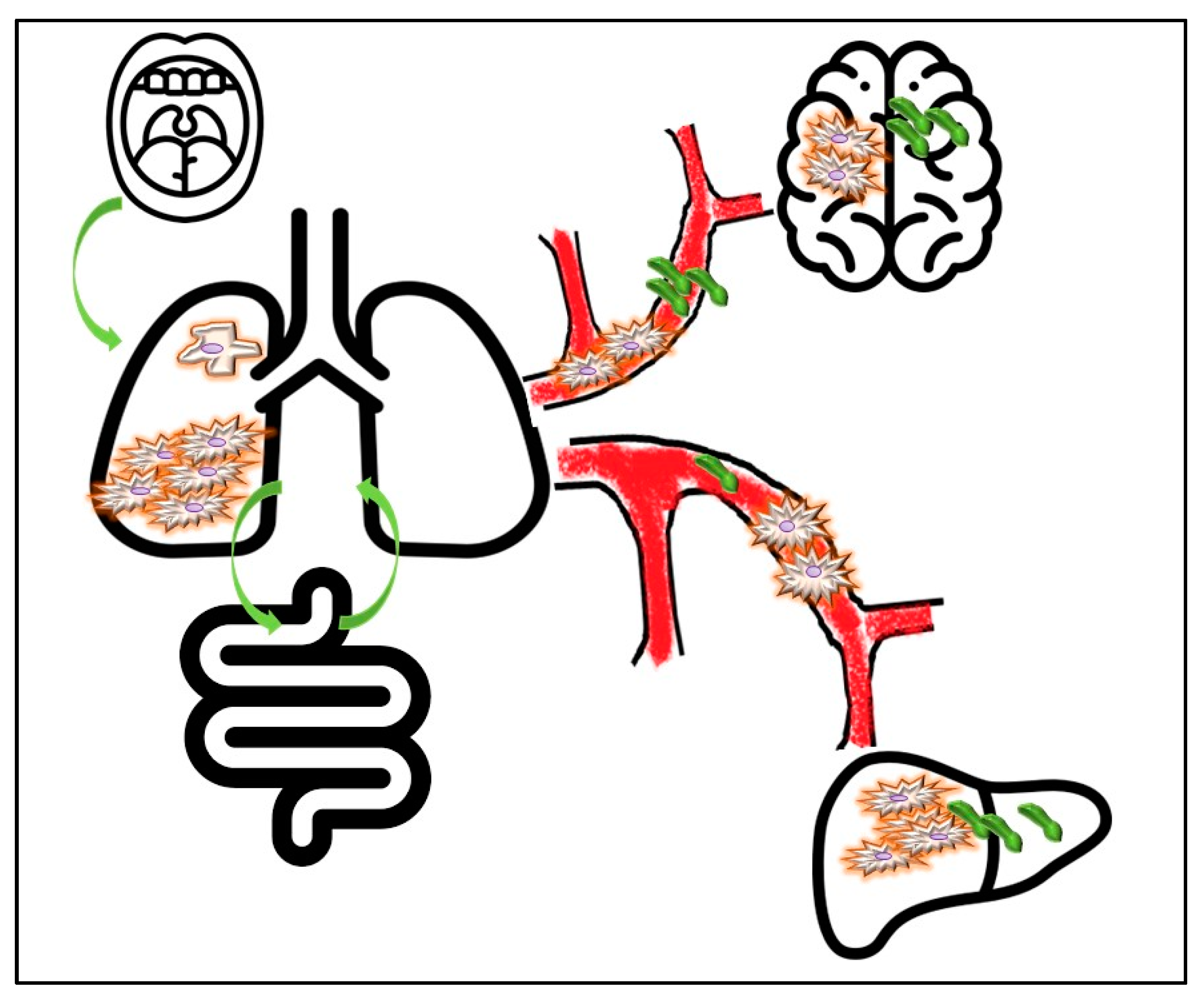
3.1. Tracking Primary Origin of Tumor Cells: Which Role for Microbes?
3.2. Genetic Signatures on Microbiota and Effect of Microbiota on Epigenetics
4. How, Where and When to Analyze NSCLC-Related Microbiota
| HOW | WHEN | WHERE | WHY |
|---|---|---|---|
| TUMOR | INITIAL PHASES | PRIMARY MASS | RESPONSE TO THERAPY |
| Significant association with the selection and transformation of primary cancer cells | Implication in early stage lung cancer, in areas with heavy air pollutions | Tumor mass | Association with response to targeted therapies Predictive biomarker for immune checkpoint inhibitor response and toxicity |
| Promotion, selection and survival tumor cell clones and cancer stem cells compartment | Peritumoral stroma | Implication in modulation of response to radiation therapy | |
| PATIENT | LATE PHASES | DISTANT SITES | OUTCOME |
| Specific association between microbial species and cancer patient gender and smoking habit | Implication in promoting tumor dissemination | • BLOOD: Free microbial circulating DNA (Liquid biopsy) • GUT: lung–gut axis • UPPER AIRWAYS | Discrimination of long-term/short-term survivors |
5. Therapeutic Perspective and Exploitation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quintanal-Villalonga, Á.; Chan, J.M.; Yu, H.A.; Pe’er, D.; Sawyers, C.L.; Sen, T.; Rudin, C.M. Lineage plasticity in cancer: A shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020, 17, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.M.; Luisetti, M.; Pozzi, E.; Comoglio, P.M. Oncogenes in non-small-cell lung cancer: Emerging connections and novel therapeutic dynamics. Lancet Respir. Med. 2013, 1, 251–261. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Herbst, R.S.; Goldberg, S.B. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat. Rev. Clin. Oncol. 2021, 18, 625–644. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New Insights Into the Cancer-Microbiome-Immune Axis: Decrypting a Decade of Discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Zitvogel, L.; Kroemer, G.; Routy, B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 2019, 10, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishaq, S.; Nunn, L. Helicobacter pylori and gastric cancer: A state of the art review. Gastroenterol. Hepatol. Bed Bench 2015, 8 (Suppl. S1), S6–S14. [Google Scholar] [PubMed]
- Accordino, G.; Lettieri, S.; Bortolotto, C.; Benvenuti, S.; Gallotti, A.; Gattoni, E.; Agustoni, F.; Pozzi, E.; Rinaldi, P.; Primiceri, C.; et al. From Interconnection between Genes and Microenvironment to Novel Immunotherapeutic Approaches in Upper Gastro-Intestinal Cancers-A Multidisciplinary Perspective. Cancers 2020, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, M.; Pratesi, C.; Bidoli, E.; Maiero, S.; Magris, R.; Steffan, A.; Basaglia, G.; Canzonieri, V.; De Re, V.; Cannizzaro, R.; et al. Differential Helicobacter pylori Plasticity in the Gastric Niche of Subjects at Increased Gastric Cancer Risk. Pathogens 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, Y.; Kunita, A.; Iwata, C.; Komura, D.; Nishiyama, T.; Shimazu, K.; Takeshita, K.; Shibahara, J.; Kii, I.; Morishita, Y.; et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 2014, 184, 859–870. [Google Scholar] [CrossRef]
