CD109 Is a Critical Determinant of EGFR Expression and Signaling, and Tumorigenicity in Squamous Cell Carcinoma Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Knockout Cell Line with CRISPR/Cas9
2.3. siRNA Transfection
2.4. Toluidine Blue Staining
2.5. In Vivo Animal Studies
2.6. In Vivo Tumor Xenograft Formation
2.7. Tail Vein (Metastasis) Assay
2.8. Immunohistochemistry (IHC) Analysis
2.9. Immunofluorescence Staining
2.10. Western Blot Analysis
2.11. Co-Immunoprecipitation Experiments
2.12. TGF-β, EGFR and AKT Inhibition Studies
2.13. RNA Isolation and Quantitative Real-Time PCR
2.14. Generation of CD109 KO A431 Cells That Express EGFR (CD109KOEGFR)
2.15. Tumor Spheres Formation Assay
2.16. Statistical Analysis
3. Results
3.1. The Loss of CD09 Diminishes the Tumorigenicity and Abrogates the Metastatic Ability of SCC Cells In Vivo
3.2. CD109 Is Required for Maintaining the Stemness of SCC Cells, and the Loss of CD109 Diminishes the Cancer Stem Cell Population In Vitro in SCC Cells
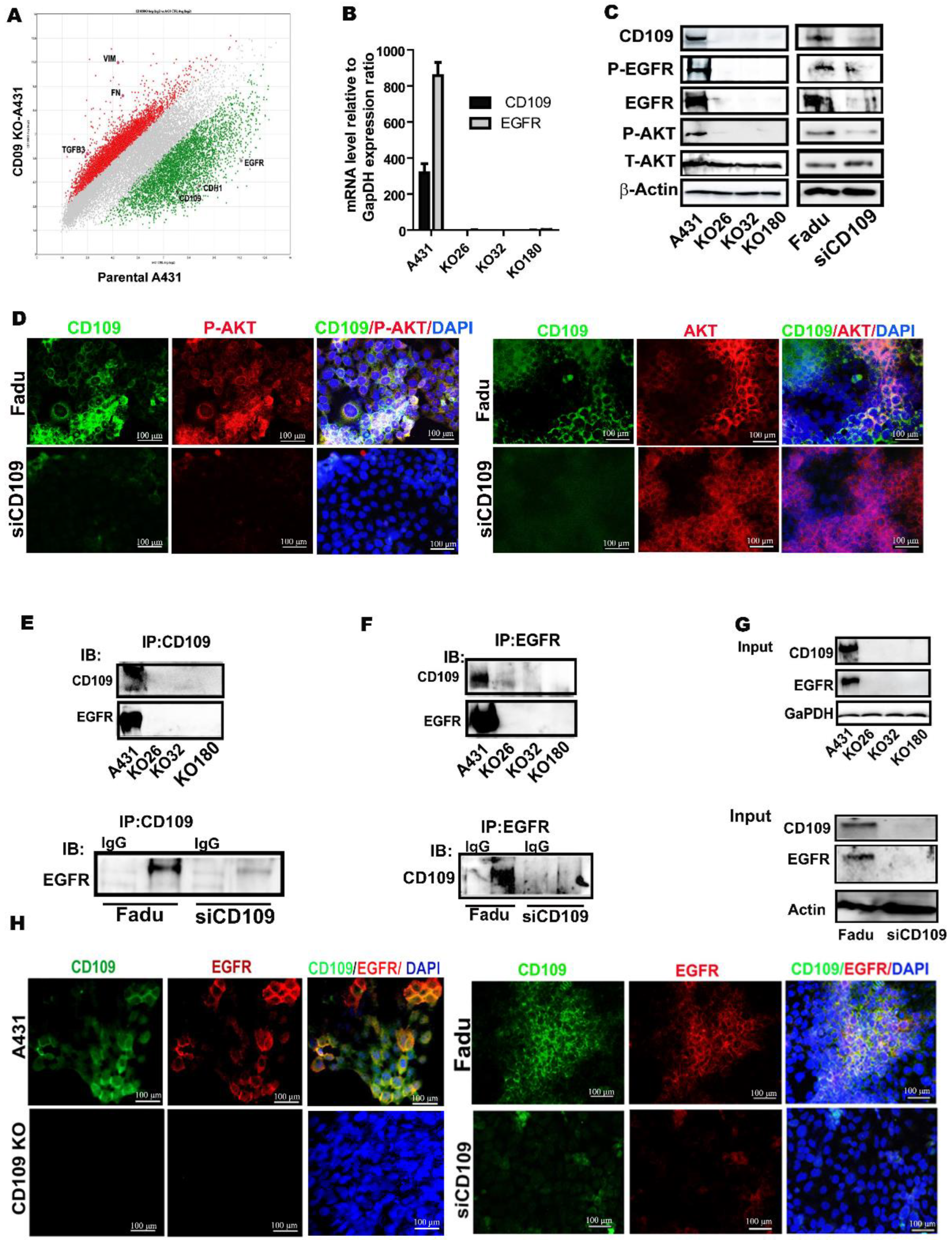
3.3. CD109 Associates and Co-Localizes with EGFR, and Loss of CD109 Diminishes EGFR Expression in SCC Cells
3.4. The AKT Pathway, but Not the ERK1/2 and STAT3 Pathways, Is Inactivated in CD109 KO SCC Cells, While in Control SCC Cells, EGFR Kinase Inhibition Phenocopies AKT Inhibition in Inducing Cell Death and CD109KO-Like Mesenchymal State
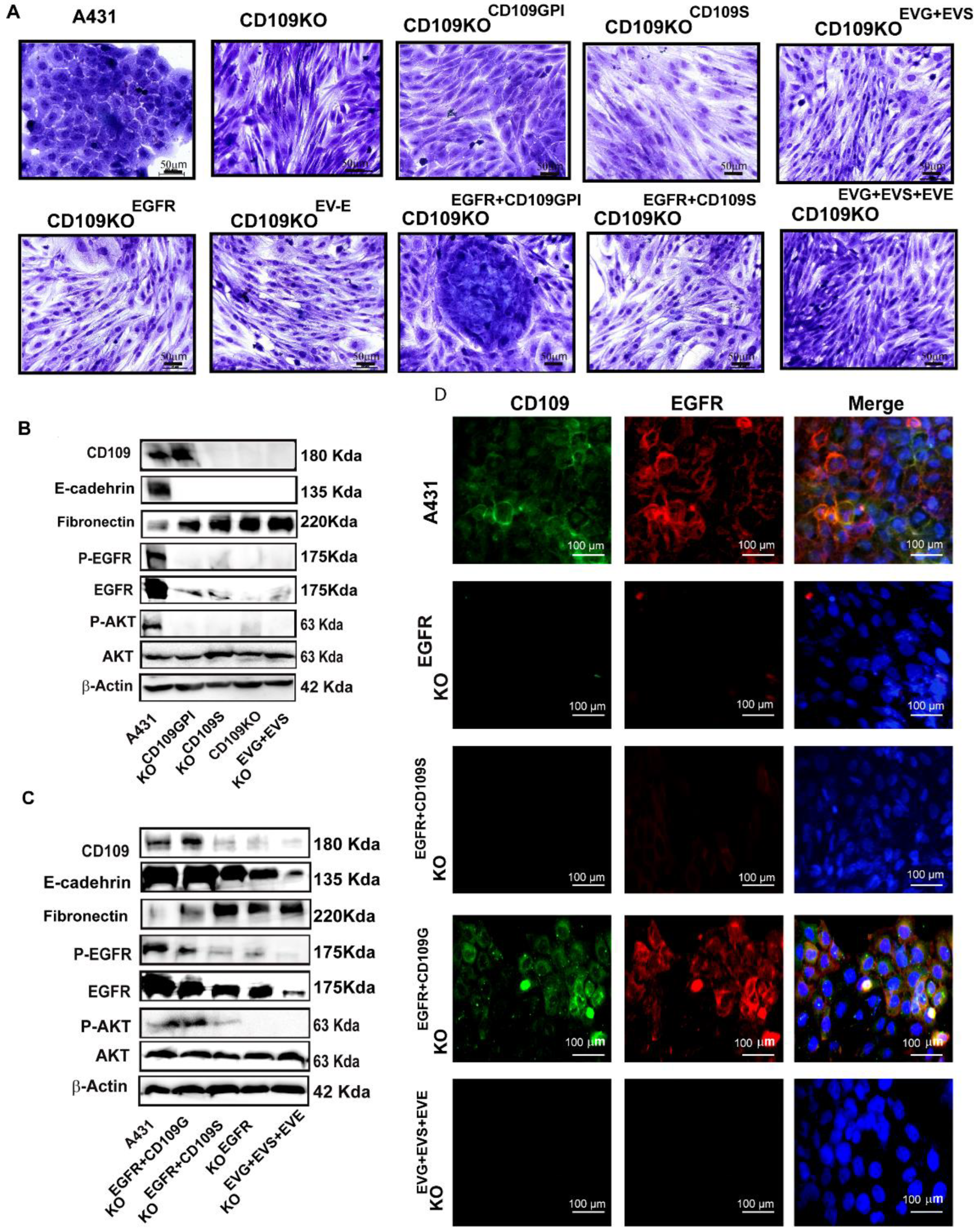
3.5. GPI-Anchored CD109 Is Required for Maintaining EGFR Expression, Levels of Phospho-EGFR, and Phospho-AKT, and the Epithelial Phenotype in CD109KO SCC Cells
