N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Melanoma Cells and Keratinocytes
2.2. Mice and Orthotopic Xenografts
2.3. NAC Administration to Tumor-Bearing Mice
2.4. Determination of NAC, Cys and Cystine Levels in Plasma
2.5. Isolation of Melanoma Cells Using Enzymatic Digestion and a Double Ficoll Gradient
2.6. Experimental Metastases
2.7. Enzyme Assays
2.8. RT-PCR and Detection of mRNA
2.9. Western Blots
2.10. GSH and GSSG Determination
2.11. Knockdown of GCLC, SLC7A10 and SLC7A11genes
2.12. Analysis of Amino Acids
2.13. Rate of Cyst(e)ine Uptake
2.14. Rates of Glucose and Glutamine Utilization
2.15. H2O2 and O2·− Generation Assays
2.16. Rate of Oxygen Utilization
2.17. Statistics
3. Results
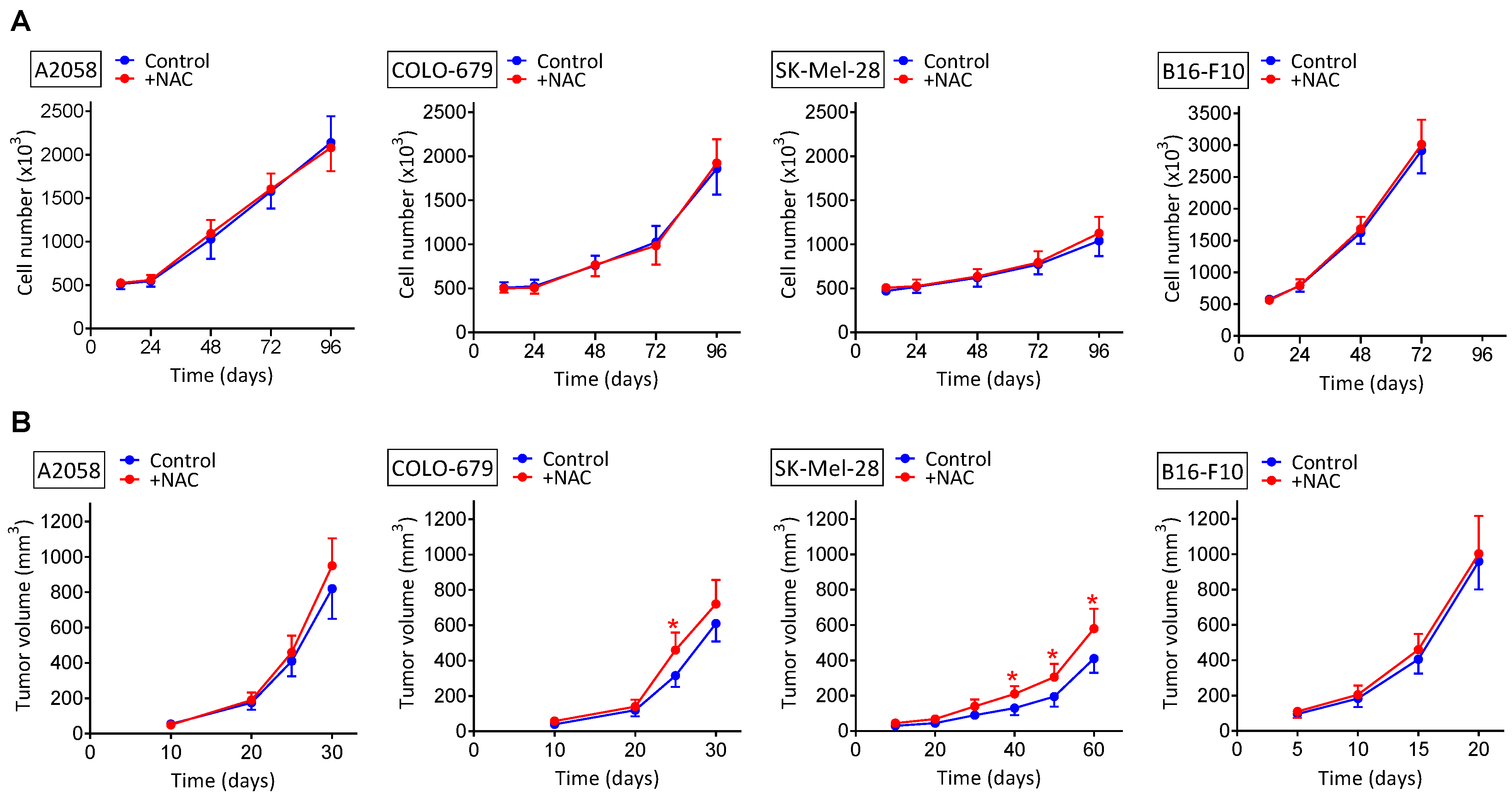
3.1. NAC Supplementation Does Not Increase the Growth of Melanoma Xenografts
3.2. NAC Supplementation Promotes Metastatic Spread despite Surgical Removal of Primary Growing Melanoma Xenografts
3.3. shRNA-Induced Downregulation of Cystine Uptake or γ-GCS Activity Decreases Metastatic Melanoma Growth
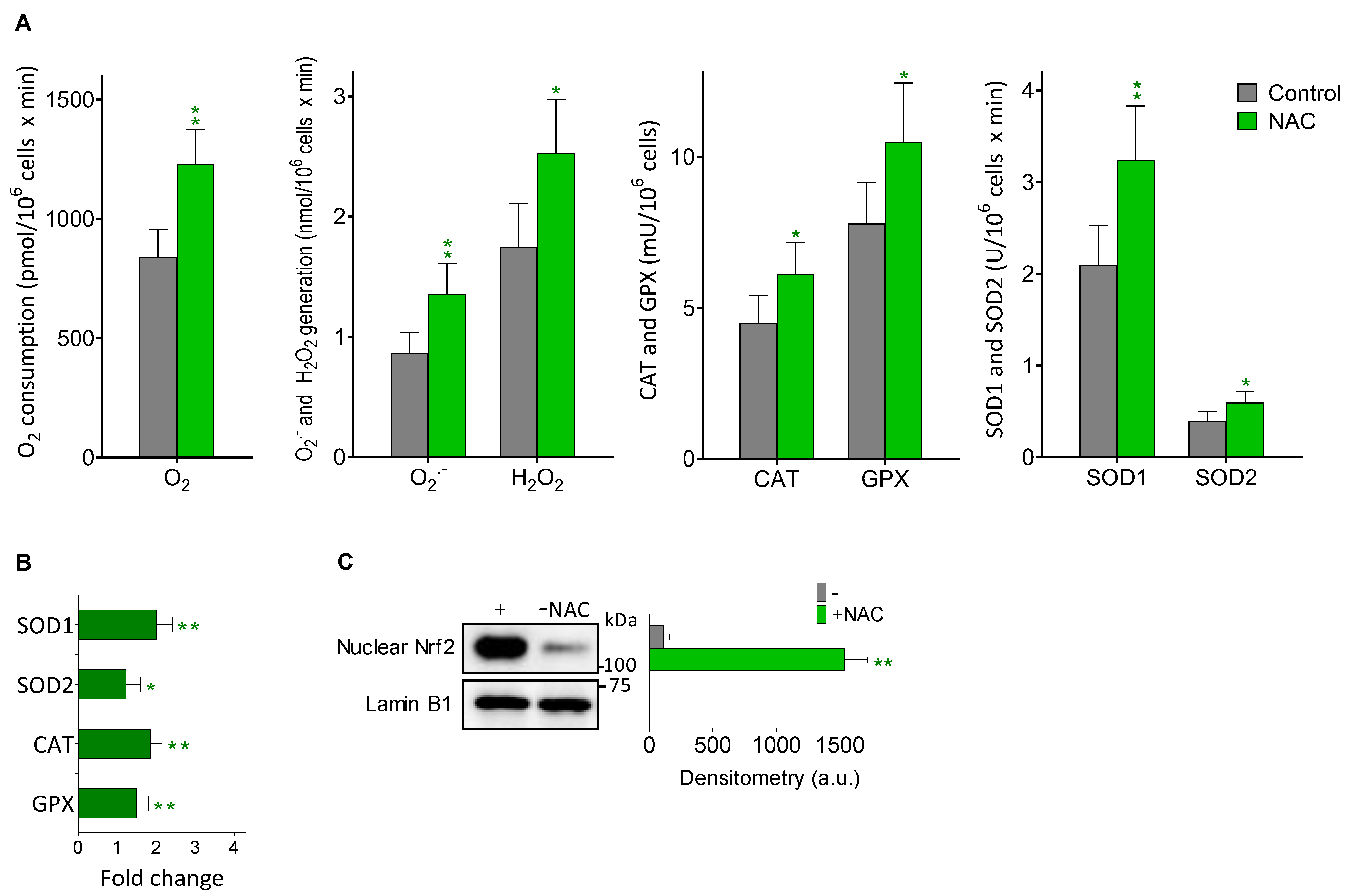
3.4. Melanoma Metastases Show Higher ROS Generation in Mice Treated with NAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Anasagasti, M.J.; Martin, J.J.; Mendoza, L.; Obrador, E.; Estrela, J.M.; McCuskey, R.S.; Vidal-Vanaclocha, F. Glutathione Protects Metastatic Melanoma Cells against Oxidative Stress in the Murine Hepatic Microvasculature. Hepatology 1998, 27, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Obrador, E.; Anasagasti, M.J.; Martin, J.J.; Vidal-Vanaclocha, F.; Estrela, J.M. Growth-Associated Changes in Glutathione Content Correlate with Liver Metastatic Activity of B16 Melanoma Cells. Clin. Exp. Metastasis 1999, 17, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione Metabolism. Methods Enzym. 1995, 251, 3–7. [Google Scholar] [CrossRef]
- Estrela, J.M.; Sáez, G.T.; Such, L.; Viña, J. The Effect of Cysteine and N-Acetyl Cysteine on Rat Liver Glutathione (GSH). Biochem. Pharm. 1983, 32, 3483–3485. [Google Scholar] [CrossRef]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kölle, P.; Tschoep, K.; Issels, R.D.; Daniel, P.T.; et al. The Cystine/Cysteine Cycle: A Redox Cycle Regulating Susceptibility versus Resistance to Cell Death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef] [Green Version]
