Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
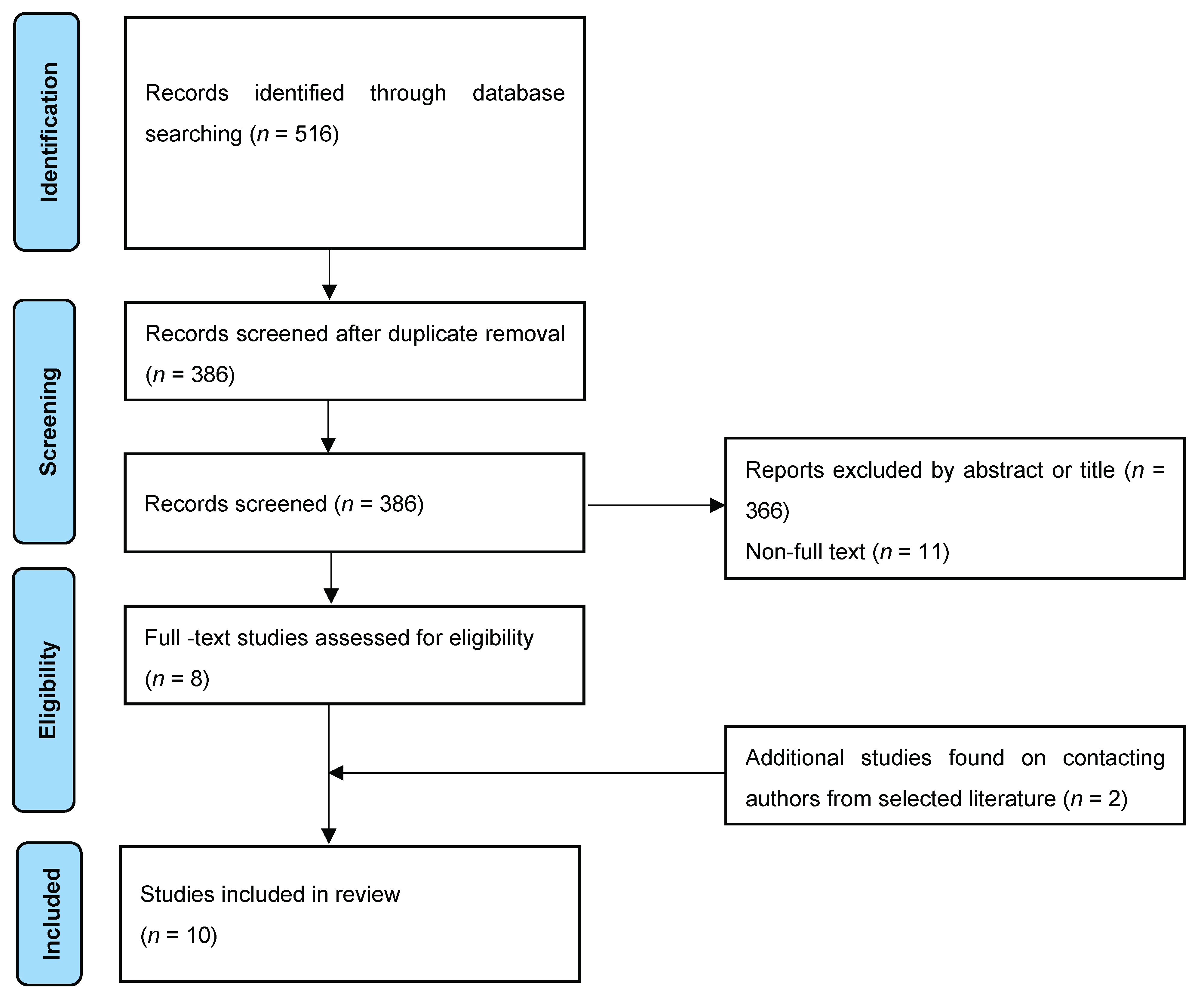
2. Evidence Acquisition
2.1. Study Design
2.2. Literature Search
2.3. Study Selection
2.4. Data Collection
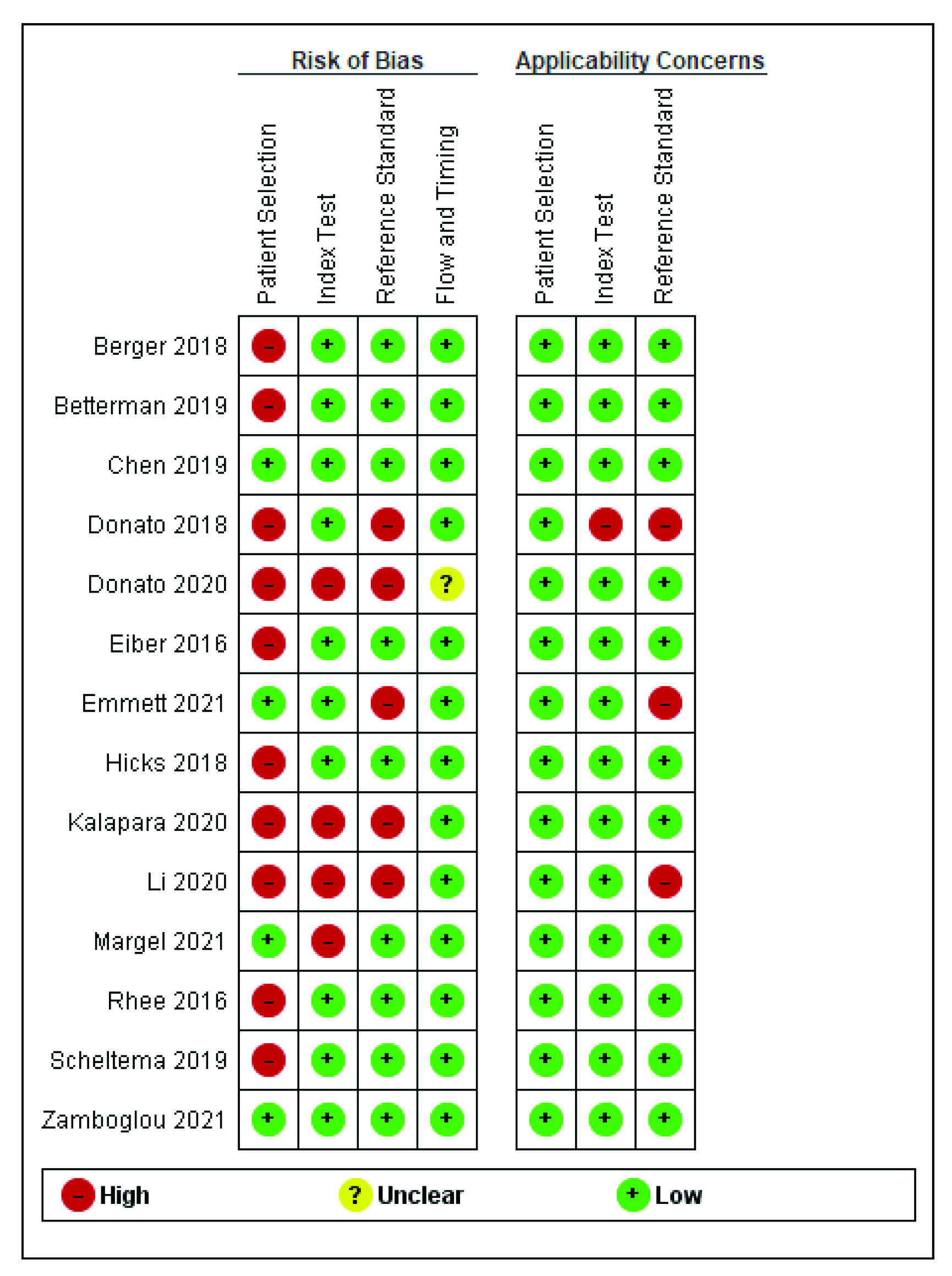
2.5. Quality Assessment
2.6. Data Synthesis
2.7. Meta-Analysis
3. Evidence Synthesis
3.1. Study Characteristics
3.2. Meta-Analysis
3.2.1. Per-Patient Analysis
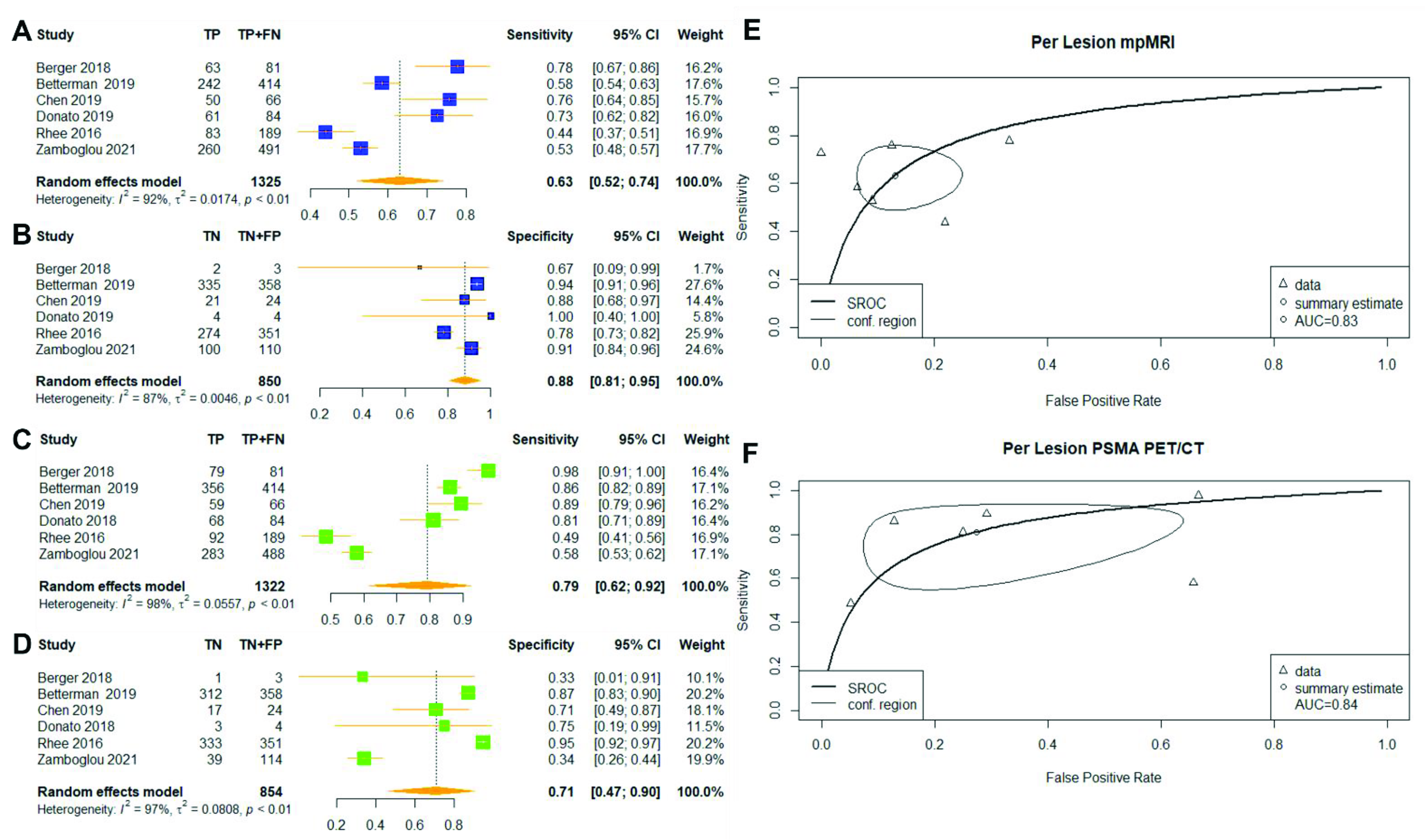
3.2.2. Per-Lesion Analysis
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic Accuracy of Multi-Parametric MRI and TRUS Biopsy in Prostate Cancer (PROMIS): A Paired Validating Confirmatory Study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, S.; Singh, H.; Kumar, R.; Mittal, B.R. Diagnostic Accuracy of 68Ga-PSMA PET/CT for Initial Detection in Patients with Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2021, 216, 599–607. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in PSMA Positron Emission Tomography (PET) of Prostate Cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol. Oncol. Semin. Orig. Investig. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.; Gabe, R.; Padhani, A.R.; Rockall, A.; Sokhi, H.; Tam, H.; Winkler, M.; Ahmed, H.U. A Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Biparametric Prostate MRI for Prostate Cancer in Men at Risk. Prostate Cancer Prostatic Dis. 2020, 1–16. [Google Scholar] [CrossRef]
- Radtke, J.P.; Kuru, T.H.; Boxler, S.; Alt, C.D.; Popeneciu, I.V.; Huettenbrink, C.; Klein, T.; Steinemann, S.; Bergstraesser, C.; Roethke, M.; et al. Comparative Analysis of Transperineal Template Saturation Prostate Biopsy versus Magnetic Resonance Imaging Targeted Biopsy with Magnetic Resonance Imaging-Ultrasound Fusion Guidance. J. Urol. 2015, 193, 87–94. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-Specific Membrane Antigen PET-CT in Patients with High-Risk Prostate Cancer before Curative-Intent Surgery or Radiotherapy (ProPSMA): A Prospective, Randomised, Multicentre Study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Zhao, Y.; Morka, N.; Simpson, B.S.S.; Freeman, A.; Kirkham, A.; Kelly, D.; Whitaker, H.C.; Emberton, M.; Norris, J.M. Prostate-Specific Membrane Antigen Positron Emission Tomography Compared to Multiparametric MRI for Prostate Cancer Diagnosis: A Protocol for a Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e052277. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Parry, M.A.; Allen, C.; Ball, R.; Freeman, A.; Kelly, D.; Kim, H.L.; Kirkham, A.; You, S.; et al. Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric Magnetic Resonance Imaging: A Systematic Review and Bioinformatic Analysis. Eur. Urol. Open Sci. 2020, 20, 37–47. [Google Scholar] [CrossRef]
- Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric MRI: A Protocol for a Systematic Review and Bioinformatic Analysis. BMJ Open 2020, 10, e034611. Available online: https://bmjopen.bmj.com/content/10/1/e034611.abstract (accessed on 1 March 2021). [CrossRef] [PubMed] [Green Version]
- Berger, I.; Annabattula, C.; Lewis, J.; Shetty, D.V.; Kam, J.; Maclean, F.; Arianayagam, M.; Canagasingham, B.; Ferguson, R.; Khadra, M.; et al. 68 Ga-PSMA PET/CT vs. MpMRI for Locoregional Prostate Cancer Staging: Correlation with Final Histopathology. Prostate Cancer Prostatic Dis. 2018, 21, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bettermann, A.S.; Zamboglou, C.; Kiefer, S.; Jilg, C.A.; Spohn, S.; Kranz-Rudolph, J.; Fassbender, T.F.; Bronsert, P.; Nicolay, N.H.; Gratzke, C.; et al. [68Ga-]PSMA-11 PET/CT and Multiparametric MRI for Gross Tumor Volume Delineation in a Slice by Slice Analysis with Whole Mount Histopathology as a Reference Standard—Implications for Focal Radiotherapy Planning in Primary Prostate Cancer. Radiother. Oncol. 2019, 141, 214–219. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Q.; Zhang, C.; Zhao, X.; Marra, G.; Gao, J.; Lv, X.; Zhang, B.; Fu, Y.; Wang, F.; et al. Combination of 68Ga-PSMA PET/CT and Multiparametric MRI Improves the Detection of Clinically Significant Prostate Cancer: A Lesion-by-Lesion Analysis. J. Nucl. Med. 2019, 60, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Donato, P.; Roberts, M.J.; Morton, A.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; Yaxley, J. Improved Specificity with 68Ga PSMA PET/CT to Detect Clinically Significant Lesions “Invisible” on Multiparametric MRI of the Prostate: A Single Institution Comparative Analysis with Radical Prostatectomy Histology. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 20–30. [Google Scholar] [CrossRef]