3.6. CD109 Is Required for EGF-Mediated Regulation of EGFR Levels, AKT Signaling, and to Maintain Stemness in SCC Cells
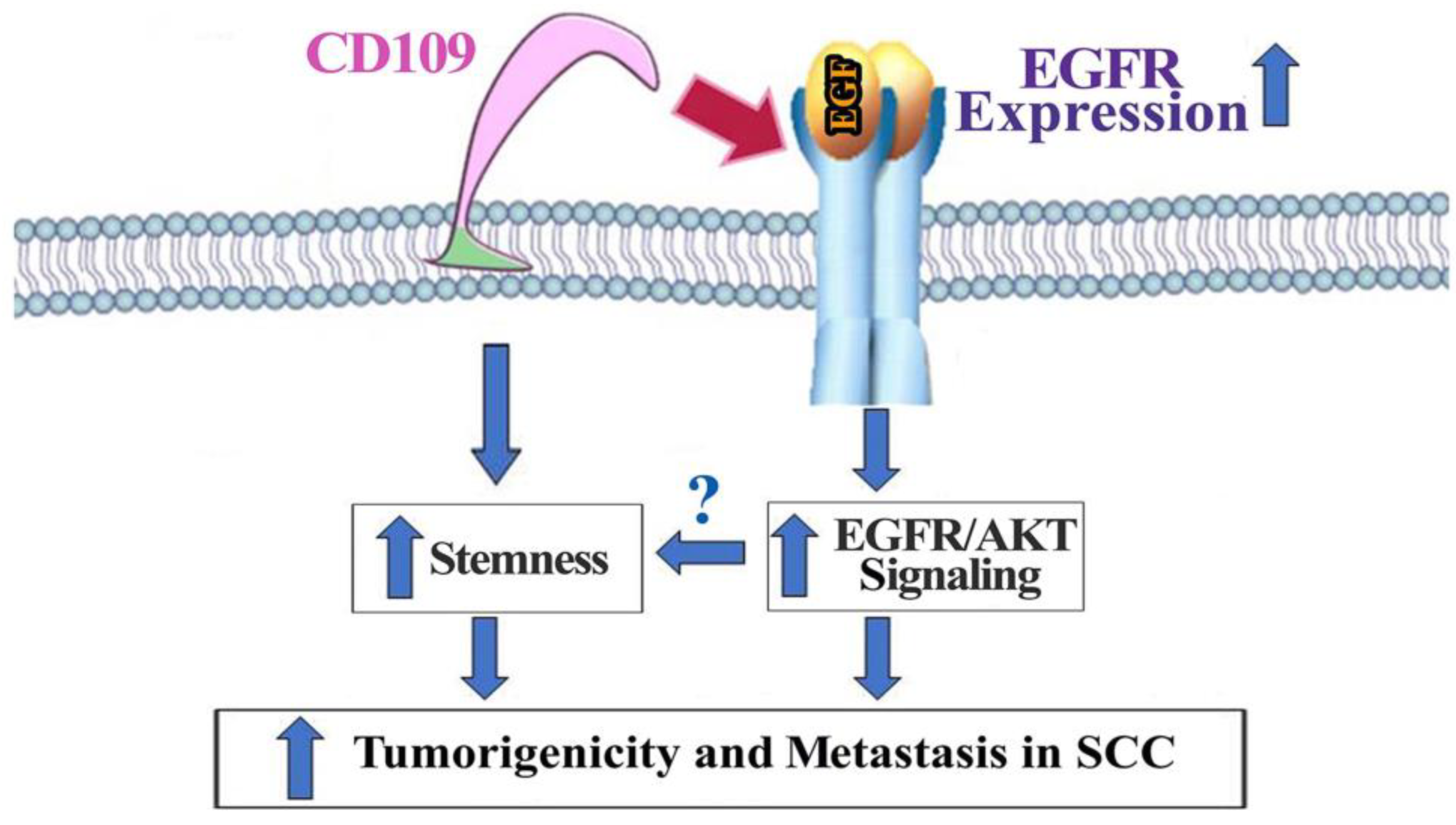
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, L.D. Laryngeal Dysplasia, Squamous Cell Carcinoma, and Variants. Surg. Pathol. Clin. 2017, 10, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Dong, F.; Liu, Q.; Murakumo, Y.; Liu, J. CD109 and squamous cell carcinoma. J. Transl. Med. 2018, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Sutherland, D.R.; Horsfall, W.; Totty, N.; Yeo, E.; Nayar, R.; Wu, X.-F.; Schuh, A.C. Cell surface antigen CD109 is a novel member of the α2 macroglobulin/C3, C4, C5 family of thioester-containing proteins. Blood 2002, 99, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.R.; Yeo, E.; Ryan, A.; Mills, G.B.; Bailey, D.; Baker, M.A. Identification of a cell-surface antigen associated with activated T lymphoblasts and activated platelets. Blood 1991, 77, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bizet, A.A.; Liu, K.; Tran-Khanh, N.; Saksena, A.; Vorstenbosch, J.; Finnson, K.W.; Buschmann, M.D.; Philip, A. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim. Biophys. Acta 2011, 1813, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Bizet, A.A.; Tran-Khanh, N.; Saksena, A.; Liu, K.; Buschmann, M.D.; Philip, A. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J. Cell. Biochem. 