- Gal, K.L.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants Can Increase Melanoma Metastasis in Mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- De Flora, S.; D’Agostini, F.; Masiello, L.; Giunciuglio, D.; Albini, A. Synergism between N-Acetylcysteine and Doxorubicin in the Prevention of Tumorigenicity and Metastasis in Murine Models. Int. J. Cancer 1996, 67, 842–848. [Google Scholar] [CrossRef]
- Cai, T.; Fassina, G.; Morini, M.; Aluigi, M.G.; Masiello, L.; Fontanini, G.; D’Agostini, F.; De Flora, S.; Noonan, D.M.; Albini, A. N-Acetylcysteine Inhibits Endothelial Cell Invasion and Angiogenesis. Lab. Investig. 1999, 79, 1151–1159. [Google Scholar]
- Morini, M.; Cai, T.; Aluigi, M.G.; Noonan, D.M.; Masiello, L.; De Flora, S.; D’Agostini, F.; Albini, A.; Fassina, G. The Role of the Thiol N-Acetylcysteine in the Prevention of Tumor Invasion and Angiogenesis. Int. J. Biol. Markers 1999, 14, 268–271. [Google Scholar] [CrossRef]
- Goldman, Y.; Peled, A.; Shinitzky, M. Effective Elimination of Lung Metastases Induced by Tumor Cells Treated with Hydrostatic Pressure and N-Acetyl-L-Cysteine. Cancer Res. 2000, 60, 350–358. [Google Scholar] [PubMed]
- Futakuchi, M.; Ogawa, K.; Tamano, S.; Takahashi, S.; Shirai, T. Suppression of Metastasis by Nuclear Factor KappaB Inhibitors in an in Vivo Lung Metastasis Model of Chemically Induced Hepatocellular Carcinoma. Cancer Sci. 2004, 95, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Lee, H.J. Allyl Isothiocyanate and Its N-Acetylcysteine Conjugate Suppress Metastasis via Inhibition of Invasion, Migration, and Matrix Metalloproteinase-2/-9 Activities in SK-Hep 1 Human Hepatoma Cells. Exp. Biol. Med. 2006, 231, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Supabphol, A.; Supabphol, R. Antimetastatic Potential of N-Acetylcysteine on Human Prostate Cancer Cells. J. Med. Assoc. Thai 2012, 95 (Suppl. 12), S56–S62. [Google Scholar]
- Agarwal, A.; Muñoz-Nájar, U.; Klueh, U.; Shih, S.-C.; Claffey, K.P. N-Acetyl-Cysteine Promotes Angiostatin Production and Vascular Collapse in an Orthotopic Model of Breast Cancer. Am. J. Pathol. 2004, 164, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Teng, T.; Kamal, M.; Iriondo, O.; Amzaleg, Y.; Luo, C.; Thomas, A.; Lee, G.; Hsu, C.-J.; Nguyen, J.D.; Kang, I.; et al. N-Acetyl-L-Cysteine Promotes Ex Vivo Growth and Expansion of Single Circulating Tumor Cells by Mitigating Cellular Stress Responses. Mol. Cancer Res. 2021, 19, 441–450. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef]