- Sokolova, O.; Naumann, M. Matrix Metalloproteinases in Helicobacter pylori-Associated Gastritis and Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 1883. [Google Scholar] [CrossRef]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, R.; Zhu, Y.; Li, Z.; She, X.; Jian, X.; Yu, F.; Deng, X.; Sai, B.; Wang, L.; et al. Lung microbiome alterations in NSCLC patients. Sci. Rep. 2021, 11, 11736. [Google Scholar] [CrossRef]
- Tsay, J.J.; Wu, B.G.; Sulaiman, I.; Gershner, K.; Schluger, R.; Li, Y.; Yie, T.A.; Meyn, P.; Olsen, E.; Perez, L.; et al. Lower Airway Dysbiosis Affects Lung Cancer Progression. Cancer Discov. 2021, 11, 293–307. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, E.S.; Zhao, C.; Jin, C. Host-Microbiome Interaction in Lung Cancer. Front. Immunol. 2021, 12, 679829. [Google Scholar] [CrossRef]
- Najafi, S.; Abedini, F.; Azimzadeh Jamalkandi, S.; Shariati, P.; Ahmadi, A.; Gholami Fesharaki, M. The composition of lung microbiome in lung cancer: A systematic review and meta-analysis. BMC Microbiol. 2021, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Ma, Q.; Ge, Y.; Yi, C.X.; Wei, L.Q.; Tan, J.C.; Chu, Q.; Li, J.Q.; Zhang, P.; Wang, H. Microbiome dysbiosis in lung cancer: From composition to therapy. NPJ Precis. Oncol. 2020, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Goto, T. Airway Microbiota as a Modulator of Lung Cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef] [PubMed]
- Dumont-Leblond, N.; Veillette, M.; Racine, C.; Joubert, P.; Duchaine, C. Non-small cell lung cancer microbiota characterization: Prevalence of enteric and potentially pathogenic bacteria in cancer tissues. PLoS ONE 2021, 16, e0249832. [Google Scholar] [CrossRef]
- Wang, S.; Du, M.; Zhang, J.; Xu, W.; Yuan, Q.; Li, M.; Wang, J.; Zhu, H.; Wang, Y.; Wang, C.; et al. Tumor evolutionary trajectories during the acquisition of invasiveness in early stage lung adenocarcinoma. Nat. Commun. 2020, 11, 6083. [Google Scholar] [CrossRef]
- Rubanova, Y.; Shi, R.; Harrigan, C.F.; Li, R.; Wintersinger, J.; Sahin, N.; Deshwar, A.; PCAWG Evolution and Heterogeneity Working Group; Morris, Q.; PCAWG Consortium. Reconstructing evolutionary trajectories of mutation signature activities in cancer using TrackSig. Nat. Commun. 2020, 11, 731. [Google Scholar] [CrossRef]
- Cheung, W.K.; Nguyen, D.X. Lineage factors and differentiation states in lung cancer progression. Oncogene 2015, 34, 5771–5780. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Qian, H.; Shao, G.; Lu, X.; Chen, Q.; Sun, X.; Chen, D.; Yin, R.; Zhu, H.; et al. Stemness and inducing differentiation of small cell lung cancer NCI-H446 cells. Cell Death Dis. 2013, 4, e633. [Google Scholar] [CrossRef] [Green Version]
- Stella, G.M.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-Seeking Metastases. Cancers 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Stella, G.M.; Benvenuti, S.; Gentile, A.; Comoglio, P.M. MET Activation and Physical Dynamics of the Metastatic Process: The Paradigm of Cancers of Unknown Primary Origin. EBioMedicine 2017, 24, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Moran, S.; Martínez-Cardús, A.; Sayols, S.; Musulén, E.; Balañá, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef]
