Positive Correlation of Peripheral CD8+ T Lymphocytes with Immune-Related Adverse Events and Combinational Prognostic Value in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Study Assessment
2.3. Statistical Analysis
3. Results
3.1. Profiles of IrAEs
3.2. Association of CD8+ T Lymphocytes at Baseline with the Occurrence of IrAEs
3.3. Factors Predictive of Clinical Outcomes
3.4. Association among Baseline CD8+ T Lymphocytes, IrAEs and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. SEER Cancer Statistics Review 1975–2018. Available online: https://seer.cancer.gov/csr/1975_2018/ (accessed on 15 April 2021).
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zang, X.Y.; Wang, J.C.; Huang, S.S.; Xu, J.; Zhang, P. Diagnosis and Management of Immune Related Adverse Events (irAEs) in Cancer Immunotherapy. Biomed. Pharmacother. 2019, 120, 109437. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Rao, B.; Dong, A.H.; Liang, W.; Zhu, J.X.; Qin, M.P.; Huang, W.W.; Lu, J.M.; Li, Z.F.; et al. Landscape of immune checkpoint inhibitor-related adverse events in Chinese population. Sci. Rep. 2020, 10, 15567. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Emens, L.; Loi, S.; Rugo, H.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.; Nechaeva, M.; Molinero, L.; Nguyen Duc, A.; et al. Abstract GS1-04: IMpassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer. Cancer Res. 2019, 79, GS1-04. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zeng, D.; Ou, Q.; Liu, S.; Li, A.; Chen, Y.; Lin, D.; Gao, Q.; Zhou, H.; Liao, W.; et al. Association of Survival and Immune-Related Biomarkers with Immunotherapy in Patients with Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw. Open 2019, 2, e196879. [Google Scholar] [CrossRef] [Green Version]
- Manjarrez-Orduño, N.; Menard, L.C.; Kansal, S.; Fischer, P.; Kakrecha, B.; Jiang, C.; Cunningham, M.; Greenawalt, D.; Patel, V.; Yang, M.; et al. Circulating T Cell Subpopulations Correlate With Immune Responses at the Tumor Site and Clinical Response to PD1 Inhibition in Non-Small Cell Lung Cancer. Front. Immunol. 2018, 9, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Fu, Y.; Zhu, B.; Wang, J.; Zhang, B. Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities. Front. Immunol. 2020, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab Versus Docetaxel in Previously Treated Patients with Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes from Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, F.; Zhao, C.; Cheng, L.; Zhou, C.; Qiao, M.; Li, X.; Chen, X. Interleukin-10 Is a Promising Marker for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving Immunotherapy. Front. Immunol. 2022, 13, 840313. [Google Scholar] [CrossRef]