- Breau, M.; Houssaini, A.; Lipskaia, L.; Abid, S.; Born, E.; Marcos, E.; Czibik, G.; Attwe, A.; Beaulieu, D.; Palazzo, A.; et al. The Antioxidant N-Acetylcysteine Protects from Lung Emphysema but Induces Lung Adenocarcinoma in Mice. JCI Insight 2019, 4, 127647. [Google Scholar] [CrossRef]
- Zhang, L.-J. Isolation, Culture, and Characterization of Primary Mouse Epidermal Keratinocytes. Methods Mol. Biol. 2019, 1940, 205–215. [Google Scholar] [CrossRef]
- Tsikas, D.; Sandmann, J.; Ikic, M.; Fauler, J.; Stichtenoth, D.O.; Frölich, J.C. Analysis of Cysteine and N-Acetylcysteine in Human Plasma by High-Performance Liquid Chromatography at the Basal State and after Oral Administration of N-Acetylcysteine. J. Chromatogr. B Biomed. Sci. Appl. 1998, 708, 55–60. [Google Scholar] [CrossRef]
- Estrela, J.M.; Salvador, R.; Marchio, P.; Valles, S.L.; López-Blanch, R.; Rivera, P.; Benlloch, M.; Alcácer, J.; Pérez, C.L.; Pellicer, J.A.; et al. Glucocorticoid Receptor Antagonism Overcomes Resistance to BRAF Inhibition in BRAFV600E-Mutated Metastatic Melanoma. Am. J. Cancer Res. 2019, 9, 2580–2598. [Google Scholar] [PubMed]
- López-Blanch, R.; Salvador-Palmer, R.; Estrela, J.M.; Obrador, E. An Intercellular Flow of Glutathione Regulated by Interleukin 6 Links Astrocytes and the Liver in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Valles, S.L.; Benlloch, M.; Sirerol, J.A.; Pellicer, J.A.; Alcácer, J.; Coronado, J.A.-F.; Estrela, J.M. Glucocorticoid Receptor Knockdown Decreases the Antioxidant Protection of B16 Melanoma Cells: An Endocrine System-Related Mechanism That Compromises Metastatic Cell Resistance to Vascular Endothelium-Induced Tumor Cytotoxicity. PLoS ONE 2014, 9, e96466. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016, 24, 974–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Hernandez, J.I.; Almeida, A.; Delgado-Esteban, M.; Fernandez, E.; Bolaños, J.P. Knockdown of Glutamate-Cysteine Ligase by Small Hairpin RNA Reveals That Both Catalytic and Modulatory Subunits Are Essential for the Survival of Primary Neurons. J. Biol. Chem. 2005, 280, 38992–39001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastro, M.; Kourtidis, A.; Farley, K.; Conklin, D.S. XCT Expression Reduces the Early Cell Cycle Requirement for Calcium Signaling. Cell. Signal. 