- Li, R.; Liao, B.; Wang, B.; Dai, C.; Liang, X.; Tian, G.; Wu, F. Identification of Tumor Tissue of Origin with RNA-Seq Data and Using Gradient Boosting Strategy. BioMed Res. Int. 2021, 2021, 6653793. [Google Scholar] [CrossRef] [PubMed]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Zakharkina, T.; Heinzel, E.; Koczulla, R.A.; Greulich, T.; Rentz, K.; Pauling, J.K.; Baumbach, J.; Herrmann, M.; Grünewald, C.; Dienemann, H.; et al. Analysis of the airway microbiota of healthy individuals and patients with chronic obstructive pulmonary disease by T-RFLP and clone sequencing. PLoS ONE 2013, 8, e68302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Mu, X.; Wang, Y.; Zhu, D.; Zhang, J.; Liang, C.; Chen, B.; Wang, J.; Zhao, C.; Zuo, Z.; et al. Dysbiosis of the Salivary Microbiome Is Associated with Non-smoking Female Lung Cancer and Correlated with Immunocytochemistry Markers. Front. Oncol. 2018, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Tong, P.; Liu, C.; Wang, W.; Lu, C.; Han, Y.; Sun, X.; Kuang, X.; Li, N.; Dai, J. The characteristics of gut microbiota and commensal Enterobacteriaceae isolates in tree shrew (Tupaia belangeri). BMC Microbiol. 2019, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, Y.; Li, S.; Peng, Z.; Liu, X.; Chen, J.; Zheng, X. Role of lung and gut microbiota on lung cancer pathogenesis. J. Cancer Res. Clin. Oncol. 2021, 147, 2177–2186. [Google Scholar] [CrossRef]
- Mao, Q.; Jiang, F.; Yin, R.; Wang, J.; Xia, W.; Dong, G.; Ma, W.; Yang, Y.; Xu, L.; Hu, J. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018, 415, 40–48. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Wiencke, J.K. DNA adduct burden and tobacco carcinogenesis. Oncogene 2002, 21, 7376–7391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Flora, S.; Balansky, R.M.; D’Agostini, F.; Izzotti, A.; Camoirano, A.; Bennicelli, C.; Zhang, Z.; Wang, Y.; Lubet, R.A.; You, M. Molecular alterations and lung tumors in p53 mutant mice exposed to cigarette smoke. Cancer Res. 2003, 63, 793–800. [Google Scholar] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Intern. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Chen, L.; Cao, L.; Li, K.J.; Huang, Y.; Luan, X.Q.; Li, G. Effects of smoking on the lower respiratory tract microbiome in mice. Respir. Res. 2018, 19, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Z.; Zhang, L.; Hou, R.; Ma, X.; Yu, J.; Zhang, W.; Zhuang, C. Exposure to a mixture of cigarette smoke carcinogens disturbs gut microbiota and influences metabolic homeostasis in A/J mice. Chem. Biol. Interact. 2021, 344, 109496. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Hand, C.K.; Shanahan, F.; Murphy, T.; O’Toole, P.W. Mutagenesis by Microbe: The Role of the Microbiota in Shaping the Cancer Genome. Trends Cancer 2020, 6, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Garmendia, J.; Morey, P.; Bengoechea, J.A. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur. Respir. J. 2012, 39, 467–477. [Google Scholar] [CrossRef]
- Stella, G.M.; Luisetti, M.; Inghilleri, S.; Cemmi, F.; Scabini, R.; Zorzetto, M.; Pozzi, E. Targeting EGFR in non-small-cell lung cancer: Lessons, experiences, strategies. Respir. Med. 2012, 106, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, I.B. Addiction to oncogenes—The Achilles heal of cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef]