- Maekura, T.; Naito, M.; Tahara, M.; Ikegami, N.; Kimura, Y.; Sonobe, S.; Kobayashi, T.; Tsuji, T.; Minomo, S.; Tamiya, A.; et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo 2017, 31, 1035–1039. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Suazo-Zepeda, E.; Bokern, M.; Vinke, P.C.; Hiltermann, T.J.N.; de Bock, G.H.; Sidorenkov, G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021, 70, 3069–3080. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Aparicio, A.; Gao, J.; Zurita, A.J.; Araujo, J.C.; Logothetis, C.J.; Tahir, S.A.; Korivi, B.R.; Slack, R.S.; Vence, L.; et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl. Acad. Sci. USA 2016, 113, 11919–11924. [Google Scholar] [CrossRef] [Green Version]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e622. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Naidoo, J.; Zhong, Q.; Xiong, Y.; Mammen, J.; de Flores, M.V.; Cappelli, L.; Balaji, A.; Palmer, T.; Forde, P.M.; et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019, 129, 4305–4315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zen, Y.; Yeh, M.M. Hepatotoxicity of immune checkpoint inhibitors: A histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 2018, 31, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, G.M.; Zhang, L.; Zhang, H.; Kazandjian, D.; Khozin, S.; Tang, S.; Goldberg, K.; Sridhara, R.; Keegan, P.; Pazdur, R. Milestone Analyses of Immune Checkpoint Inhibitors, Targeted Therapy, and Conventional Therapy in Metastatic Non-Small Cell Lung Cancer Trials: A Meta-analysis. JAMA Oncol. 2017, 3, e171029. [Google Scholar] [CrossRef]

| Characteristics | Subsets | No. | % |
|---|---|---|---|
| Gender | Male | 88 | 80.7% |
| Female | 21 | 19.3% | |
| Age (years) | Median | 65 | |
| Range | 36–85 | ||
| ECOG PS score | 0–1 | 92 | 84.4% |
| 2 | 17 | 15.6% | |
| Smoking status | Current/former | 63 | 57.8% |
| Never | 46 | 42.2% | |
| Histology | Adenocarcinoma | 60 | 55.0% |
| Squamous carcinoma | 46 | 42.2% | |
| Large cell carcinoma | 3 | 2.8% | |
| Stage | Ⅳ | 102 | 93.6% |
| ⅢB | 7 | 6.4% | |
| PD-L1 states test | ≥50% | 23 | 21.1% |
| 1–49% | 33 | 30.3% | |
| <1% | 17 | 15.6% | |
| Unknown | 36 | 33.0% | |
| Driver gene alteration | EGFR/ALK/ROS1 positive | 9 | 8.3% |
| Negative/unknown | 100 | 91.7% | |
| Treatment line | First | 41 | 37.6% |
| Second | 39 | 35.8% | |
| Third | 15 | 13.8% | |
| Fourth or later line | 14 | 12.8% | |
| Combination treatment | Yes | 63 | 57.8% |
| No | 46 | 42.2% |
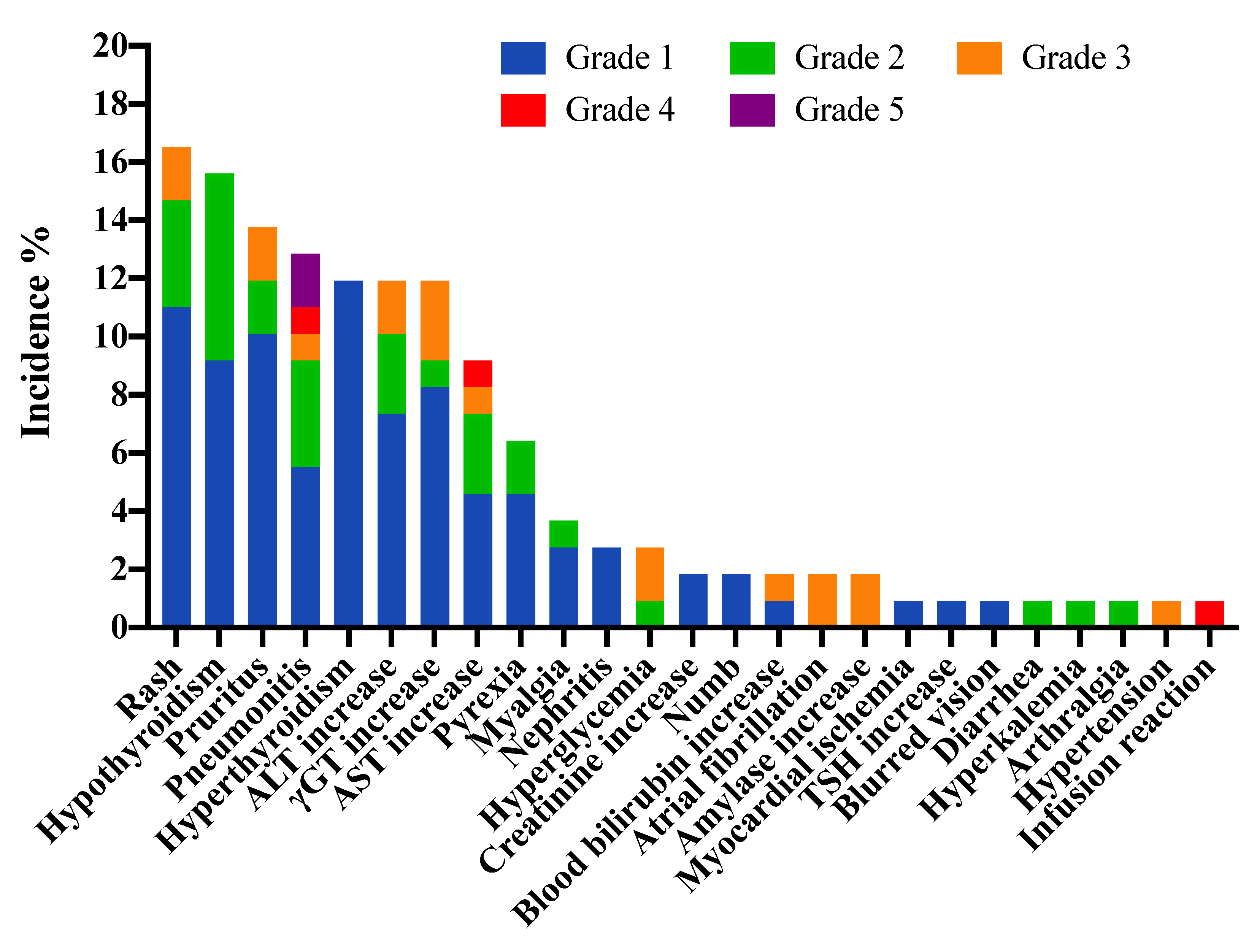
| IrAEs Category | No. of Patients, n = 109 | No. of Resolved Events, n = 148, (%) | |
|---|---|---|---|
| Any Grade, n (%) | Grade 3–5, n (%) | ||
| Any irAEs | 55 (50.5%) | 16 (14.7%) | 104 (70.3%) |
| Single site irAEs | 17 (15.6%) | / | |
| Multiple site irAEs | 38 (34.9%) | / | |
| Pulmonary | |||
| Pneumonitis | 14 (12.8%) | 4 (3.7%) | 8 (57.1%) |
| Cardiovascular | |||
| Myocardial ischemia | 1 (0.9%) | 0 | 1 (100.0%) |
| Atrial fibrillation | 2 (1.8%) | 2 (1.8%) | 1 (50.0%) |