- Donato, P.; Morton, A.; Yaxley, J.; Ranasinghe, S.; Teloken, P.E.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT Better Characterises Localised Prostate Cancer after MRI and Transperineal Prostate Biopsy: Is 68Ga-PSMA PET/CT Guided Biopsy the Future? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1843–1851. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-Specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Kalapara, A.A.; Nzenza, T.; Pan, H.Y.C.; Ballok, Z.; Ramdave, S.; O’Sullivan, R.; Ryan, A.; Cherk, M.; Hofman, M.S.; Konety, B.R.; et al. Detection and Localisation of Primary Prostate Cancer Using 68 Gallium Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography Compared with Multiparametric Magnetic Resonance Imaging and Radical Prostatectomy Specimen Pathology. Br. J. Urol. 2020, 126, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Wu, P.; Ren, J.; Ma, S.; Zhang, J.; Song, W.; Lin, X.; Jiao, D.; Shi, S.; et al. Comparison of 68Ga-PSMA-617 PET/CT with MpMRI for the Detection of PCa in Patients with a PSA Level of 4–20 Ng/Ml before the Initial Biopsy. Sci. Rep. 2020, 10, 10963. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Thomas, P.; Shepherd, B.; Gustafson, S.; Vela, I.; Russell, P.J.; Nelson, C.; Chung, E.; Wood, G.; Malone, G.; et al. Prostate Specific Membrane Antigen Positron Emission Tomography May Improve the Diagnostic Accuracy of Multiparametric Magnetic Resonance Imaging in Localized Prostate Cancer. J. Urol. 2016, 196, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Kramer, M.; Kiefer, S.; Bronsert, P.; Ceci, L.; Sigle, A.; Schultze-Seemann, W.; Jilg, C.A.; Sprave, T.; Fassbender, T.F.; et al. The Impact of the Co-Registration Technique and Analysis Methodology in Comparison Studies between Advanced Imaging Modalities and Whole-Mount-Histology Reference in Primary Prostate Cancer. Sci. Rep. 2021, 11, 5836. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.M.; Simko, J.P.; Westphalen, A.C.; Nguyen, H.G.; Greene, K.L.; Zhang, L.; Carroll, P.R.; Hope, T.A. Diagnostic Accuracy of 68Ga-PSMA-11 PET/MRI Compared with Multiparametric MRI in the Detection of Prostate Cancer. Radiology 2018, 289, 730–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Chang, J.I.; Stricker, P.D.; van Leeuwen, P.J.; Nguyen, Q.A.; Ho, B.; Delprado, W.; Lee, J.; Thompson, J.E.; Cusick, T.; et al. Diagnostic Accuracy of 68Ga-Prostate-Specific Membrane Antigen (PSMA) Positron-Emission Tomography (PET) and Multiparametric (Mp)MRI to Detect Intermediate-Grade Intra-Prostatic Prostate Cancer Using Whole-Mount Pathology: Impact of the Addition of 68Ga-PSMA PET to MpMRI. BJU Int. 2019, 124, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; You, S.; Nguyen, C.; Wang, Y.; Kim, J.; Sirohi, D.; Ziembiec, A.; Luthringer, D.; Lin, S.-C.; Daskivich, T.; et al. Genes Involved in Prostate Cancer Progression Determine MRI Visibility. Theranostics 2018, 8, 1752–1765. [Google Scholar] [CrossRef]
- Zhen, L.; Liu, X.; Yegang, C.; Yongjiao, Y.; Yawei, X.; Jiaqi, K.; Xianhao, W.; Yuxuan, S.; Rui, H.; Wei, Z.; et al. Accuracy of Multiparametric Magnetic Resonance Imaging for Diagnosing Prostate Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 1244. [Google Scholar] [CrossRef] [Green Version]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Stavrinides, V.; Syer, T.; Hu, Y.; Giganti, F.; Freeman, A.; Karapanagiotis, S.; Bott, S.R.J.; Brown, L.C.; Burns-Cox, N.; Dudderidge, T.J.; et al. False Positive Multiparametric Magnetic Resonance Imaging Phenotypes in the Biopsy-Naïve Prostate: Are They Distinct from Significant Cancer-Associated Lesions? Lessons from PROMIS. Eur. Urol. 2021, 79, 20–29. [Google Scholar] [CrossRef]
- Litjens, G.J.S.; Elliott, R.; Shih, N.N.C.; Feldman, M.D.; Kobus, T.; Hulsbergen-van de Kaa, C.; Barentsz, J.O.; Huisman, H.J.; Madabhushi, A. Computer-Extracted Features Can Distinguish Non-cancerous Confounding Disease from Prostatic Adenocarcinoma at Multiparametric MR Imaging. Radiology 2016, 278, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Prabhu, V.; Li, J.; Babb, J.S.; Taneja, S.S.; Rosenkrantz, A.B. Prostate Cancer: Diffusion-Weighted MR Imaging for Detection and Assessment of Aggressiveness-Comparison between Conventional and Kurtosis Models. Radiology 2017, 284, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic Diseases on PSMA PET Imaging: A Spectrum of Benign and Malignant Findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, Z.; Yu, H.; Wang, Y.; Zhang, Y.; Song, B. Comparison of PET/MRI with Multiparametric MRI in Diagnosis of Primary Prostate Cancer: A Meta-Analysis. Eur. J. Radiol. 