- Baselga, J. The EGFR as a target for anticancer therapy: Focus on cetuximab. Eur. J. Cancer 2001, 37, S16–S22. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Martini, M.; Molinari, F.; Sartore-Bianchi, A.; Arena, S.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; Frattini, M.; Siena, S.; et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.H.; He, J.; Su, X.F.; Wen, Y.N.; Zhang, S.J.; Liu, L.Y.; Zhao, H.; Ye, C.P.; Wu, J.H.; Cai, S.; et al. The airway microbiota of non-small cell lung cancer patients and its relationship to tumor stage and EGFR gene mutation. Thorac. Cancer 2022, 13, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, Y.; Liu, B.; Li, L.; Huang, X.; Wang, M.; Wang, G.; Gao, X.; Zhang, L.; Bao, X.; et al. The relationship between KRAS gene mutation and intestinal flora in tumor tissues of colorectal cancer patients. Ann. Transl. Med. 2020, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Pupo, E.; Avanzato, D.; Middonti, E.; Bussolino, F.; Lanzetti, L. KRAS-Driven Metabolic Rewiring Reveals Novel Actionable Targets in Cancer. Front. Oncol. 2019, 9, 848. [Google Scholar] [CrossRef] [Green Version]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef] [Green Version]
- Reita, D.; Pabst, L.; Pencreach, E.; Guérin, E.; Dano, L.; Rimelen, V.; Voegeli, A.C.; Vallat, L.; Mascaux, C.; Beau-Faller, M. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: Focus on Resistance. Cancers 2022, 14, 1321. [Google Scholar] [CrossRef]
- Roberts, P.J.; Stinchcombe, T.E.; Der, C.J.; Socinski, M.A. Personalized medicine in non-small-cell lung cancer: Is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J. Clin. Oncol. 2010, 28, 4769–4777. [Google Scholar] [CrossRef]
- Kempf, E.; Rousseau, B.; Besse, B.; Paz-Ares, L. KRAS oncogene in lung cancer: Focus on molecularly driven clinical trials. Eur. Respir. Rev. 2016, 25, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peters, B.A.; Dominianni, C.; Zhang, Y.; Pei, Z.; Yang, L.; Ma, Y.; Purdue, M.P.; Jacobs, E.J.; Gapstur, S.M.; et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016, 10, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.A.; Lhakhang, T.; Olsen, E.; et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198. [Google Scholar] [CrossRef]
- Kerk, S.A.; Papagiannakopoulos, T.; Shah, Y.M.; Lyssiotis, C.A. Metabolic networks in mutant KRAS-driven tumours: Tissue specificities and the microenvironment. Nat. Rev. Cancer 2021, 21, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef]
- Mehta, A.; Dobersch, S.; Romero-Olmedo, A.J.; Barreto, G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015, 34, 229–241. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetic lesions causing genetic lesions in human cancer: Promoter hypermethylation of DNA repair genes. Eur. J. Cancer 2000, 36, 2294–2300. [Google Scholar] [CrossRef]
- Futscher, B.W. Epigenetic changes during cell transformation. Adv. Exp. Med. Biol. 2013, 754, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, C.Z.; Wan, J.Y.; Yao, H.; Yuan, C.S. Dissecting the Interplay Mechanism between Epigenetics and Gut Microbiota: Health Maintenance and Disease Prevention. Int. J. Mol. Sci. 2021, 22, 6933. [Google Scholar] [CrossRef]
- Khan, F.H.; Bhat, B.A.; Sheikh, B.A.; Tariq, L.; Padmanabhan, R.; Verma, J.P.; Shukla, A.C.; Dowlati, A.; Abbas, A. Microbiome dysbiosis and epigenetic modulations in lung cancer: From pathogenesis to therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 13, 2485–2493. [Google Scholar] [CrossRef]
- Haque, S.; Raina, R.; Afroze, N.; Hussain, A.; Alsulimani, A.; Singh, V.; Mishra, B.N.; Kaul, S.; Kharwar, R.N. Microbial dysbiosis and epigenetics modulation in cancer development—A chemopreventive approach. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef]
