State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Aims of This Study
- The indications, strengths and limitations of various imaging modalities for diagnosis of MSCC, focusing primarily on MRI and including techniques for metal artifact suppression on MRI and CT;
- The role of imaging in SBRT with regards to treatment planning, image-guidance during treatment and post-treatment follow-up;
- Recent advances in deep learning (DL) tools for image acquisition and analysis, which hold potential to reduce time to MSCC diagnosis, enabling more efficient patient referrals and treatment selection.
3. Imaging Modalities for Diagnosis of Metastatic Spinal Cord Compression (MSCC)
3.1. Goals of Imaging
- Confirm the diagnosis of MSCC;
- Identify the level of involvement (especially because localization by clinical examination may not be reliable) [2] as well as areas of epidural and paraspinal involvement;
- Assess for other sites of metastatic disease in the vertebral column, which has implications for both prognosis and treatment planning [2];
- Determine the presence of mechanical instability. This is achieved via the Spine Instability Neoplastic Score (SINS) which was also developed by the SOSG [22] (Table 1). It uses radiologic criteria and pain characteristics to identify patients at high risk of spinal instability from underlying neoplasm.
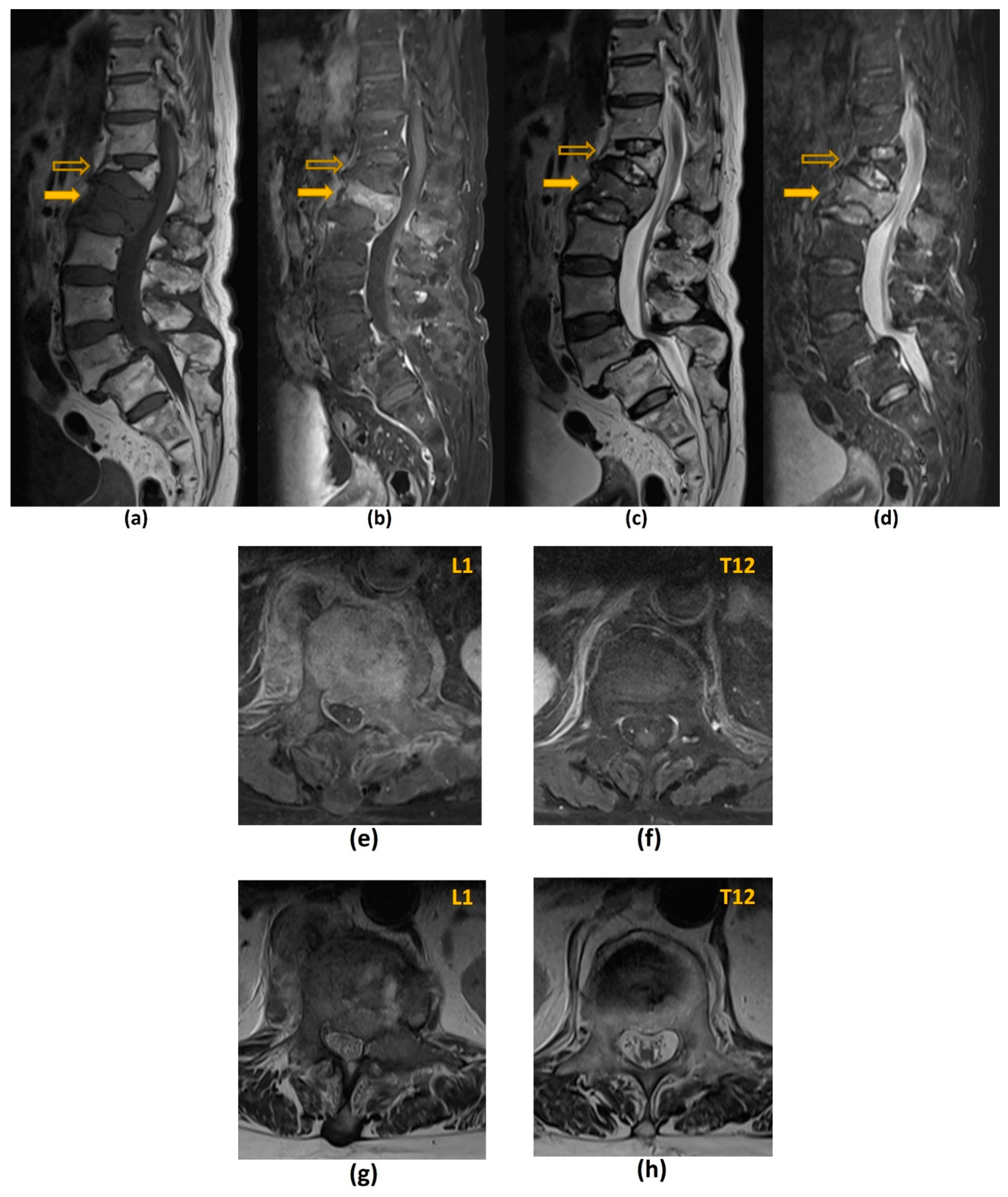
3.2. Magnetic Resonance Imaging (MRI)
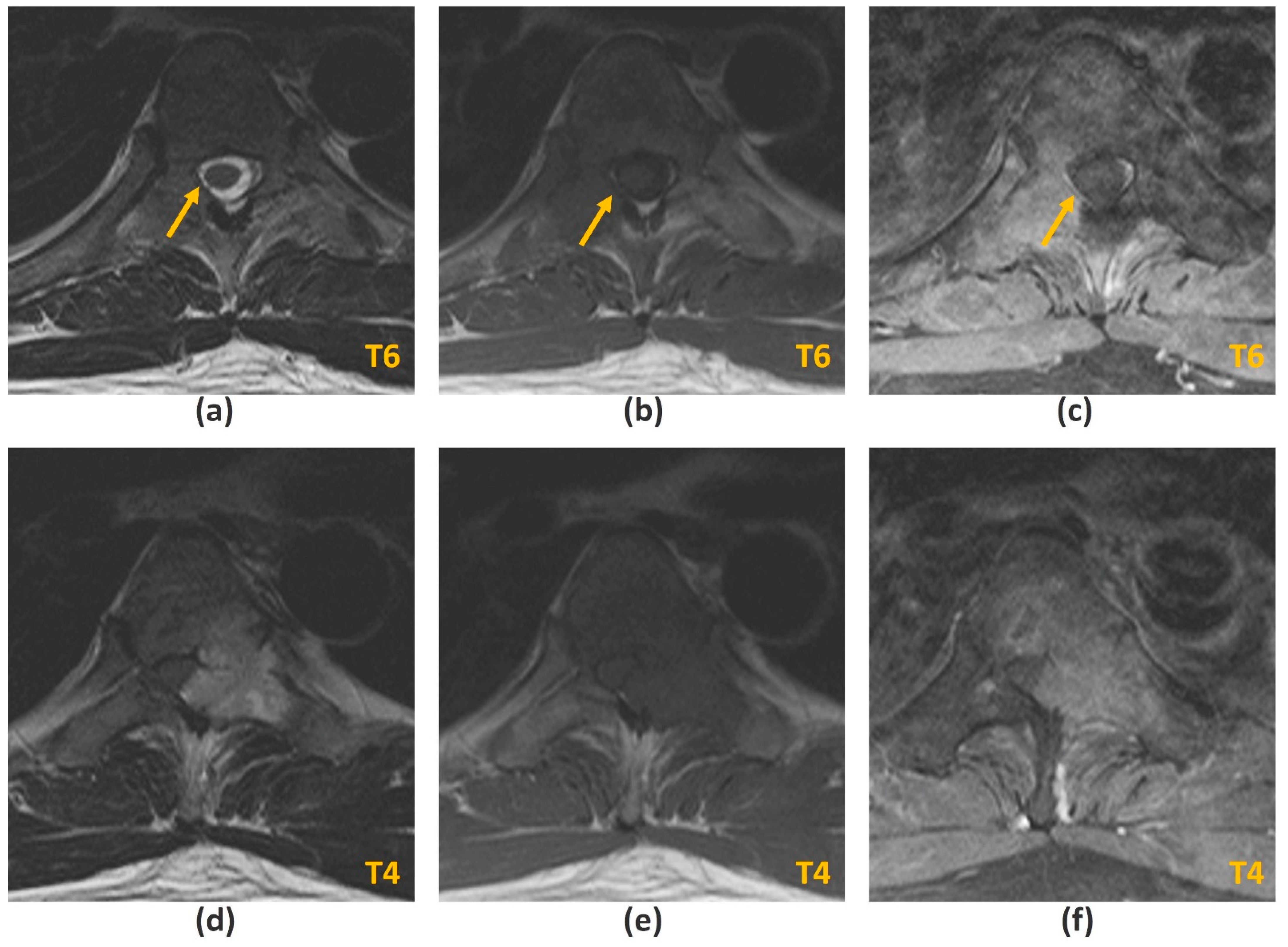
3.2.1. Challenges of MRI: Metal-Related Artifacts
| Title 1 | Advantages | Disadvantages |
|---|---|---|
| Spectral fat-saturation/ CHESS | Versatile, can be applied to any pulse sequence including post-contrast sequences; High SNR; Relatively fast technique; Relatively high resolution | Sensitive to magnetic field inhomogeneities1, especially around metal implants |
| STIR | Less sensitive to magnetic field inhomogeneities 1 | Reduced SNR; Relatively long imaging times; Relatively low resolution; High SAR; Inability to image post-contrast administration (other materials with a short T1 relaxation time including protein, melanin, and methaemoglobin would also be suppressed) |
| Dixon TSE | Less sensitive to magnetic field inhomogeneities 1; Ability to image post-contrast administration; SNR improved compared to STIR sequences | Less effective than STIR at homogeneous fat suppression around metal implants; Relatively long imaging times; Fat-water swap may occur |
3.2.2. Challenges of MRI: Long Scan Acquisition Times
3.2.3. Challenges of MRI: Others
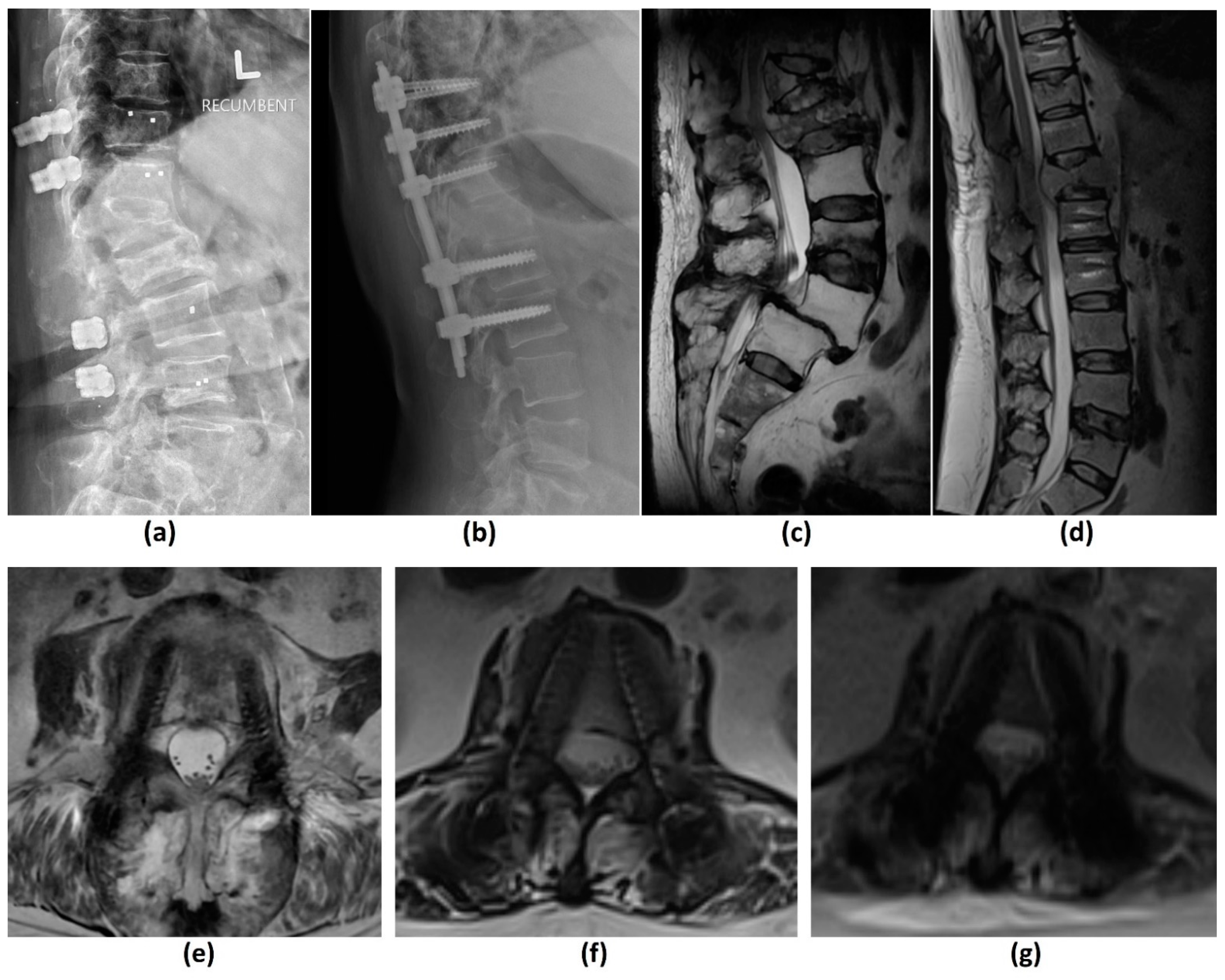
3.3. Computed Tomography (CT) Myelogram
- MRI is contraindicated (e.g., due to extreme claustrophobia, large body habitus, inability to lie still for a prolonged period of time, metallic foreign body in orbit, or a noncompatible cardiac device);
- Poor diagnostic yield of MRI due to metal artifacts from spinal implants (CT will also require MAR techniques);
3.4. Conventional CT Imaging
3.4.1. Challenges of CT: Metal-Related Artifacts
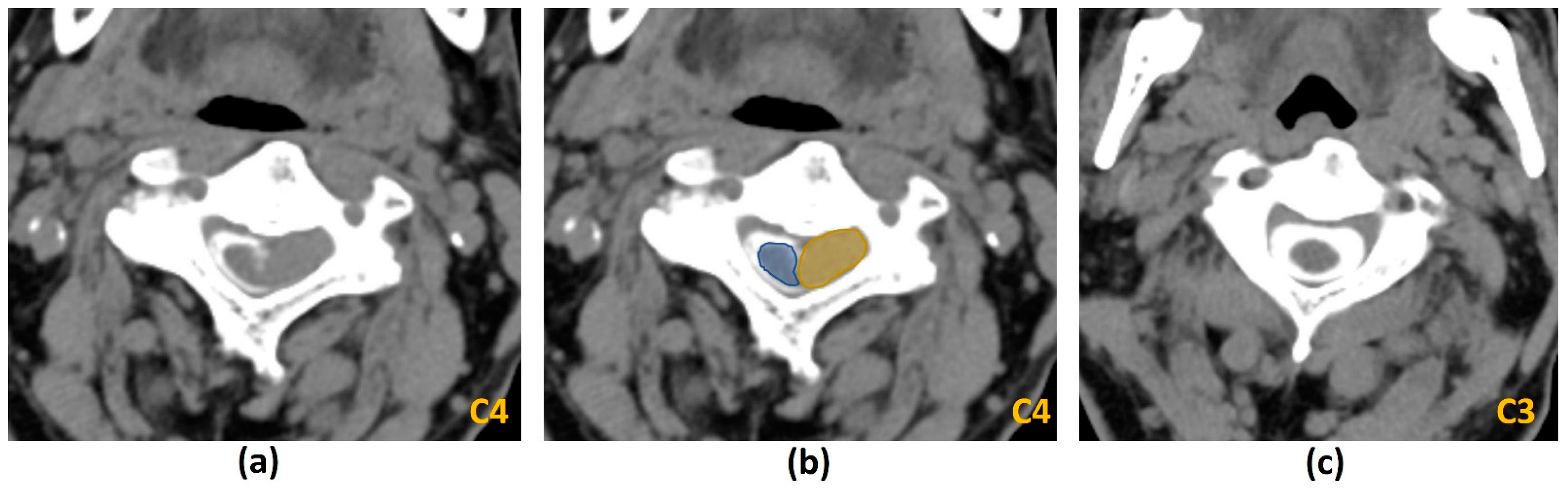
3.4.2. Photon-Counting CT
3.5. Other Imaging Modalities