2019, 113, 225–231. [Google Scholar] [CrossRef]
- Matsumoto, K.; Omura, M.; Takeda, T.; Kosaka, T.; Hashiguchi, A.; Takamatsu, K.; Yasumizu, Y.; Tanaka, N.; Morita, S.; Mizuno, R.; et al. Grading of Multifocal Prostate Cancer Cases in Which the Largest Volume and the Highest Grade Do not Coincide within One Lesion. J. Urol. 2021, 206, 338–345. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; Dal Moro, F.; Zucchetta, P. PET/MRI in Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 859–873. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primers 2021, 7, 1–27. [Google Scholar] [CrossRef]
- Zamboglou, C.; Sachpazidis, I.; Koubar, K.; Drendel, V.; Wiehle, R.; Kirste, S.; Mix, M.; Schiller, F.; Mavroidis, P.; Meyer, P.T.; et al. Evaluation of Intensity Modulated Radiation Therapy Dose Painting for Localized Prostate Cancer Using 68Ga-HBED-CC PSMA-PET/CT: A Planning Study Based on Histopathology Reference. Radiother. Oncol. 2017, 123, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Chan, T.Y.; Partin, A.W.; Walsh, P.C.; Epstein, J.I. Prognostic Significance of Gleason Score 3+4 versus Gleason Score 4+3 Tumor at Radical Prostatectomy. Urology 2000, 56, 823–827. [Google Scholar] [CrossRef]
- van den Bergh, R.C.N.; Roemeling, S.; Roobol, M.J.; Aus, G.; Hugosson, J.; Rannikko, A.S.; Tammela, T.L.; Bangma, C.H.; Schröder, F.H. Gleason Score 7 Screen-Detected Prostate Cancers Initially Managed Expectantly: Outcomes in 50 Men. BJU Int. 2009, 103, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Egevad, L.; Delahunt, B.; Srigley, J.R.; Samaratunga, H. International Society of Urological Pathology (ISUP) Grading of Prostate Cancer—An ISUP Consensus on Contemporary Grading. APMIS 2016, 124, 433–435. [Google Scholar] [CrossRef] [PubMed]