2008, 20, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Gordon, S.; Newsholme, E.A. Rates of Utilization and Fates of Glucose, Glutamine, Pyruvate, Fatty Acids and Ketone Bodies by Mouse Macrophages. Biochem. J. 1987, 242, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Obrador, E.; Carretero, J.; Ortega, A.; Medina, I.; Rodilla, V.; Pellicer, J.A.; Estrela, J.M. Gamma-Glutamyl Transpeptidase Overexpression Increases Metastatic Growth of B16 Melanoma Cells in the Mouse Liver. Hepatology 2002, 35, 74–81. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Segawa, H.; Kim, J.Y.; Chairoungdua, A.; Kim, D.K.; Matsuo, H.; Cha, S.H.; Endou, H.; Kanai, Y. Identification and Characterization of a Na(+)-Independent Neutral Amino Acid Transporter That Associates with the 4F2 Heavy Chain and Exhibits Substrate Selectivity for Small Neutral D- and L-Amino Acids. J. Biol. Chem. 2000, 275, 9690–9698. [Google Scholar] [CrossRef] [Green Version]
- Bannai, S. Exchange of Cystine and Glutamate across Plasma Membrane of Human Fibroblasts. J. Biol. Chem. 1986, 261, 2256–2263. [Google Scholar] [CrossRef]
- Keenan, M.M.; Chi, J.-T. Alternative Fuels for Cancer Cells. Cancer J. 2015, 21, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, D.R.; Thompson, C.B. Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Alcácer, J.; Benlloch, M.; Pellicer, J.A.; Estrela, J.M. Melanoma in the Liver: Oxidative Stress and the Mechanisms of Metastatic Cell Survival. Semin. Cancer Biol. 2021, 71, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Tasdogan, A.; Faubert, B.; Ramesh, V.; Ubellacker, J.M.; Shen, B.; Solmonson, A.; Murphy, M.M.; Gu, Z.; Gu, W.; Martin, M.; et al. Metabolic Heterogeneity Confers Differences in Melanoma Metastatic Potential. Nature 2020, 577, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sivinski, J.; Zhang, D.D.; Chapman, E. Targeting NRF2 to Treat Cancer. Semin. Cancer Biol. 2021, 76, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-Acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef]