- Oliva, M.; Mulet-Margalef, N.; Ochoa-De-Olza, M.; Napoli, S.; Mas, J.; Laquente, B.; Alemany, L.; Duell, E.J.; Nuciforo, P.; Moreno, V. Tumor-Associated Microbiome: Where Do We Stand? Int. J. Mol. Sci. 2021, 22, 1446. [Google Scholar] [CrossRef] [PubMed]
- Mignard, S.; Flandrois, J.P. 16S rRNA sequencing in routine bacterial identification: A 30-month experiment. J. Microbiol. Methods 2006, 67, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tse, H.; Yuen, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpton, T.J. An introduction to the analysis of shotgun metagenomic data. Front. Plant Sci. 2014, 5, 209. [Google Scholar] [CrossRef] [Green Version]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, V.; Jiang, C.; Zhou, W.; Jahanbani, F.; Batzoglou, S.; Snyder, M. Synthetic long-read sequencing reveals intraspecies diversity in the human microbiome. Nat. Biotechnol. 2016, 34, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Gevers, D.; Pop, M.; Schloss, P.D.; Huttenhower, C. Bioinformatics for the Human Microbiome Project. PLoS Comput. Biol. 2012, 8, e1002779. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.B.; Chen, T.; Xu, Y.P.; Chen, L.; Sun, F.X.; Lu, M.P.; Liu, Y.X. A guide to human microbiome research: Study design, sample collection, and bioinformatics analysis. Chin. Med. J. 2020, 133, 1844–1855. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Hypothesis Testing and Statistical Analysis of Microbiome. Genes Dis. 2017, 4, 138–148. [Google Scholar] [CrossRef]
- Clausen, D.S.; Willis, A.D. Evaluating replicability in microbiome data. Biostatistics 2021, kxab048. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lei, X.; Zhang, Y. Association predictions of genomics, proteinomics, transcriptomics, microbiome, metabolomics, pathomics, radiomics, drug, symptoms, environment factor, and disease networks: A comprehensive approach. Med. Res. Rev. 2022, 42, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Endesfelder, D.; Schloter-Hai, B.; Kublik, S.; Granitsiotis, M.S.; Boschetto, P.; Stendardo, M.; Barta, I.; Dome, B.; Deleuze, J.F.; et al. Influence of lung CT changes in chronic obstructive pulmonary disease (COPD) on the human lung microbiome. PLoS ONE 2017, 12, e0180859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, S.; Maringe, C.; Coleman, M.P.; Peake, M.D.; Butler, J.; Young, N.; Bergström, S.; Hanna, L.; Jakobsen, E.; Kölbeck, K.; et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 2013, 68, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Omar, T.; Michelow, P. Effectiveness of the cell block technique in diagnostic cytopathology. J. Cytol. 2012, 29, 177–182. [Google Scholar] [CrossRef]
- Sale, M.S.; Kulkarni, V.V.; Kulkarni, P.V.; Patil, C.A. Efficacy of modified cell block cytology compared to fine needle aspiration cytology for diagnostic oral cytopathology. Biotech. Histochem. 2021, 96, 197–201. [Google Scholar] [CrossRef]
- Bortolotto, C.; Maglia, C.; Ciuffreda, A.; Coretti, M.; Catania, R.; Antonacci, F.; Carnevale, S.; Sarotto, I.; Dore, R.; Filippi, A.R.; et al. The growth of non-solid neoplastic lung nodules is associated with low PD L1 expression, irrespective of sampling technique. J. Transl. Med. 2020, 18, 54. [Google Scholar] [CrossRef] [Green Version]
- Stella, G.M.; Bortolotto, C.; Filippi, A.R. Intrathoracic core needle biopsy and repeat biopsy for PD-L1 evaluation in non-small cell lung cancer. J. Thorac. Dis. 2018, 10 (Suppl. S33), S4031–S4033. [Google Scholar] [CrossRef]