- van Leenders, G.J.L.H.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Jo, N.; Bamashmos, A.S.; Ahmed, M.; Sun, J.; Ward, J.F.; Choi, H. Accuracy of Prostate Magnetic Resonance Imaging: Reader Experience Matters. Eur. Urol. Open Sci. 2021, 27, 53–60. [Google Scholar] [CrossRef]
- Muller, B.G.; Shih, J.H.; Sankineni, S.; Marko, J.; Rais-Bahrami, S.; George, A.K.; de la Rosette, J.J.M.C.H.; Merino, M.J.; Wood, B.J.; Pinto, P.; et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology 2015, 277, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Sonn, G.A.; Fan, R.E.; Ghanouni, P.; Wang, N.N.; Brooks, J.D.; Loening, A.M.; Daniel, B.L.; To’o, K.J.; Thong, A.E.; Leppert, J.T. Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. Eur. Urol. Focus 2019, 5, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Giannarini, G.; Valotto, C.; Girometti, R.; Dal Moro, F.; Briganti, A.; Padhani, A.R. Measuring the Quality of Diagnostic Prostate Magnetic Resonance Imaging: A Urologist’s Perspective. Eur. Urol. 2021, 79, 440–441. [Google Scholar] [CrossRef]
- Giganti, F.; Kasivisvanathan, V.; Kirkham, A.; Punwani, S.; Emberton, M.; Moore, C.M.; Allen, C. Prostate MRI Quality: A Critical Review of the Last 5 Years and the Role of the PI-QUAL Score. BJR 2022, 95, 20210415. [Google Scholar] [CrossRef]
- Giganti, F.; Lindner, S.; Piper, J.W.; Kasivisvanathan, V.; Emberton, M.; Moore, C.M.; Allen, C. Multiparametric Prostate MRI Quality Assessment Using a Semi-Automated PI-QUAL Software Program. Eur. Radiol. Exp. 2021, 5, 48. [Google Scholar] [CrossRef]
- Toriihara, A.; Nobashi, T.; Baratto, L.; Duan, H.; Moradi, F.; Park, S.; Hatami, N.; Aparici, C.M.; Davidzon, G.; Iagaru, A. Comparison of 3 Interpretation Criteria for 68Ga-PSMA11 PET Based on Inter- and Intrareader Agreement. J. Nucl. Med. 2020, 61, 533–539. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM Standardized Reporting Guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen–Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Salami, S.S.; Kaplan, J.B.; Nallandhighal, S.; Takhar, M.; Tosoian, J.J.; Lee, M.; Yoon, J.; Hovelson, D.H.; Plouffe, K.R.; Kaffenberger, S.D.; et al. Biologic Significance of Magnetic Resonance Imaging Invisibility in Localized Prostate Cancer. JCO Precis. Oncol. 2019, 3, PO.19.00054. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Rosenkrantz, A.B.; Taneja, S.S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for Prostate Cancer Diagnosis: Current Status and Future Directions. Nat. Rev. Urol. 2020, 17, 41–61. [Google Scholar] [CrossRef]