3.5.1. Plain Radiograph
3.5.2. Skeletal Scintigraphy
3.5.3. Positron Emission Tomography (PET)/CT and PET/MRI
4. The Role of Imaging in Stereotactic Body Radiotherapy (SBRT)
- Pre-treatment planning;
- In-room imaging guidance;
- Post-treatment follow-up.
4.1. Pre-Treatment Planning
4.2. Image-Guided Radiotherapy (IGRT)
4.3. Post-Treatment Follow-Up
5. Deep Learning (DL) in MSCC Imaging
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- National Institute for Health and Care Excellence. Metastatic Spinal Cord Compression: Diagnosis and Management of Adults at Risk of and with Metastatic Spinal Cord Compression NICE Guidelines (CG75); NICE: London, UK, 2008. [Google Scholar]
- Laufer, I.; Schiff, D.; Kelly, H.R.; Bilsky, M. Clinical Features and Diagnosis of Neoplastic Epidural spinal Cord Compression; Eichler, A.F., Ed.; UpToDate: Waltham, MA, USA.
- McLinton, A.; Hutchison, C. Malignant spinal cord compression: A retrospective audit of clinical practice at a UK regional cancer centre. Br. J. Cancer. 2006, 94, 486–491. [Google Scholar] [CrossRef]
- Laur, O.; Nandu, H.; Titelbaum, D.S.; Nunez, D.B.; Khurana, B. Nontraumatic Spinal Cord Compression: MRI Primer for Emergency Department Radiologists. Radiographics 2019, 39, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.G.; Lynch, D.; Garbett, I.; Nazeer, N. Malignant spinal cord compression. J. R Coll. Physicians Edinb. 2019, 49, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilai, O.; Fisher, C.G.; Bilsky, M.H. State of the Art Treatment of Spinal Metastatic Disease. Neurosurgery 2018, 82, 757–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, K.S.; Lee, L.K.; Mak, R.H.; Wang, S.; Pile-Spellman, J.; Abrahm, J.L.; Prigerson, H.G.; Balboni, T.A. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998–2006. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 824–831. [Google Scholar] [CrossRef]
- Savage, P.; Sharkey, R.; Kua, T.; Schofield, L.; Richardson, D.; Panchmatia, N.; Papanastasopoulos, P.; Williams, M.; Falconer, A.; Power, D.; et al. Malignant spinal cord compression: NICE guidance, improvements and challenges. QJM 2014, 107, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Schiff, D.; O’Neill, B.P.; Suman, V.J. Spinal epidural metastasis as the initial manifestation of malignancy: Clinical features and diagnostic approach. Neurology 1997, 49, 452–456. [Google Scholar] [CrossRef]
- Robson, P. Metastatic spinal cord compression: A rare but important complication of cancer. Clin. Med. 2014, 14, 542–545. [Google Scholar] [CrossRef]
- Decroisette, C.; Monnet, I.; Berard, H.; Quere, G.; Le Caer, H.; Bota, S.; Audigier-Valette, C.; Geriniere, L.; Vernejoux, J.M.; Chouaid, C. Groupe Français de Pneumo-Cancérologie 0601 Team. Epidemiology and treatment costs of bone metastases from lung cancer: A French prospective, observational, multicenter study (GFPC 0601). J. Thorac. Oncol. 2011, 6, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Laufer, I.; Bilsky, M.; Schiff, D.; Brown, P. Treatment and Prognosis of Neoplastic Epidural Spinal Cord Compression; Eichler, A.F., Savarese, D.M.F., Eds.; UpToDate: Waltham, MA, USA.