| Hypertension | 1 (0.9%) | 1 (0.9%) | 0 |
| Gastrointestinal | |||
| Diarrhea | 1 (0.9%) | 0 | 1 (100.0%) |
| Amylase increase | 2 (1.8%) | 2 (1.8%) | 1 (50.0%) |
| Hepatic | |||
| ALT increase | 13 (11.9%) | 2 (1.8%) | 11 (84.6%) |
| AST increase | 10 (9.2%) | 2 (1.8%) | 7 (70.0%) |
| γGT increase | 13 (11.9%) | 3 (2.8%) | 10 (76.9%) |
| Blood bilirubin increase | 2 (1.8%) | 1 (0.9%) | 2 (100.0%) |
| Renal | |||
| Nephritis | 3 (2.8%) | 0 | 1 (33.3%) |
| Creatinine increase | 2 (1.8%) | 0 | 1 (50.0%) |
| Hyperkalemia | 1 (0.9%) | 0 | 1 (100.0%) |
| Musculoskeletal | |||
| Myalgia | 4 (3.7%) | 0 | 3 (75.0%) |
| Arthralgia | 1 (0.9%) | 0 | 1 (100.0%) |
| Endocrine | |||
| Hypothyroidism | 17 (15.6%) | 0 | 4 (23.5%) |
| TSH increase | 1 (0.9%) | 0 | 1 (100.0%) |
| Hyperthyroidism | 13 (11.9%) | 0 | 12 (92.3%) |
| Hyperglycemia | 3 (2.8%) | 2 (1.8%) | 1 (33.3%) |
| Skin | |||
| Rash | 18 (16.5%) | 2 (1.8%) | 15 (83.3%) |
| Pruritus | 15 (13.8%) | 2 (1.8%) | 13 (86.7%) |
| Eye | |||
| Blurred vision | 1 (0.9%) | 0 | 1 (100.0%) |
| Neurology | |||
| Numb | 2 (1.8%) | 0 | 0 |
| Others | |||
| Infusion reaction | 1 (0.9%) | 1 (0.9%) | 1 (100.0%) |
| Pyrexia | 7 (6.4%) | 0 | 7 (100.0%) |
| Variable | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Gender | Male | 1.151 | 0.444–2.985 | 0.772 | |||
| Age (years) | ≥65 years | 1.292 | 0.606–2.752 | 0.507 | |||
| Histology | Non-adenocarcinoma | 1.630 | 0.762–3.487 | 0.208 | |||
| ECOG PS score | 2 | 0.350 | 0.114–1.074 | 0.066 | 0.355 | 0.109–1.163 | 0.087 |
| Smoking status | Former/current | 0.656 | 0.305–1.410 | 0.280 | |||
| Treatment line | ≥Second | 0.593 | 0.271–1.299 | 0.192 | |||
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 0.253 | 0.050–1.280 | 0.097 | 0.308 | 0.055–1.709 | 0.178 |
| Combination treatment | Yes | 0.833 | 0.389–1.784 | 0.639 | |||
| PD-L1 states test | ≥50% | 1.362 | 0.539–3.440 | 0.513 | |||
| CD4+ T lymphocytes | ≥266 M/L | 1.392 | 0.655–2.957 | 0.390 | |||
| CD8+ T lymphocytes | ≥288 M/L | 2.975 | 1.365–6.484 | 0.006 | 2.953 | 1.324–6.587 | 0.008 |
| Regulatory T lymphocytes | ≥17 M/L | 0.773 | 0.364–1.640 | 0.502 | |||
| (A) | |||||||
| Variable | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Gender | Male | 1.091 | 0.584–2.040 | 0.785 | |||
| Age (years) | ≥65 years | 0.975 | 0.612–1.551 | 0.913 | |||
| Histology | Non-adenocarcinoma | 1.114 | 0.700–1.774 | 0.648 | |||
| ECOG PS score | 2 | 2.146 | 1.208–3.812 | 0.009 | 1.882 | 1.036–3.420 | 0.038 |
| Smoking status | Former/current | 1.284 | 0.793–2.080 | 0.309 | |||
| Treatment line | ≥Second | 3.211 | 1.857–5.551 | <0.001 | 3.479 | 1.906–6.349 | <0.001 |
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 1.934 | 0.831–4.500 | 0.126 | |||
| Combination treatment | Yes | 0.636 | 0.385–1.053 | 0.078 | 0.638 | 0.369–1.102 | 0.107 |
| PD-L1 states test | ≥50% | 0.877 | 0.714–1.078 | 0.213 | |||