- Will, L.; Sonni, I.; Kopka, K.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. Radiolabeled Prostate-Specific Membrane Antigen Small-Molecule Inhibitors. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 168–180. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-Specific Membrane Antigen Cleavage of Vitamin B9 Stimulates Oncogenic Signaling through Metabotropic Glutamate Receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [Green Version]

| Authors | Year | Ref | No. of Patients | No. of Lesions | MRI Imaging Power | PI-RADS Version | mpMRI Positivity Criteria | PSMA PET Scoring System | PSMA Tracer | PSMA Tracer Injected (MBq) | PSMA PET/CT Positivity Criteria | Mean Age (yrs) | Mean PSA Value (ng/dL) | Reference Standard | Clinically Significant Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berger | 2018 | [14] | 50 | 84 | 3T | v2.0 | PI-RAD ≥ 3 | SUVmax | 68Ga-PSMA-11 | - | SUVmax > 2.5 | 64.9 (59.3–70.5) | 10.6 (2.5–18.7) | WMP | ISUP ≥ 1 |
| Bettermann | 2019 | [15] | 17 | 193 (772 quadrants) | 3T/1.5T | v2.0 | PI-RADS ≥ 3 | Uptake against background | 68Ga-PSMA-11 | 172 (138–206) | Uptake superior to the background activity in >1 slice | 67 (48–76) | 17.4 (6.01–218.0) | WMP | Lesions extending > 3 mm into another quadrant |
| Chen | 2019 | [16] | 54 | 90 | 3T | v2.0 | PI-RADS ≥ 4 | MI-ES Score | 68Ga-PSMA-11 | 131.7 (130.6–177.6) | MI-ES ≥ 2 | 69 (55–84) | 13.53 (4.04–110.00) | WMP | Cancer volume ≥ 0.5 cm3/GS ≥ 3 + 4 /Stage ≥ pT3 |
| Donato 2019 | 2019 | [17] | 58 | 88 | 3T | v2.0 | PI-RADS ≥ 3 | 3-point Likert Scale a | 68Ga-PSMA-11 | 150.0 (142.5–157.5) | SUVmax > 5 (Equivocal) | 65.5 (60–68) | 7.35 (5.6–12) | WMP | GS ≥ 3 + 4 |
| Donato 2020 | 2020 | [18] | 144 | - | 3T | v2.0 | PI-RADS ≥ 3 | 3-point Likert Scale a | 68Ga-PSMA-11 | 150.0 (142.5–157.5) | >Equivocal | 66.5 (61.7–71.25) | 8.6 (6–12.25) | Ultrasound-guided transperineal targeted biopsies | GS ≥ 3 + 4 |