- Laufer, I.; Zuckerman, S.L.; Bird, J.E.; Bilsky, M.H.; Lazáry, Á.; Quraishi, N.A.; Fehlings, M.G.; Sciubba, D.M.; Shin, J.H.; Mesfin, A.; et al. Predicting Neurologic Recovery after Surgery in Patients with Deficits Secondary to MESCC: Systematic Review. Spine 2016, 41 (Suppl. 20), S224–S230. [Google Scholar] [CrossRef]
- Hussain, I.; Barzilai, O.; Reiner, A.S.; DiStefano, N.; McLaughlin, L.; Ogilvie, S.; Bilsky, M.; Laufer, I. Patient-reported outcomes after surgical stabilization of spinal tumors: Symptom-based validation of the Spinal Instability Neoplastic Score (SINS) and surgery. Spine J. 2018, 18, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. A Prospective Audit of the Diagnosis, Management and Outcome of Malignant Spinal Cord Compression; Clinical Resource and Audit Group (CRAG) 97/08; CRAG: Edinburgh, Scotland, 2001. [Google Scholar]
- van Tol, F.R.; Versteeg, A.L.; Verkooijen, H.M.; Öner, F.C.; Verlaan, J.J. Time to Surgical Treatment for Metastatic Spinal Disease: Identification of Delay Intervals. Glob. Spine J. 2021, 2192568221994787. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussios, S.; Hayward, C.; Cooke, D.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Karathanasi, A. Spinal Ewing Sarcoma Debuting with Cord Compression: Have We Discovered the Thread of Ariadne? Anticancer Res. 2018, 38, 5589–5597. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. 2019 Surveillance of Metastatic Spinal Cord Compression in Adults: Risk Assessment, Diagnosis and Management NICE Guidelines (CG75); NICE: London, UK, 2019. [Google Scholar]
- Shah, S.; Kutka, M.; Lees, K.; Abson, C.; Hadaki, M.; Cooke, D.; Neill, C.; Sheriff, M.; Karathanasi, A.; Boussios, S. Management of Metastatic Spinal Cord Compression in Secondary Care: A Practice Reflection from Medway Maritime Hospital, Kent, UK. J. Pers. Med. 2021, 11, 110. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [Green Version]
- Shah, L.M.; Salzman, K.L. Imaging of spinal metastatic disease. Int. J. Surg. Oncol. 2011, 2011, 769753. [Google Scholar] [CrossRef] [Green Version]
- Isaac, A.; Dalili, D.; Dalili, D.; Weber, M.A. State-of-the-art imaging for diagnosis of metastatic bone disease. Radiologe 2020, 60 (Suppl. 1), 1–16. [Google Scholar] [CrossRef] [Green Version]
- Carroll, K.W.; Feller, J.F.; Tirman, P.F. Useful internal standards for distinguishing infiltrative marrow pathology from hematopoietic marrow at MRI. J. Magn. Reson. Imaging 1997, 7, 394–398. [Google Scholar] [CrossRef]
- Khan, M.; Garg, R.; Gui, C.; Lee, Y.; Sahgal, A.; Mossa-Basha, M.; Mayr, N.; Lo, S.; Redmond, K. Neuroimaging and Stereotactic Body Radiation Therapy (SBRT) for Spine Metastasis. Top. Magn. Reson. Imaging 2019, 28, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Arana, E.; Kovacs, F.M.; Royuela, A.; Asenjo, B.; Pérez-Ramírez, Ú.; Zamora, J. Spanish Back Pain Research Network Task Force for the Improvement of Inter-Disciplinary Management of Spinal Metastasis. Spine Instability Neoplastic Score: Agreement across different medical and surgical specialties. Spine J. 2016, 16, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; Schouten, R.; Versteeg, A.L.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Kawahara, N.; Fourney, D.; Weir, L.; Reynolds, J.J.; et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: An assessment of instability secondary to spinal metastases. Radiat. Oncol. 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; Versteeg, A.L.; Schouten, R.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Heran, M.K.; Kawahara, N.; Fourney, D.; Reynolds, J.J.; et al. Reliability of the spinal instability neoplastic scale among radiologists: An assessment of instability secondary to spinal metastases. AJR Am. J. Roentgenol. 2014, 203, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.F.; Yang, P.J.; Seeley, G.W.; Seeger, J.F.; Unger, E.C.; Johnson, J.E. Spinal cord compression due to metastatic disease: Diagnosis with MR imaging versus myelography. Radiology 1989, 173, 225–229. [Google Scholar] [CrossRef]
- Husain, Z.A.; Sahgal, A.; Chang, E.L.; Maralani, P.J.; Kubicky, C.D.; Redmond, K.J.; Fisher, C.; Laufer, I.; Lo, S.S. Modern approaches to the management of metastatic epidural spinal cord compression. CNS Oncol. 2017, 6, 231–241. [Google Scholar] [CrossRef]
- van der Sande, J.J.; Kröger, R.; Boogerd, W. Multiple spinal epidural metastases; an unexpectedly frequent finding. J. Neurol. Neurosurg. Psychiatry 1990, 53, 1001–1003. [Google Scholar] [CrossRef] [Green Version]
- Stradiotti, P.; Curti, A.; Castellazzi, G.; Zerbi, A. Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: State of the art. Eur. Spine J. 2009, 18 (Suppl. 1), 102–108. [Google Scholar] [CrossRef] [Green Version]
- Krätzig, T.; Mende, K.C.; Mohme, M.; Kniep, H.; Dreimann, M.; Stangenberg, M.; Westphal, M.; Gauer, T.; Eicker, S.O. Carbon fiber-reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg. Rev. 2021, 44, 2163–2170. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Agten, C.A.; Pfirrmann, C.W.; Sutter, R. Advances in MRI around metal. J. Magn. Reson. Imaging 2017, 46, 972–991. [Google Scholar] [CrossRef]
- Talbot, B.S.; Weinberg, E.P. MR Imaging with Metal-suppression Sequences for Evaluation of Total Joint Arthroplasty. Radiographics 2016, 36, 209–225. [Google Scholar] [CrossRef]
- Do, T.D.; Sutter, R.; Skornitzke, S.; Weber, M.A. CT and MRI Techniques for Imaging Around Orthopedic Hardware. Rofo 2018, 190, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koff, M.F.; Shah, P.; Koch, K.M.; Potter, H.G. Quantifying image distortion of orthopedic materials in magnetic resonance imaging. J. Magn. Reson. Imaging 2013, 38, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, R.K.; Serhan, H.; Zarda, B.; Patel, R.; Albert, T.; Vaccaro, A.R. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation. Spine J. 2009, 9, 263–267. [Google Scholar] [CrossRef]
- Kumar, N.; Lopez, K.G.; Alathur Ramakrishnan, S.; Hallinan, J.T.P.D.; Fuh, J.Y.H.; Pandita, N.; Madhu, S.; Kumar, A.; Benneker, L.M.; Vellayappan, B.A. Evolution of materials for implants in metastatic spine disease till date—Have we found an ideal material? Radiother Oncol. 2021, 163, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ramakrishnan, S.A.; Lopez, K.G.; Madhu, S.; Ramos, M.R.D.; Fuh, J.Y.H.; Hallinan, J.; Nolan, C.P.; Benneker, L.M.; Vellayappan, B.A. Can Polyether Ether Ketone Dethrone Titanium as the Choice Implant Material for Metastatic Spine Tumor Surgery? World Neurosurg. 2021, 148, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Zimel, M.N.; Hwang, S.; Riedel, E.R.; Healey, J.H. Carbon fiber intramedullary nails reduce artifact in postoperative advanced imaging. Skelet. Radiol. 2015, 44, 1317–1325. [Google Scholar] [CrossRef]
- Osterhoff, G.; Huber, F.A.; Graf, L.C.; Erdlen, F.; Pape, H.C.; Sprengel, K.; Guggenberger, R. Comparison of metal artifact reduction techniques in magnetic resonance imaging of carbon-reinforced PEEK and titanium spinal implants. Acta Radiol. 2021, 2841851211029077. [Google Scholar] [CrossRef]
- Kumar, N.M.; de Cesar Netto, C.; Schon, L.C.; Fritz, J. Metal Artifact Reduction Magnetic Resonance Imaging Around Arthroplasty Implants: The Negative Effect of Long Echo Trains on the Implant-Related Artifact. Investig. Radiol. 2017, 52, 310–316. [Google Scholar] [CrossRef]
- Liebl, H.; Heilmeier, U.; Lee, S.; Nardo, L.; Patsch, J.; Schuppert, C.; Han, M.; Rondak, I.C.; Banerjee, S.; Koch, K.; et al. In vitro assessment of knee MRI in the presence of metal implants comparing MAVRIC-SL and conventional fast spin echo sequences at 1.5 and 3 T field strength. J. Magn. Reson. Imaging 2015, 41, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Koch, K.M.; Hargreaves, B.A.; Stevens, K.J.; Gold, G.E. Metal artifact reduction with MAVRIC SL at 3-T MRI in patients with hip arthroplasty. AJR Am. J. Roentgenol. 2015, 204, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, L.B.; Do, B.H.; Gold, G.E.; Hargreaves, B.A.; Koch, K.M.; Worters, P.W.; Stevens, K.J. MR imaging near metallic implants using MAVRIC SL: Initial clinical experience at 3T. Acad. Radiol. 2015, 22, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzschmar, M.; Nardo, L.; Han, M.M.; Heilmeier, U.; Sam, C.; Joseph, G.B.; Koch, K.M.; Krug, R.; Link, T.M. Metal artefact suppression at 3 T MRI: Comparison of MAVRIC-SL with conventional fast spin echo sequences in patients with Hip joint arthroplasty. Eur. Radiol. 2015, 25, 2403–2411. [Google Scholar] [CrossRef]
- Delfaut, E.M.; Beltran, J.; Johnson, G.; Rousseau, J.; Marchandise, X.; Cotten, A. Fat suppression in MR imaging: Techniques and pitfalls. Radiographics 1999, 19, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Del Grande, F.; Santini, F.; Herzka, D.A.; Aro, M.R.; Dean, C.W.; Gold, G.E.; Carrino, J.A. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics 2014, 34, 217–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchgesner, T.; Acid, S.; Perlepe, V.; Lecouvet, F.; Vande Berg, B. Two-point Dixon fat-water swapping artifact: Lesion mimicker at musculoskeletal T2-weighted MRI. Skelet. Radiol. 2020, 49, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Skeoch, G.D.; Tobin, M.K.; Khan, S.; Linninger, A.A.; Mehta, A.I. Corticosteroid Treatment for Metastatic Spinal Cord Compression: A Review. Global Spine J. 2017, 7, 272–279. [Google Scholar] [CrossRef]