- Mena, S.; Benlloch, M.; Ortega, A.; Carretero, J.; Obrador, E.; Asensi, M.; Petschen, I.; Brown, B.D.; Estrela, J.M. Bcl-2 and Glutathione Depletion Sensitizes B16 Melanoma to Combination Therapy and Eliminates Metastatic Disease. Clin. Cancer Res. 2007, 13, 2658–2666. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.I.; Lee, J.; Jo, G.; Kim, S.W.; Minn, K.W.; Hong, K.Y.; Jo, S.J.; Cho, K.H.; Kim, B.J.; Mun, J.-H. Local Recurrence and Metastasis in Patients with Malignant Melanomas after Surgery: A Single-Center Analysis of 202 Patients in South Korea. PLoS ONE 2019, 14, e0213475. [Google Scholar] [CrossRef]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive Oxygen Species: A Volatile Driver of Field Cancerization and Metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative Stress and Antioxidants in the Pathophysiology of Malignant Melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuki, F.; Yasutake, A.; Umehara, F.; Tokunaga, H.; Matsumoto, M.; Eto, K.; Ishiura, S.; Higuchi, I. In Vivo Protection of a Water-Soluble Derivative of Vitamin E, Trolox, against Methylmercury-Intoxication in the Rat. Neurosci. Lett. 2001, 304, 199–203. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.-D.; Zhou, X.-M.; Fang, J.; Zhu, L.; Ding, K. N-Acetylcysteine Amide Provides Neuroprotection via Nrf2-ARE Pathway in a Mouse Model of Traumatic Brain Injury. Drug Des. Dev. 2018, 12, 4117–4127. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Lou, Q.; Wang, F.; Li, E.; Sun, J.; Fang, H.; Xi, J.; Ju, L. N-Acetylcysteine Protects against Liver Injure Induced by Carbon Tetrachloride via Activation of the Nrf2/HO-1 Pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8655–8662. [Google Scholar]
- Ko, E.; Kim, D.; Min, D.W.; Kwon, S.-H.; Lee, J.-Y. Nrf2 Regulates Cell Motility through RhoA-ROCK1 Signalling in Non-Small-Cell Lung Cancer Cells. Sci. Rep. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.-X.; Singh, G. Expression of XCT and Activity of System Xc(-) Are Regulated by NRF2 in Human Breast Cancer Cells in Response to Oxidative Stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Brooks, M.; Wicha, M.S. Asparagine and Glutamine: Co-Conspirators Fueling Metastasis. Cell Metab. 2018, 27, 947–949. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective Glutamine Metabolism Inhibition in Tumor Cells Improves Antitumor T Lymphocyte Activity in Triple-Negative Breast Cancer. J. Clin. Investig. 2021, 131, 140100. [Google Scholar] [CrossRef]


| A | ||||||
| Amino Acid Concentration (μM) in Plasma | ||||||