- Comito, G.; Ippolito, L.; Chiarugi, P.; Cirri, P. Nutritional Exchanges within Tumor Microenvironment: Impact for Cancer Aggressiveness. Front. Oncol. 2020, 10, 396. [Google Scholar] [CrossRef]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Milotti, E.; Fredrich, T.; Chignola, R.; Rieger, H. Oxygen in the Tumor Microenvironment: Mathematical and Numerical Modeling. Adv. Exp. Med. Biol. 2020, 1259, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Facciotti, F.; Cavalcoli, F.; Amoroso, C.; Rausa, E.; Centonze, G.; Cribiù, F.M.; Invernizzi, P.; Milione, M. Intratumor Microbiome in Neuroendocrine Neoplasms: A New Partner of Tumor Microenvironment? A Pilot Study. Cells 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, J. A comprehensive analysis of intratumor microbiome in head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2022, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Han, Y.; Zhao, X.; Sun, Y. Lung microbiota features of stage III and IV non-small cell lung cancer patients without lung infection. Transl. Cancer Res. 2022, 11, 426–434. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, I.; Schofield, Z.; Hall, L.J. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg. Top. Life Sci. 2017, 1, 333–349. [Google Scholar] [CrossRef] [Green Version]
- Rodes, L.; Khan, A.; Paul, A.; Coussa-Charley, M.; Marinescu, D.; Tomaro-Duchesneau, C.; Shao, W.; Kahouli, I.; Prakash, S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: An in vitro study using a human colonic microbiota model. J. Microbiol. Biotechnol. 2013, 23, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Su, H.; She, Y.; Dai, C.; Xie, D.; Narrandes, S.; Huang, S.; Chen, C.; Xu, W. Whole genome sequencing revealed microbiome in lung adenocarcinomas presented as ground-glass nodules. Transl. Lung Cancer Res. 2019, 8, 235–246. [Google Scholar] [CrossRef]
- de Bruin, E.C.; Taylor, T.B.; Swanton, C. Intra-tumor heterogeneity: Lessons from microbial evolution and clinical implications. Genome Med. 2013, 5, 101. [Google Scholar] [CrossRef] [Green Version]
- Zong, Y.; Zhou, Y.; Liao, B.; Liao, M.; Shi, Y.; Wei, Y.; Huang, Y.; Zhou, X.; Cheng, L.; Ren, B. The Interaction between the Microbiome and Tumors. Front. Cell. Infect. Microbiol. 2021, 11, 673724. [Google Scholar] [CrossRef] [PubMed]
- Vande Voorde, J.; Balzarini, J.; Liekens, S. Mycoplasmas and cancer: Focus on nucleoside metabolism. EXCLI J. 2014, 13, 300–322. [Google Scholar] [PubMed]
- Jiang, S.; Zhang, S.; Langenfeld, J.; Lo, S.C.; Rogers, M.B. Mycoplasma infection transforms normal lung cells and induces bone morphogenetic protein 2 expression by post-transcriptional mechanisms. J. Cell. Biochem. 2008, 104, 580–594. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Damato, M.; Maffia, M.; Lanuti, P.; Trerotola, M. The Cancer Microbiota: EMT and Inflammation as Shared Molecular Mechanisms Associated with Plasticity and Progression. J. Oncol. 2019, 2019, 1253727. [Google Scholar] [CrossRef]