- Fritz, J.; Fritz, B.; Thawait, G.K.; Raithel, E.; Gilson, W.D.; Nittka, M.; Mont, M.A. Advanced metal artifact reduction MRI of metal-on-metal hip resurfacing arthroplasty implants: Compressed sensing acceleration enables the time-neutral use of SEMAC. Skelet. Radiol. 2016, 45, 1345–1356. [Google Scholar] [CrossRef]
- Hargreaves, B.A.; Chen, W.; Lu, W.; Alley, M.T.; Gold, G.E.; Brau, A.C.; Pauly, J.M.; Pauly, K.B. Accelerated slice encoding for metal artifact correction. J. Magn. Reson. Imaging 2010, 31, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Worters, P.W.; Sung, K.; Stevens, K.J.; Koch, K.M.; Hargreaves, B.A. Compressed-sensing multispectral imaging of the postoperative spine. J. Magn. Reson. Imaging 2013, 37, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, K.G. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Phys. Med. Biol. 2015, 60, R297–R322. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Tan, E.T.; Mintz, D.N.; Sahr, M.; Endo, Y.; Nguyen, J.; Lebel, R.M.; Carrino, J.A.; Sneag, D.B. Evaluation of deep learning reconstructed high-resolution 3D lumbar spine MRI. Eur. Radiol. 2022, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.D. The use of methylmethacrylate for vertebral-body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J. Bone Joint. Surg. Am. 1981, 63, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Jee, W.H.; McCauley, T.R.; Ha, K.Y.; Choi, K.H. Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR imaging. Radiographics 2003, 23, 179–187. [Google Scholar] [CrossRef]
- Mauch, J.T.; Carr, C.M.; Cloft, H.; Diehn, F.E. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. AJNR Am. J. Neuroradiol. 2018, 39, 1584–1592. [Google Scholar] [CrossRef]
- Arana, E.; Kovacs, F.M.; Royuela, A.; Asenjo, B.; Nagib, F.; Pérez-Aguilera, S.; Dejoz, M.; Cabrera-Zubizarreta, A.; García-Hidalgo, Y.; Estremera, A. Spanish Back Pain Research Network Task Force for the Improvement of Inter-Disciplinary Management of Spinal Metastasis. Metastatic Versus Osteoporotic Vertebral Fractures on MRI: A Blinded, Multicenter, and Multispecialty Observer Agreement Evaluation. J. Natl. Compr. Cancer Netw. 2020, 18, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Jabehdar Maralani, P.; Lo, S.S.; Redmond, K.; Soliman, H.; Myrehaug, S.; Husain, Z.A.; Heyn, C.; Kapadia, A.; Chan, A.; Sahgal, A. Spinal metastases: Multimodality imaging in diagnosis and stereotactic body radiation therapy planning. Future Oncol. 2017, 13, 77–91. [Google Scholar] [CrossRef]
- Ciray, I.; Lindman, H.; Aström, G.K.; Wanders, A.; Bergh, J.; Ahlström, H.K. Effect of granulocyte colony-stimulating factor (G-CSF)-supported chemotherapy on MR imaging of normal red bone marrow in breast cancer patients with focal bone metastases. Acta Radiol. 2003, 44, 472–484. [Google Scholar] [CrossRef]
- Patel, D.M.; Weinberg, B.D.; Hoch, M.J. CT Myelography: Clinical Indications and Imaging Findings. Radiographics 2020, 40, 470–484. [Google Scholar] [CrossRef]
- Hollis, P.H.; Malis, L.I.; Zappulla, R.A. Neurological deterioration after lumbar puncture below complete spinal subarachnoid block. J. Neurosurg. 1986, 64, 253–256. [Google Scholar] [CrossRef]
- Hagenau, C.; Grosh, W.; Currie, M.; Wiley, R.G. Comparison of spinal magnetic resonance imaging and myelography in cancer patients. J. Clin. Oncol. 1987, 5, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Loblaw, D.A.; Perry, J.; Chambers, A.; Laperriere, N.J. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J. Clin. Oncol. 2005, 23, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.P.; Cherryman, G.R.; Husband, J.E. Magnetic resonance imaging in suspected metastatic spinal cord compression. Clin. Radiol. 1989, 40, 286–290. [Google Scholar] [CrossRef]
- Salvo, N.; Christakis, M.; Rubenstein, J.; de Sa, E.; Napolskikh, J.; Sinclair, E.; Ford, M.; Goh, P.; Chow, E. The role of plain radiographs in management of bone metastases. J. Palliat. Med. 2009, 12, 195–198. [Google Scholar] [CrossRef]
- Pezaro, C.; Omlin, A.; Perez-Lopez, R.; Mukherji, D.; Attard, G.; Bianchini, D.; Lorente, D.; Parker, C.; Dearnaley, D.; de Bono, J.S.; et al. Progressive computed tomography (CT) appearances preceding malignant spinal cord compression (MSCC) in men with castration-resistant prostate cancer. Clin. Radiol. 2015, 70, 359–365. [Google Scholar] [CrossRef]
- Freeman, C.W.; Lazor, J.W.; Loevner, L.A.; Nabavizadeh, S.A. Variations of the CNS Venous System Mimicking Pathology: Spectrum of Imaging Findings. J. Neuroimaging 2019, 29, 673–688. [Google Scholar] [CrossRef]
- Crocker, M.; Anthantharanjit, R.; Jones, T.L.; Shoeb, M.; Joshi, Y.; Papadopoulos, M.C.; Bell, B.A.; Rich, P. An extended role for CT in the emergency diagnosis of malignant spinal cord compression. Clin. Radiol. 2011, 66, 922–927. [Google Scholar] [CrossRef]
- Hove, B.; Gyldensted, C. Spiculated vertebral metastases from prostatic carcinoma. Report of first two cases. Neuroradiology 1990, 32, 337–339. [Google Scholar] [CrossRef]
- Catherine, G.; MacLeod, N.; Sheridan, S. Is CT adequate to assess for malignant cord compression? Clin. Radiol. 2015, 70 (Suppl. 1), s7. [Google Scholar] [CrossRef]
- Thomas, S.J. Relative electron density calibration of CT scanners for radiotherapy treatment planning. Br. J. Radiol. 1999, 72, 781–786. [Google Scholar] [CrossRef]
- Buhmann Kirchhoff, S.; Becker, C.; Duerr, H.R.; Reiser, M.; Baur-Melnyk, A. Detection of osseous metastases of the spine: Comparison of high resolution multi-detector-CT with MRI. Eur. J. Radiol. 2009, 69, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, F.A.; Sprengel, K.; Müller, L.; Graf, L.C.; Osterhoff, G.; Guggenberger, R. Comparison of different CT metal artifact reduction strategies for standard titanium and carbon-fiber reinforced polymer implants in sheep cadavers. BMC Med. Imaging 2021, 21, 29. [Google Scholar] [CrossRef]
- Andersson, K.M.; Nowik, P.; Persliden, J.; Thunberg, P.; Norrman, E. Metal artefact reduction in CT imaging of hip prostheses—An evaluation of commercial techniques provided by four vendors. Br. J. Radiol. 2015, 88, 20140473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Noel, C.; Chen, H.; Harold Li, H.; Low, D.; Moore, K.; Klahr, P.; Michalski, J.; Gay, H.A.; Thorstad, W.; et al. Clinical evaluation of a commercial orthopedic metal artifact reduction tool for CT simulations in radiation therapy. Med. Phys. 2012, 39, 7507–7517. [Google Scholar] [CrossRef]
- Axente, M.; Paidi, A.; Von Eyben, R.; Zeng, C.; Bani-Hashemi, A.; Krauss, A.; Hristov, D. Clinical evaluation of the iterative metal artifact reduction algorithm for CT simulation in radiotherapy. Med. Phys. 2015, 42, 1170–1183. [Google Scholar] [CrossRef]
- Wagenaar, D.; van der Graaf, E.R.; van der Schaaf, A.; Greuter, M.J. Quantitative comparison of commercial and non-commercial metal artifact reduction techniques in computed tomography. PLoS ONE 2015, 10, e0127932. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Xu, D.; Zamyatin, A. Metal artifact reduction algorithm for single energy and dual energy CT scans. In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), Anaheim, CA, USA, 29 October–3 November 2013; IEEE: Piscataway, NJ, USA, 2012; pp. 3426–3429. [Google Scholar] [CrossRef]
- Han, S.C.; Chung, Y.E.; Lee, Y.H.; Park, K.K.; Kim, M.J.; Kim, K.W. Metal artifact reduction software used with abdominopelvic dual-energy CT of patients with metal hip prostheses: Assessment of image quality and clinical feasibility. AJR Am. J. Roentgenol. 2014, 203, 788–795. [Google Scholar] [CrossRef]
- Wang, F.; Xue, H.; Yang, X.; Han, W.; Qi, B.; Fan, Y.; Qian, W.; Wu, Z.; Zhang, Y.; Jin, Z. Reduction of metal artifacts from alloy hip prostheses in computer tomography. J. Comput. Assist. Tomogr. 2014, 38, 828–833. [Google Scholar] [CrossRef]
- Schenzle, J.C.; Sommer, W.H.; Neumaier, K.; Michalski, G.; Lechel, U.; Nikolaou, K.; Becker, C.R.; Reiser, M.F.; Johnson, T.R. Dual energy CT of the chest: How about the dose? Investig. Radiol. 2010, 45, 347–353. [Google Scholar] [CrossRef]
- Henzler, T.; Fink, C.; Schoenberg, S.O.; Schoepf, U.J. Dual-energy CT: Radiation dose aspects. AJR Am. J. Roentgenol. 2012, 199 (Suppl. 5), S16–S25. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Weng, A.M.; Heidemeier, A.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Multiple Myeloma and Dual-Energy CT: Diagnostic Accuracy of Virtual Noncalcium Technique for Detection of Bone Marrow Infiltration of the Spine and Pelvis. Radiology 2018, 286, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, N.; Große Hokamp, N.; Lennartz, S.; Holz, J.A.; Romman, Z.; Pahn, G.; Neuhaus, V.; Maintz, D.; Krug, B.; Borggrefe, J. Improvements of diagnostic accuracy and visualization of vertebral metastasis using multi-level virtual non-calcium reconstructions from dual-layer spectral detector computed tomography. Eur. Radiol. 2019, 29, 5941–5949. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.C.; Garg, A.; Youngner, J.M.; Deshmukh, S.D.; Omar, I.M. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT. Skelet. Radiol. 2019, 48, 605–613. [Google Scholar] [CrossRef]