| Non-Tumor-Bearing Mice | B16-F10-Bearing Mice | |||||
| - | +NAC | - | +NAC | |||
| Gln | 543 ± 31 | 526 ± 39 | 416 ± 51 * | 385 ± 32 * | ||
| Glu | 132 ± 26 | 125 ± 17 | 87 ± 23 * | 106 ± 21 | ||
| Gly | 327 ± 43 | 318 ± 40 | 306 ± 38 | 312± 36 | ||
| Ser | 169 ± 25 | 159 ± 37 | 152 ± 26 | 149 ± 24 | ||
| Met | 64 ± 14 | 59 ± 15 | 37 ± 10 * | 30 ± 6 * | ||
| Cyst(e)ine | 16 ± 3 | 184 ± 35 + | 21 ± 5 | 227 ± 42 + | ||
| B | ||||||
| GGT Activity and Cyst(e)ine Uptake in B16-F10 Cells | ||||||
| - | +NAC | |||||
| GGT (mU/106 cells) | 35.5 ± 6.4 | 36.0 ± 5.3 | ||||
| Cys uptake (nmol/mg protein × min) | 4.3 ± 1.2 | 8.5 ± 2.3 + | ||||
| Cystine uptake (nmol/mg protein × min) | 27.2 ± 4.6 | 60.7 ± 9.7 + | ||||
| Cys Uptake (nmol/mg prot. × min) | Cystine Uptake (nmol/mg prot. × min) | GSH (nmol/106 cells) | Metastatic Activity (% of the Liver Volume Occupied by Metastases) | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | - | +NAC | - | +NAC | - | +NAC | - | +NAC |
| Control | 4.0 ± 0.9 | 8.3 ± 1.7 + | 25.4 ± 3.8 | 62.3 ± 8.2 + | 26.2 ± 4.1 | 40.4 ± 5.0 + | 17.4 ± 3.6 | 30.2 ± 4.5 + |
| Anti-ASC1-shRNA | 0.7 ± 0.2 * | 0.9 ± 0.3 * | 26.2 ± 3.5 | 60.4 ± 7.7 + | 23.5 ± 3.3 | 38.3 ± 5.7 + | 15.9 ± 2.8 | 27.4 ± 3.6 + |
| Anti-xCT-shRNA | 3.9 ± 0.7 | 8.5 ± 2.0 + | 3.5 ± 0.6 * | 6.4 ± 1.2 *,+ | 7.9 ± 1.3 * | 9.6 ± 3.4 * | 9.2 ± 1.5 * | 16.7 ± 2.8 *,+ |
| Anti-GCLC-shRNA | 4.2 ± 1.0 | 8.5 ± 1.8 + | 27.9 ± 4.2 | 61.7 ± 6.9 + | 2.0 ± 0.5 * | 3.5 ± 0.7 *,+ | 4.1 ± 0.7 * | 5.8 ± 1.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers 2022, 14, 3614. https://doi.org/10.3390/cancers14153614
Obrador E, Salvador-Palmer R, López-Blanch R, Oriol-Caballo M, Moreno-Murciano P, Estrela JM. N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers. 2022; 14(15):3614. https://doi.org/10.3390/cancers14153614
Chicago/Turabian StyleObrador, Elena, Rosario Salvador-Palmer, Rafael López-Blanch, María Oriol-Caballo, Paz Moreno-Murciano, and José M. Estrela. 2022. "N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice" Cancers 14, no. 15: 3614. https://doi.org/10.3390/cancers14153614
APA StyleObrador, E., Salvador-Palmer, R., López-Blanch, R., Oriol-Caballo, M., Moreno-Murciano, P., & Estrela, J. M. (2022). N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers, 14(15), 3614. https://doi.org/10.3390/cancers14153614