- Hofman, P.; Vouret-Craviari, V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 2012, 3, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Anderton, J.M.; Rajam, G.; Romero-Steiner, S.; Summer, S.; Kowalczyk, A.P.; Carlone, G.M.; Sampson, J.S.; Ades, E.W. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog 2007, 42, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Wessler, S.; Necchi, V.; Rohde, M.; Harrer, A.; Rau, T.T.; Asche, C.I.; Boehm, M.; Loessner, H.; Figueiredo, C.; et al. Helicobacter pylori employs a unique basolateral type IV secretion mechanism for CagA delivery. Cell Host Microbe 2017, 22, 552–560.e5. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Fujiwara, N.; Mouri, Y.; Kisoda, S.; Yoshida, K.; Yoshida, K.; Yumoto, H.; Ozaki, K.; Ishimaru, N.; Kudo, Y. Conversion from epithelial to partial-EMT phenotype by Fusobacterium nucleatum infection promotes invasion of oral cancer cells. Sci. Rep. 2021, 11, 14943. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Marzano, M.; Fosso, B.; Piancone, E.; Defazio, G.; Pesole, G.; De Robertis, M. Stem Cell Impairment at the Host-Microbiota Interface in Colorectal Cancer. Cancers 2021, 13, 996. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Guccione, C.; Laplane, L.; Pradeu, T.; Curtius, K.; Knight, R. Cancer’s second genome: Microbial cancer diagnostics and redefining clonal evolution as a multispecies process: Humans and their tumors are not aseptic, and the multispecies nature of cancer modulates clinical care and clonal evolution. Bioessays 2022, 44, e2100252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Gonzalez-Kozlova, E.E. Molecular Profiling of Liquid Biopsies for Precision Oncology. Adv. Exp. Med. Biol. 2022, 1361, 235–247. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Liu, Z.; Li, J.; Li, X.; Yang, F.; Qiu, M. Circulating microbiome DNA: An emerging paradigm for cancer liquid biopsy. Cancer Lett. 2021, 521, 82–87. [Google Scholar] [CrossRef]
- Newsome, R.C.; Jobin, C. Microbiome-Derived Liquid Biopsy: New Hope for Cancer Screening? Clin. Chem. 2021, 67, 463–465. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Okuma, Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin. Transl. Oncol. 2018, 20, 966–974. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.Q.; Inchakalody, V.P.; Mestiri, S.; Yoosuf, Z.S.K.M.; Bedhiafi, T.; El-Ella, D.M.A.; Taib, N.; Hydrose, S.; Akbar, S.; et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 99. [Google Scholar] [CrossRef] [PubMed]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Hermida, L.C.; Gertz, E.M.; Ruppin, E. Predicting cancer prognosis and drug response from the tumor microbiome. Nat. Commun. 2022, 13, 2896. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, L.E.; Ellermann, M.; Arthur, J.C. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017, 13, e1006480. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [Green Version]
- Nougayrède, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Huycke, M.M. Commensal bacteria drive endogenous transformation and tumour stem cell marker expression through a bystander effect. Gut 2015, 64, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, M.C.; Sarkar, C. Morphology of angiogenesis in human cancer: A conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology 2005, 46, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gardlik, R.; Behuliak, M.; Palffy, R.; Celec, P.; Li, C.J. Gene therapy for cancer: Bacteria-mediated anti-angiogenesis therapy. Gene Ther. 2011, 18, 425–431. [Google Scholar] [CrossRef]