- Long, Z.; Bruesewitz, M.R.; DeLone, D.R.; Morris, J.M.; Amrami, K.K.; Adkins, M.C.; Glazebrook, K.N.; Kofler, J.M.; Leng, S.; McCollough, C.H.; et al. Evaluation of projection- and dual-energy-based methods for metal artifact reduction in CT using a phantom study. J. Appl. Clin. Med. Phys. 2018, 19, 252–260. [Google Scholar] [CrossRef]
- Puvanasunthararajah, S.; Fontanarosa, D.; Wille, M.L.; Camps, S.M. The application of metal artifact reduction methods on computed tomography scans for radiotherapy applications: A literature review. J. Appl. Clin. Med. Phys. 2021, 22, 198–223. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- O’Sullivan, G.J.; Carty, F.L.; Cronin, C.G. Imaging of bone metastasis: An update. World J. Radiol. 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Choi, J.; Raghavan, M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Control. 2012, 19, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.Y.; Dalal, I.; Griffith, B. [18F]FDG-PET Evaluation of Spinal Pathology in Patients in Oncology: Pearls and Pitfalls for the Neuroradiologist. AJNR Am. J. Neuroradiol. 2022, 43, 332–340. [Google Scholar] [CrossRef]
- Wallace, A.N.; Greenwood, T.J.; Jennings, J.W. Use of Imaging in the Management of Metastatic Spine Disease with Percutaneous Ablation and Vertebral Augmentation. AJR Am. J. Roentgenol. 2015, 205, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Thibault, I.; Chang, E.L.; Sheehan, J.; Ahluwalia, M.S.; Guckenberger, M.; Sohn, M.J.; Ryu, S.; Foote, M.; Lo, S.S.; Muacevic, A.; et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: A report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015, 16, e595–e603. [Google Scholar] [CrossRef]
- Bredella, M.A.; Essary, B.; Torriani, M.; Ouellette, H.A.; Palmer, W.E. Use of FDG-PET in differentiating benign from malignant compression fractures. Skelet. Radiol. 2008, 37, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Zhao, L.; Guo, X.; Zhao, L.; Wu, J.; Huang, J.; Sun, L.; Xie, C.; Chen, H. Differential diagnostic value of 18F-FDG PET/CT for benign and malignant vertebral compression fractures: Comparison with magnetic resonance imaging. Cancer Manag. Res. 2018, 10, 2105–2115. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.I.; Chang, U.K. Comparison of MR imaging and FDG-PET/CT in the differential diagnosis of benign and malignant vertebral compression fractures. J. Neurosurg. Spine 2011, 14, 177–183. [Google Scholar] [CrossRef]
- Gwak, H.S.; Youn, S.M.; Chang, U.; Lee, D.H.; Cheon, G.J.; Rhee, C.H.; Kim, K.; Kim, H.J. Usefulness of (18)F-fluorodeoxyglucose PET for radiosurgery planning and response monitoring in patients with recurrent spinal metastasis. Min-Minim. Invasive Neurosurg. 2006, 49, 127–134. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where might it replace PET/CT? J. Magn. Reson. Imaging 2017, 46, 1247–1262. [Google Scholar] [CrossRef] [Green Version]
- Batouli, A.; Braun, J.; Singh, K.; Gholamrezanezhad, A.; Casagranda, B.U.; Alavi, A. Diagnosis of non-osseous spinal metastatic disease: The role of PET/CT and PET/MRI. J. Neurooncol. 2018, 138, 221–230. [Google Scholar] [CrossRef]
- Tseng, C.L.; Eppinga, W.; Charest-Morin, R.; Soliman, H.; Myrehaug, S.; Maralani, P.J.; Campbell, M.; Lee, Y.K.; Fisher, C.; Fehlings, M.G.; et al. Spine Stereotactic Body Radiotherapy: Indications, Outcomes, and Points of Caution. Glob. Spine J. 2017, 7, 179–197. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Nakamura, N.; Shimizuguchi, T.; Ogawa, H.; Karasawa, K. Appropriate endpoints for stereotactic body radiotherapy for bone metastasis: Classification into five treatment groups. Rep. Pr. Oncol. Radiother. 2020, 25, 150–153. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Mendel, E.; Yamada, Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine 2009, 34 (Suppl. 22), S78–S92. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ogawa, H.; Shimizuguchi, T.; Nihei, K.; Furuya, T.; Tanaka, H.; Karasawa, K. Stereotactic Body Radiotherapy for Spinal Metastases: Clinical Experience in 134 Cases from a Single Japanese Institution. Technol. Cancer Res. Treat. 2018, 17, 1533033818806472. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.M.; Fraser, I.M.; Liu, M. Stereotactic body radiation therapy for lung, spine and oligometastatic disease: Current evidence and future directions. Ann. Transl. Med. 2018, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Vellayappan, B.A.; Kumar, N.; Chang, E.L.; Sahgal, A.; Sloan, A.E.; Lo, S.S. Novel multidisciplinary approaches in the management of metastatic epidural spinal cord compression. Future Oncol. 2018, 14, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Katsoulakis, E.; Laufer, I.; Lovelock, M.; Barzilai, O.; McLaughlin, L.A.; Zhang, Z.; Schmitt, A.M.; Higginson, D.S.; Lis, E.; et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg. Focus. 2017, 42, E6. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Bilsky, M.H.; Lovelock, D.M.; Venkatraman, E.S.; Toner, S.; Johnson, J.; Zatcky, J.; Zelefsky, M.J.; Fuks, Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 484–490. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Burton, S.A.; Ozhasoglu, C.; Welch, W.C. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine 2007, 32, 193–199. [Google Scholar] [CrossRef]
- Sahgal, A.; Larson, D.A.; Chang, E.L. Stereotactic body radiosurgery for spinal metastases: A critical review. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 652–665. [Google Scholar] [CrossRef]
- Husain, Z.A.; Sahgal, A.; De Salles, A.; Funaro, M.; Glover, J.; Hayashi, M.; Hiraoka, M.; Levivier, M.; Ma, L.; Mar-tínez-Alvarez, R.; et al. Stereotactic body radiotherapy for de novo spinal metastases: Systematic review. J. Neurosurg. Spine 2017, 27, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. trial investigators. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef]
- Mossa-Basha, M.; Gerszten, P.C.; Myrehaug, S.; Mayr, N.A.; Yuh, W.T.; Jabehdar Maralani, P.; Sahgal, A.; Lo, S.S. Spinal metastasis: Diagnosis, management and follow-up. Br. J. Radiol. 2019, 92, 20190211. [Google Scholar] [CrossRef]
- Das, I.J.; McGee, K.P.; Tyagi, N.; Wang, H. Role and future of MRI in radiation oncology. Br. J. Radiol. 2019, 92, 20180505. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.O.; Giese, H.; Biller, A.; Nagel, A.M.; Kiening, K. Spatial Distortion in MRI-Guided Stereotactic Procedures: Evaluation in 1.5-, 3- and 7-Tesla MRI Scanners. Stereotact. Funct. Neurosurg. 2015, 93, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rogé, M.; Henni, A.H.; Neggaz, Y.A.; Mallet, R.; Hanzen, C.; Dubray, B.; Colard, E.; Gensanne, D.; Thureau, S. Evaluation of a Dedicated Software “Elements™ Spine SRS, Brainlab®” for Target Volume Definition in the Treatment of Spinal Bone Metastases with Stereotactic Body Radiotherapy. Front. Oncol. 2022, 12, 827195. [Google Scholar] [CrossRef] [PubMed]
- Aselmaa, A.; van Herk, M.; Song, Y.; Goossens, R.H.M.; Laprie, A. The influence of automation on tumor contouring. Cogn. Technol. Work 2017, 19, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Sterzing, F.; Engenhart-Cabillic, R.; Flentje, M.; Debus, J. Image-guided radiotherapy: A new dimension in radiation oncology. Dtsch. Arztebl. Int. 2011, 108, 274–280. [Google Scholar] [CrossRef]
- Cubillos Mesí as, M.; Boda-Heggemann, J.; Thoelking, J.; Lohr, F.; Wenz, F.; Wertz, H. Quantification and Assessment of Interfraction Setup Errors Based on Cone Beam CT and Determination of Safety Margins for Radiotherapy. PLoS ONE 2016, 11, e0150326. [Google Scholar] [CrossRef]
- Tseng, C.L.; Sussman, M.S.; Atenafu, E.G.; Letourneau, D.; Ma, L.; Soliman, H.; Thibault, I.; Cho, B.C.; Simeonov, A.; Yu, E.; et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 995–1002. [Google Scholar] [CrossRef]
- Hyde, D.; Lochray, F.; Korol, R.; Davidson, M.; Wong, C.S.; Ma, L.; Sahgal, A. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: Intrafraction motion analysis accounting for all six degrees of freedom. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e555–e562. [Google Scholar] [CrossRef]
- Oztek, M.A.; Mayr, N.A.; Mossa-Basha, M.; Nyflot, M.; Sponseller, P.A.; Wu, W.; Hofstetter, C.P.; Saigal, R.; Bowen, S.R.; Hippe, D.S.; et al. The Dancing Cord: Inherent Spinal Cord Motion and Its Effect on Cord Dose in Spine Stereotactic Body Radiation Therapy. Neurosurgery 2020, 87, 1157–1166. [Google Scholar] [CrossRef]
- Boda-Heggemann, J.; Lohr, F.; Wenz, F.; Flentje, M.; Guckenberger, M. kV cone-beam CT-based IGRT: A clinical review. Strahlenther Onkol. 2011, 187, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shanavas, M.; Sidappa, A.; Kiran, M. Cone beam computed tomography—Know its secrets. J. Int. Oral. Health 2015, 7, 64–68. [Google Scholar] [PubMed]