| CD4+ T lymphocytes | ≥266 M/L | 0.850 | 0.533–1.356 | 0.496 | |||
| CD8+ T lymphocytes | ≥288 M/L | 0.386 | 0.237–0.629 | <0.001 | 0.364 | 0.217–0.612 | <0.001 |
| Regulatory T lymphocytes | ≥17 M/L | 0.911 | 0.572–1.452 | 0.696 | |||
| IrAEs | Yes | 0.368 | 0.227–0.596 | <0.001 | 0.344 | 0.204–0.578 | <0.001 |
| (B) | |||||||
| Variable | Category | Univariate | Multivariate | ||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Gender | Male | 1.379 | 0.615–3.094 | 0.436 | |||
| Age (years) | ≥65 years | 0.958 | 0.548–1.673 | 0.879 | |||
| Histology | Non-adenocarcinoma | 1.095 | 0.627–1.910 | 0.750 | |||
| ECOG PS score | 2 | 1.948 | 1.004–3.781 | 0.049 | 1.596 | 0.796–3.200 | 0.188 |
| Smoking status | Former/current | 1.248 | 0.692–2.252 | 0.462 | |||
| Treatment line | ≥Second | 2.413 | 1.234–4.715 | 0.010 | 1.952 | 0.977–3.902 | 0.058 |
| Driver gene alterlation | EGFR/ALK/ROS1 positive | 2.474 | 1.042–5.872 | 0.040 | 1.890 | 0.775–4.609 | 0.162 |
| Combination treatment | Yes | 0.693 | 0.367–1.310 | 0.259 | |||
| PD-L1 states test | ≥50% | 0.883 | 0.702–1.110 | 0.287 | |||
| CD4+ T lymphocytes | ≥266 M/L | 0.674 | 0.385–1.178 | 0.166 | |||
| CD8+ T lymphocytes | ≥288 M/L | 0.485 | 0.273–0.862 | 0.014 | 0.647 | 0.348–1.202 | 0.169 |
| Regulatory T lymphocytes | ≥17 M/L | 0.655 | 0.373–1.149 | 0.140 | |||
| IrAEs | Yes | 0.439 | 0.247–0.782 | 0.005 | 0.599 | 0.320–1.120 | 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Xia, B.; Zhang, J.; Li, X.; Yang, S.; Zhang, M.; Zhu, L.; Wang, B.; Xu, X.; Ma, S.; et al. Positive Correlation of Peripheral CD8+ T Lymphocytes with Immune-Related Adverse Events and Combinational Prognostic Value in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Cancers 2022, 14, 3568. https://doi.org/10.3390/cancers14153568
Wu K, Xia B, Zhang J, Li X, Yang S, Zhang M, Zhu L, Wang B, Xu X, Ma S, et al. Positive Correlation of Peripheral CD8+ T Lymphocytes with Immune-Related Adverse Events and Combinational Prognostic Value in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Cancers. 2022; 14(15):3568. https://doi.org/10.3390/cancers14153568
Chicago/Turabian StyleWu, Kan, Bing Xia, Jing Zhang, Xin Li, Shaoyu Yang, Minna Zhang, Lucheng Zhu, Bing Wang, Xiao Xu, Shenglin Ma, and et al. 2022. "Positive Correlation of Peripheral CD8+ T Lymphocytes with Immune-Related Adverse Events and Combinational Prognostic Value in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors" Cancers 14, no. 15: 3568. https://doi.org/10.3390/cancers14153568
APA StyleWu, K., Xia, B., Zhang, J., Li, X., Yang, S., Zhang, M., Zhu, L., Wang, B., Xu, X., Ma, S., & Chen, X. (2022). Positive Correlation of Peripheral CD8+ T Lymphocytes with Immune-Related Adverse Events and Combinational Prognostic Value in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Cancers, 14(15), 3568. https://doi.org/10.3390/cancers14153568