| Emmett | 2021 | [19] | 291 | - | 3T/1.5T | v2.0 | PI-RADS ≥ 3 | 4-point Certainty Scale b | 68Ga-PSMA-11 | 1.8–2.2 MBq/kg | Positive (Probably/Definite) | 64.0 (58.7–69.9) | 5.6 (4.2–7.5) | Systematic transperineal biopsies | ISUP ≥ 2 |
| Kalapara | 2020 | [20] | 205 | - | 3T | v2.0 | PI-RAD ≥ 3 | Binary Scale | 68Ga-PSMA-11 | 1.8–2.2 MBq/kg | Lesion with the highest avidity by SUVmax | 67 (61–72) | 7.18 (4.90–10.20) | WMP | ISUP ≥ 3 |
| Li | 2020 | [21] | 67 | - | 3T | v2.0 | PI-RAD ≥ 3 | Uptake against background | 68Ga-PSMA-617 | 111–185 | Uptake superior to the background activity | 68 (42–85) | 10.48 (3.15–19.76) | Transrectal ultrasound biopsy | GS ≥ 7 |
| Rhee | 2016 | [22] | 22 | 71 (540 segments) | 3T | v1.0 | PI-RAD ≥ 3 | Uptake against background | 68Ga-PSMA-11 | 150 | Uptake superior to the background activity | 62 (55–69) | 6.1 (0–14.6) | WMP | GS ≥ 4 + 3 +/− tumour size ≥ 6 mm |
| Zamboglou | 2021 | [23] | 10 | 14 (601 segments) * | 3T | v2.1 | PI-RAD ≥ 3 | Uptake against background | [18F]PSMA-1007 | 310 (249–370) | Uptake superior to the background activity | - | - | WMP | GS ≥ 7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Simpson, B.S.; Morka, N.; Freeman, A.; Kirkham, A.; Kelly, D.; Whitaker, H.C.; Emberton, M.; Norris, J.M. Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3497. https://doi.org/10.3390/cancers14143497
Zhao Y, Simpson BS, Morka N, Freeman A, Kirkham A, Kelly D, Whitaker HC, Emberton M, Norris JM. Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(14):3497. https://doi.org/10.3390/cancers14143497
Chicago/Turabian StyleZhao, Yi, Benjamin S. Simpson, Naomi Morka, Alex Freeman, Alex Kirkham, Daniel Kelly, Hayley C. Whitaker, Mark Emberton, and Joseph M. Norris. 2022. "Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis" Cancers 14, no. 14: 3497. https://doi.org/10.3390/cancers14143497
APA StyleZhao, Y., Simpson, B. S., Morka, N., Freeman, A., Kirkham, A., Kelly, D., Whitaker, H. C., Emberton, M., & Norris, J. M. (2022). Comparison of Multiparametric Magnetic Resonance Imaging with Prostate-Specific Membrane Antigen Positron-Emission Tomography Imaging in Primary Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis. Cancers, 14(14), 3497. https://doi.org/10.3390/cancers14143497