- Chan, M.F.; Lim, S.B.; Li, X.; Tang, X.; Zhang, P.; Shi, C. Commissioning and Evaluation of a Third-Party 6 Degrees-of-Freedom Couch Used in Radiotherapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870778. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechuga, L.; Weidlich, G.A. Cone Beam CT vs. Fan Beam CT: A Comparison of Image Quality and Dose Delivered Between Two Differing CT Imaging Modalities. Cureus 2016, 8, e778. [Google Scholar] [CrossRef] [Green Version]
- Sajja, S.; Lee, Y.; Eriksson, M.; Nordström, H.; Sahgal, A.; Hashemi, M.; Mainprize, J.G.; Ruschin, M. Technical Principles of Dual-Energy Cone Beam Computed Tomography and Clinical Applications for Radiation Therapy. Adv. Radiat. Oncol. 2019, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nagarajappa, A.K.; Dwivedi, N.; Tiwari, R. Artifacts: The downturn of CBCT image. J. Int. Soc. Prev. Community Dent. 2015, 5, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Schulze, R.; Heil, U.; Gross, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofac. Radiol. 2011, 40, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Llorente, R.; Spieler, B.O.; Victoria, J.; Takita, C.; Yechieli, R.; Ford, J.C.; Brown, K.; Samuels, M.A.; Mellon, E.A. MRI-guided stereotactic ablative radiation therapy of spinal bone metastases: A preliminary experience. Br. J. Radiol. 2020, 93, 20190655. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, J.H.; Kim, J.I.; Park, J.M. Comparison of treatment plan quality among MRI-based IMRT with a linac, MRI-based IMRT with tri-Co-60 sources, and VMAT for spine SABR. PLoS ONE 2019, 14, e0220039. [Google Scholar] [CrossRef]
- Pollard, J.M.; Wen, Z.; Sadagopan, R.; Wang, J.; Ibbott, G.S. The future of image-guided radiotherapy will be MR guided. Br. J. Radiol. 2017, 90, 20160667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Payne, G.S. Radiotherapy planning using MRI. Phys. Med. Biol. 2015, 60, R323–R361. [Google Scholar] [CrossRef] [PubMed]
- Ranger, M.R.; Irwin, G.J.; Bunbury, K.M.; Peutrell, J.M. Changing body position alters the location of the spinal cord within the vertebral canal: A magnetic resonance imaging study. Br. J. Anaesth. 2008, 101, 804–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, J.; Nardella, L.; Degnan, M.; Andreozzi, J.; Yu, H.M.; Penagaricano, J.; Johnstone, P.A.S.; Oliver, D.; Ahmed, K.; Rosenberg, S.A.; et al. Triggered kV Imaging During Spine SBRT for Intrafraction Motion Management. Technol. Cancer Res. Treat. 2021, 20, 15330338211063033. [Google Scholar] [CrossRef]
- Xiao, Y.; Kry, S.F.; Popple, R.; Yorke, E.; Papanikolaou, N.; Stathakis, S.; Xia, P.; Huq, S.; Bayouth, J.; Galvin, J.; et al. Flattening filter-free accelerators: A report from the AAPM Therapy Emerging Technology Assessment Work Group. J. Appl. Clin. Med. Phys. 2015, 16, 5219. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Sohn, M.J.; Lee, B.H.; Kim, S.Y.; Seo, J.W.; Han, Y.H.; Lee, J.Y.; Cha, S.J.; Kim, Y.H. Radiosurgery for metastatic spinal tumors: Follow-up MR findings. AJNR Am. J. Neuroradiol. 2012, 33, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Jawad, M.S.; Harb, J.G.; Yee, S.; Yan, D.; Grills, I.S. Quantifying Follow-up T2-weighted MR Image in Local Failure Spinal Tumors after Stereotactic Body Radiation Therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, s6. [Google Scholar] [CrossRef]
- Kumar, K.A.; Peck, K.K.; Karimi, S.; Lis, E.; Holodny, A.I.; Bilsky, M.H.; Yamada, Y. A Pilot Study Evaluating the Use of Dynamic Contrast-Enhanced Perfusion MRI to Predict Local Recurrence After Radiosurgery on Spinal Metastases. Technol. Cancer Res. Treat. 2017, 16, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Karimi, S.; Peck, K.K.; Yamada, Y.; Lis, E.; Lyo, J.; Bilsky, M.; Holodny, A.I. Measurement of blood perfusion in spinal metastases with dynamic contrast-enhanced magnetic resonance imaging: Evaluation of tumor response to radiation therapy. Spine 2013, 38, E1418–E1424. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, G.S.; Yoon, Y.C.; Park, H.C.; Kim, H.S. Diffusion-weighted and dynamic contrast-enhanced magnetic resonance imaging after radiation therapy for bone metastases in patients with hepatocellular carcinoma. Sci. Rep. 2021, 11, 10459. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.M.; Shin, S.O.; Chang, Y.; Lee, S.J.; Finsterbusch, J.; Frahm, J. Diffusion-weighted MR imaging of metastatic disease of the spine: Assessment of response to therapy. AJNR Am. J. Neuroradiol. 2002, 23, 906–912. [Google Scholar] [PubMed]
- Choi, J.; Kim, J.W.; Jeon, T.J.; Lee, I.J. The 18F-FDG PET/CT response to radiotherapy for patients with spinal metastasis correlated with the clinical outcomes. PLoS ONE 2018, 13, e0204918. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; McDermott, R.; Keys, M.; O’Sullivan, M.; Armstrong, J.; Faul, C. Imaging response assessment following stereotactic body radiotherapy for solid tumour metastases of the spine: Current challenges and future directions. J. Med. Imaging Radiat. Oncol. 2020, 64, 385–397. [Google Scholar] [CrossRef]
- Correia, D.; Moullet, B.; Cullmann, J.; Heiss, R.; Ermiş, E.; Aebersold, D.M.; Hemmatazad, H. Response assessment after stereotactic body radiation therapy for spine and non-spine bone metastases: Results from a single institutional study. Radiat. Oncol. 2022, 17, 37. [Google Scholar] [CrossRef]
- Balagamwala, E.H.; Naik, M.; Reddy, C.A.; Angelov, L.; Suh, J.H.; Djemil, T.; Magnelli, A.; Chao, S.T. Pain flare after stereotactic radiosurgery for spine metastases. J. Radiosurg. SBRT 2018, 5, 99–105. [Google Scholar]
- McDonald, R.; Chow, E.; Rowbottom, L.; DeAngelis, C.; Soliman, H. Incidence of pain flare in radiation treatment of bone metastases: A literature review. J. Bone Oncol. 2014, 3, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Amini, B.; Beaman, C.B.; Madewell, J.E.; Allen, P.K.; Rhines, L.D.; Tatsui, C.E.; Tannir, N.M.; Li, J.; Brown, P.D.; Ghia, A.J. Osseous Pseudoprogression in Vertebral Bodies Treated with Stereotactic Radiosurgery: A Secondary Analysis of Prospective Phase I/II Clinical Trials. AJNR Am. J. Neuroradiol. 2016, 37, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Bahig, H.; Simard, D.; Létourneau, L.; Wong, P.; Roberge, D.; Filion, E.; Donath, D.; Sahgal, A.; Masucci, L. A Study of Pseudoprogression After Spine Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 848–856. [Google Scholar] [CrossRef]
- Stutz, E.; Wartenberg, M.; Hemmatazad, H. Epidural tumor pseudoprogression after spine SBRT: A case report and a mini review of the literature. RAS Oncol. Ther. 2021, 2. [Google Scholar] [CrossRef]
- Jabehdar Maralani, P.; Winger, K.; Symons, S.; Machnowska, M.; Heyn, C.; Helmi, A.; Chan, A.; Tseng, C.L.; Sahgal, A. Incidence and Time of Onset of Osseous Pseudoprogression in Patients with Metastatic Spine Disease from Renal Cell or Prostate Carcinoma After Treatment with Stereotactic Body Radiation Therapy. Neurosurgery 2019, 84, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, S.; Tseng, C.L.; Whyne, C.; Alghamdi, M.; Wilson, J.; Myrehaug, S.; Soliman, H.; Lee, Y.; Maralani, P.; Yang, V.; et al. Vertebral Compression Fracture After Spine Stereotactic Body Radiation Therapy: A Review of the Pathophysiology and Risk Factors. Neurosurgery 2018, 83, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, M.V.; Al-Omair, A.; Atenafu, E.G.; Masucci, G.L.; Letourneau, D.; Korol, R.; Yu, E.; Howard, P.; Lochray, F.; da Costa, L.B.; et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e343–e349. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.S.; Laufer, I.; Boland, P.J.; Hanover, A.; Bilsky, M.H.; Yamada, J.; Lis, E. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 2009, 27, 5075–5079. [Google Scholar] [CrossRef] [Green Version]
- Al-Omair, A.; Smith, R.; Kiehl, T.R.; Lao, L.; Yu, E.; Massicotte, E.M.; Keith, J.; Fehlings, M.G.; Sahgal, A. Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: Clinicopathological correlation. J. Neurosurg. Spine 2013, 18, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, Y.; Torun, N.; Guler, O.C.; Yildirim, B.A.; Besen, A.A.; Yetisken, A.G.; Onal, H.C.; Topkan, E. Local control and vertebral compression fractures following stereotactic body radiotherapy for spine metastases. J. Bone Oncol. 2019, 15, 100218. [Google Scholar] [CrossRef]
- Chen, W.T.; Shih, T.T.; Chen, R.C.; Lo, H.Y.; Chou, C.T.; Lee, J.M.; Tu, H.Y. Blood perfusion of vertebral lesions evaluated with gadolinium-enhanced dynamic MRI: In comparison with compression fracture and metastasis. J. Magn. Reson. Imaging 2002, 15, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Gui, C.; Chen, X.; Sheikh, K.; Mathews, L.; Lo, S.L.; Lee, J.; Khan, M.A.; Sciubba, D.M.; Redmond, K.J. Radiomic modeling to predict risk of vertebral compression fracture after stereotactic body radiation therapy for spinal metastases. J. Neurosurg. Spine 2021, 1–9. [Google Scholar] [CrossRef]
- AIR™ Image Quality. Available online: https://www.gehealthcare.com/products/magnetic-resonance-imaging/air-technology/air-image-quality (accessed on 4 May 2022).