- Drees, J.J.; Mertensotto, M.J.; Augustin, L.B.; Schottel, J.L.; Saltzman, D.A. Vasculature Disruption Enhances Bacterial Targeting of Autochthonous Tumors. J. Cancer 2015, 6, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Ji, G.; Li, Q.; Yang, Y.; Shui, L.; Shen, Y.; Yang, H. Bacterial particles retard tumor growth as a novel vascular disrupting agent. Biomed. Pharmacother. 2020, 122, 109757. [Google Scholar] [CrossRef]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond immune checkpoint blockade: Emerging immunological strategies. Nat. Rev. Drug Discov. 2021, 20, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Halley, A.; Leonetti, A.; Gregori, A.; Tiseo, M.; Deng, D.M.; Giovannetti, E.; Peters, G.J. The Role of the Microbiome in Cancer and Therapy Efficacy: Focus on Lung Cancer. Anticancer Res. 2020, 40, 4807–4818. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, S.Y.; Yoon, Y.; Park, C.; Sohn, J.; Jeong, J.J.; Jeon, B.N.; Jang, M.; An, C.; Lee, S.; et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021, 6, 277–288. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Maleki Vareki, S.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Slimmen, L.; Janssens, H.M.; van Rossum, A.; Unger, W. Antigen-Presenting Cells in the Airways: Moderating Asymptomatic Bacterial Carriage. Pathogens 2021, 10, 945. [Google Scholar] [CrossRef]
- Reens, A.L.; Cabral, D.J.; Liang, X.; Norton, J.E., Jr.; Therien, A.G.; Hazuda, D.J.; Swaminathan, G. Immunomodulation by the Commensal Microbiome During Immune-Targeted Interventions: Focus on Cancer Immune Checkpoint Inhibitor Therapy and Vaccination. Front. Immunol. 2021, 12, 643255. [Google Scholar] [CrossRef]
- Frankel, A.E.; Deshmukh, S.; Reddy, A.; Lightcap, J.; Hayes, M.; McClellan, S.; Singh, S.; Rabideau, B.; Glover, T.G.; Roberts, B.; et al. Cancer Immune Checkpoint Inhibitor Therapy and the Gut Microbiota. Integr. Cancer Ther. 2019, 18, 1534735419846379. [Google Scholar] [CrossRef]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.G.; et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Eng. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Helmink, B.A.; Khan, M.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, G.; Ren, Y.; Zheng, T.; Shen, C.; Li, M.; Chen, X.; Zhai, H.; Li, Z.; Xu, J.; et al. Investigating efficacy of “microbiota modulation of the gut-lung Axis” combined with chemotherapy in patients with advanced NSCLC: Study protocol for a multicenter, prospective, double blind, placebo controlled, randomized trial. BMC Cancer 2021, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.; Yadav, M.; Liu, B.; Furqan, M.; Dai, Q.; Shahi, S.; Gupta, A.; Mercer, K.N.; Eastman, E.; Hejleh, T.A.; et al. Prospective correlation between the patient microbiome with response to and development of immune-mediated adverse effects to immunotherapy in lung cancer. BMC Cancer 2021, 21, 808. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, G.M.; Scialò, F.; Bortolotto, C.; Agustoni, F.; Sanci, V.; Saddi, J.; Casali, L.; Corsico, A.G.; Bianco, A. Pragmatic Expectancy on Microbiota and Non-Small Cell Lung Cancer: A Narrative Review. Cancers 2022, 14, 3131. https://doi.org/10.3390/cancers14133131
Stella GM, Scialò F, Bortolotto C, Agustoni F, Sanci V, Saddi J, Casali L, Corsico AG, Bianco A. Pragmatic Expectancy on Microbiota and Non-Small Cell Lung Cancer: A Narrative Review. Cancers. 2022; 14(13):3131. https://doi.org/10.3390/cancers14133131
Chicago/Turabian StyleStella, Giulia Maria, Filippo Scialò, Chandra Bortolotto, Francesco Agustoni, Vincenzo Sanci, Jessica Saddi, Lucio Casali, Angelo Guido Corsico, and Andrea Bianco. 2022. "Pragmatic Expectancy on Microbiota and Non-Small Cell Lung Cancer: A Narrative Review" Cancers 14, no. 13: 3131. https://doi.org/10.3390/cancers14133131