- Hallinan, J.T.P.D.; Zhu, L.; Zhang, W.; Lim, D.S.W.; Baskar, S.; Low, X.Z.; Yeong, K.Y.; Teo, E.C.; Kumarakulasinghe, N.B.; Yap, Q.V.; et al. Deep Learning Model for Classifying Metastatic Epidural Spinal Cord Compression on MRI. Front. Oncol. 2022, 12, 849447. [Google Scholar] [CrossRef]
- Gourd, E. UK radiologist staffing crisis reaches critical levels. Lancet Oncol. 2017, 18, e651. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Lang, N.; Yuan, H.; Su, M.Y.; Baldi, P. A multi-resolution approach for spinal metastasis detection using deep Siamese neural networks. Comput. Biol. Med. 2017, 84, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammon, M.; Dankerl, P.; Tsymbal, A.; Wels, M.; Kelm, M.; May, M.; Suehling, M.; Uder, M.; Cavallaro, A. Automatic detection of lytic and blastic thoracolumbar spine metastases on computed tomography. Eur. Radiol. 2013, 23, 1862–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, S.D.; Yao, J.; Summers, R.M. Lytic metastases in thoracolumbar spine: Computer-aided detection at CT—Preliminary study. Radiology 2007, 242, 811–816. [Google Scholar] [CrossRef]
- Lindgren Belal, S.; Sadik, M.; Kaboteh, R.; Enqvist, O.; Ulén, J.; Poulsen, M.H.; Simonsen, J.; Høilund-Carlsen, P.F.; Edenbrandt, L.; Trägårdh, E. Deep learning for segmentation of 49 selected bones in CT scans: First step in automated PET/CT-based 3D quantification of skeletal metastases. Eur. J. Radiol. 2019, 113, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]


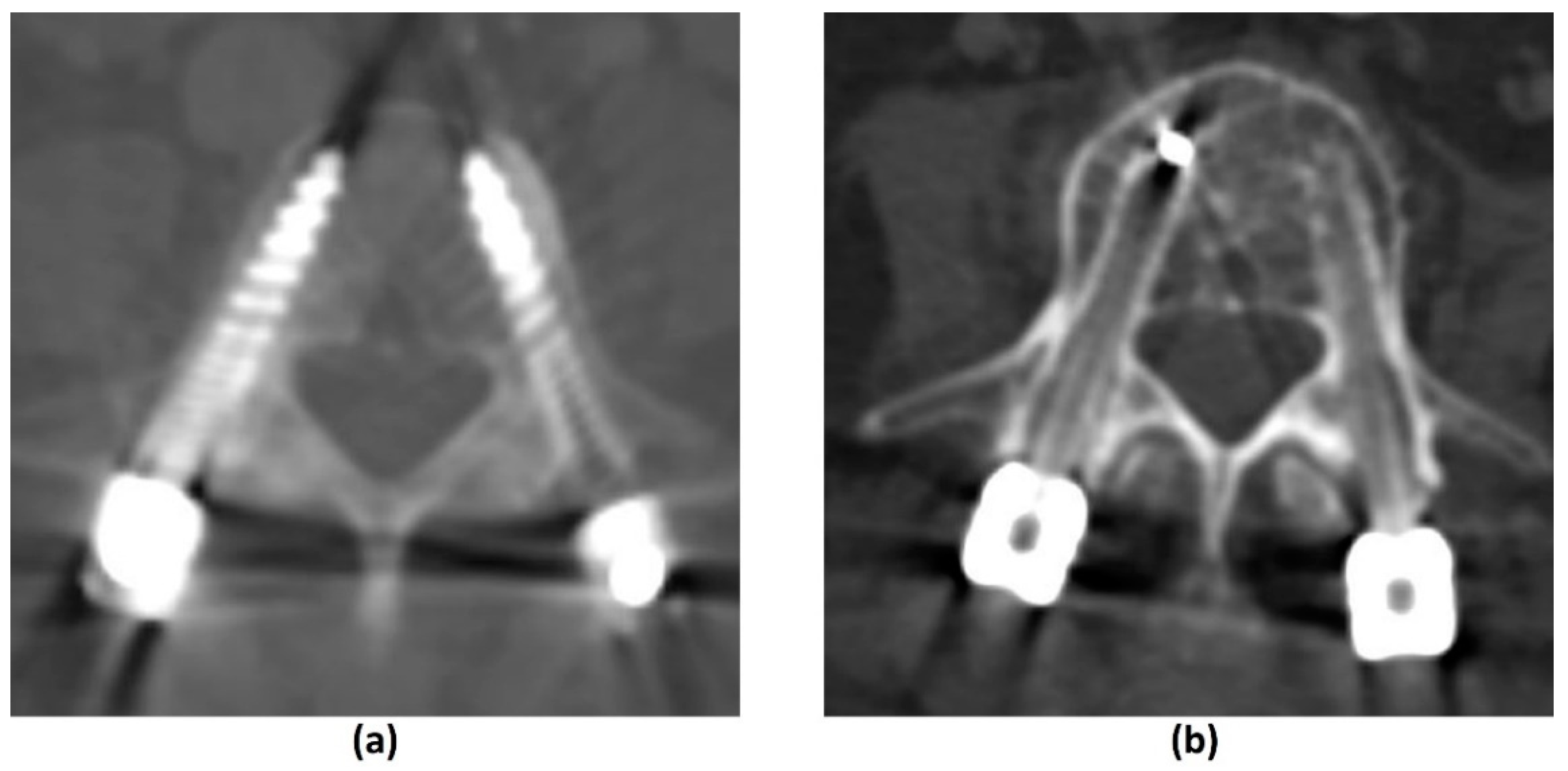
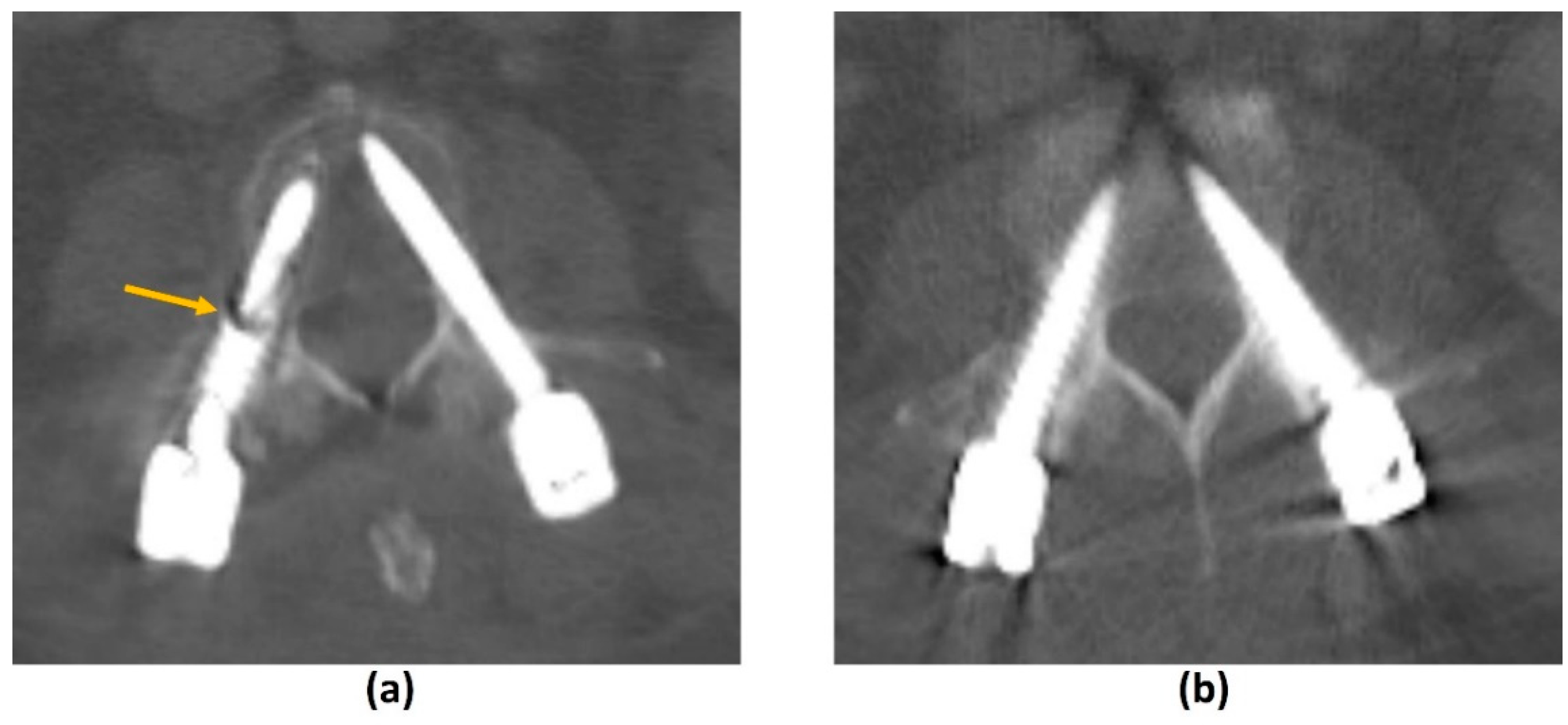



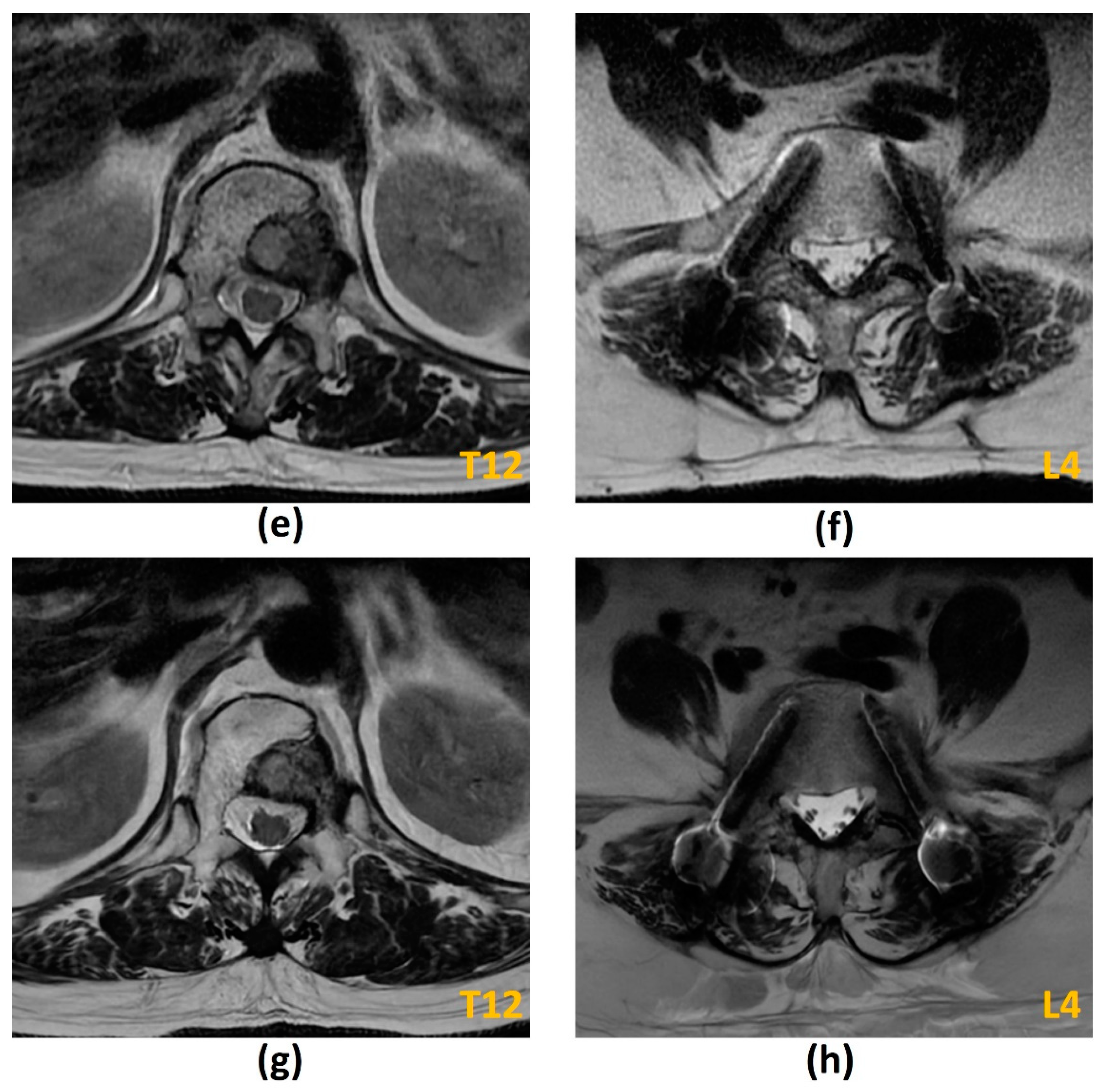
| Element of Spine Instability Neoplastic Score (SINS) | Score |
|---|---|
| Location | |
| Junctional (occiput–C2, C7–T2, T11–L1, L5–S1) | 3 |
| Mobile spine (C3–C6, L2–L4) | 2 |
| Semi-rigid (T3–T10) | 1 |
| Rigid (S2–S5) | 0 |
| Pain relief with recumbency and/or pain with movement/loading of the spine | |
| Yes | 3 |
| No (occasional pain but not mechanical) | 1 |
| Pain-free lesion | 0 |
| Bone lesion (typically assessed with CT) | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with >50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of the spinal elements (facet, pedicle or costovertebral joint fracture or replacement with tumour) | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
| Sagittal | Axial | ||||
|---|---|---|---|---|---|
| Parameters | T2-W | T1-W Pre and Post-Contrast FS | STIR | Axial T1-W Post-Contrast FS | Axial T2-W |
| TR (msec) | 3500 | 500 | 4000 | 500 | 3500 |
| TE (msec) | 90 | 10 | 60 | 10 | 90 |
| Section thickness (mm) | 3.5 | 3.5 | 3.5 | 5 | 5 |
| Gap (mm) | 1 | 1 | 1 | 2 | 2 |
| Field of view (mm2) | 400 × 400 | 400 × 400 | 400 × 400 | 160 × 160 | 160 × 160 |
| Matrix | 448 × 384 | 448 × 384 | 448 × 384 | 320 × 224 | 320 × 224 |
| Sequence | Advantages | Disadvantages |
|---|---|---|
| T2-W | Evaluation of spinal cord and nerve root compression (‘myelogram-like effect’); Detection of cord signal changes (e.g., myelomalacia or oedema) | Suboptimal for evaluation of marrow replacing lesions |
| T1-W | Identification of marrow replacing lesions including metastasis; Useful for comparison with post-contrast sequences to identify true contrast-enhancement | Suboptimal for evaluation of spinal cord and nerve root compression; Peritumoural oedema may also appear hypointense on T1-W sequences, which may limit the accuracy of measurement of the true tumour size |
| T1-W post-contrast FS | Detection of enhancing vertebral metastasis, sites of leptomeningeal and intramedullary disease; Delineation of tumour extent including identification of the epidural component, and presence of foraminal or paraspinal extension; Determination of biopsy site of highest yield (if biopsy required) | Suboptimal for evaluation of spinal cord and nerve root compression |
| STIR | Identification of marrow replacing lesions including metastasis; More accurate measurement of true tumour size from surrounding peritumoural oedema than T1-weighted sequences; Identification of macroscopic fat in lesions | Suboptimal for detection of sclerotic vertebral metastasis without oedema |
| Benign Osteoporotic Compression Fracture | Malignant Vertebral Compression Fracture |
|---|---|
| Posterior retropulsion of bony fragments or a concave posterior border of the vertebral body | Expansile convex posterior cortex |
| Normal marrow signal intensity (or a well-demarcated regular margin separating the spared marrow and abnormal marrow within the fractured vertebra) | Reduced signal intensity on T1-weighted imaging reflecting an underlying marrow replacing process, particularly if the posterior elements are involved |
| Remains isointense post-contrast imaging | Heterogeneously increased enhancement of the vertebral body |
| Usually without involvement of the posterior vertebral elements | Involvement of the posterior elements |
| Presence of multiple compression fractures (with the notable exception of multiple myeloma) | Presence of other spinal metastasis |
| Presence of a T1-weighted and T2-weighted hypointense band (thought to represent cancellous bone compaction, fluid or gas-filled clefts) | Abnormal epidural or paraspinal soft tissue or enhancement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuah, T.; Vellayappan, B.A.; Makmur, A.; Nair, S.; Song, J.; Tan, J.H.; Kumar, N.; Quek, S.T.; Hallinan, J.T.P.D. State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression. Cancers 2022, 14, 3289. https://doi.org/10.3390/cancers14133289
Kuah T, Vellayappan BA, Makmur A, Nair S, Song J, Tan JH, Kumar N, Quek ST, Hallinan JTPD. State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression. Cancers. 2022; 14(13):3289. https://doi.org/10.3390/cancers14133289
Chicago/Turabian StyleKuah, Tricia, Balamurugan A. Vellayappan, Andrew Makmur, Shalini Nair, Junda Song, Jiong Hao Tan, Naresh Kumar, Swee Tian Quek, and James Thomas Patrick Decourcy Hallinan. 2022. "State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression" Cancers 14, no. 13: 3289. https://doi.org/10.3390/cancers14133289