2012, 113, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Tam, B.Y.Y.; Liu, K.; Marcoux, A.; Lepage, P.; Roy, S.; Bizet, A.A.; Philip, A. Identification of CD109 as part of the TGF-β receptor system in human keratinocytes. FASEB J. 2006, 20, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Bizet, A.A.; Binamer, Y.; Sasseville, D.; Jones, D.A.; Philip, A. CD109 release from the cell surface in human keratinocytes regulates TGF-beta receptor expression, TGF-β signalling and STAT3 activation: Relevance to psoriasis. Exp. Dermatol. 2011, 20, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Vorstenbosch, J.; Nguyen, C.M.; Zhou, S.; Seo, Y.J.; Siblini, A.; Finnson, K.W.; Bizet, A.A.; Tran, S.D.; Philip, A. Overexpression of CD109 in the Epidermis Differentially Regulates ALK1 Versus ALK5 Signaling and Modulates Extracellular Matrix Synthesis in the Skin. J. Investig. Dermatol. 2017, 137, 641–649. [Google Scholar] [CrossRef]
- Tam, B.Y.Y.; Finnson, K.W.; Philip, A. Glycosylphosphatidylinositol-anchored proteins regulate transforming growth factor-β signaling in human keratinocytes. J. Biol. Chem. 2003, 278, 49610–49617. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.Y.; Larouche, D.; Germain, L.; Hooper, N.M.; Philip, A. Characterization of a 150 kDa accessory receptor for TGF-beta 1 on keratinocytes: Direct evidence for a GPI anchor and ligand binding of the released form. J. Cell. Biochem. 2001, 83, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cecere, R.; Philip, A. CD109 released from human bone marrow mesenchymal stem cells attenuates TGF-β-induced epithelial to mesenchymal transition and stemness of squamous cell carcinoma. Oncotarget 2017, 8, 95632. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Murakumo, Y.; Mii, S.; Shigetomi, T.; Yamamoto, N.; Furue, H.; Ueda, M.; Takahashi, M. Processing of CD109 by furin and its role in the regulation of TGF-β signaling. Oncogene 2010, 29, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Murakumo, Y.; Sato, T.; Shigetomi, T.; Mitsudo, K.; Tohnai, I.; Ueda, M.; Takahashi, M. Up-regulation of CD109 expression is associated with carcinogenesis of the squamous epithelium of the oral cavity. Cancer Sci. 2008, 99, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Lu, C.; Chen, X.; Guo, Y.; Liu, J. CD109 is a novel marker for squamous cell/adenosquamous carcinomas of the gallbladder. Diagn. Pathol. 2015, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, J.; Xu, Y.; Cheng, Z.; Chen, X.; Wang, Y.; Liu, J. CD109 expression is upregulated in penile squamous cell carcinoma. Oncol. Lett. 2017, 14, 6012–6016. [Google Scholar] [CrossRef][Green Version]
- Dong, F.; Wang, Y.; Li, L.; Wang, Y.; Liu, X.; Liu, J. CD109 expression is increased in cutaneous squamous cell carcinoma. J. Dermatol. 2014, 41, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; da Silva, S.D.; Siegel, P.M.; Philip, A. CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro. Sci. Rep. 2019, 9, 16317. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Greenside, P.G.; Rogers, Z.N.; Brady, J.J.; Yang, D.; Ma, R.K.; Caswell, D.R.; Chiou, S.-H.; Winters, A.F.; Gruner, B.M.; et al. Molecular definition of a metastatic lung cancer state reveals a targetable CD109-Janus kinase-Stat axis. Nat. Med. 2017, 23, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Murakumo, Y.; Hagiwara, S.; Jiang, P.; Mii, S.; Kalyoncu, E.; Saito, S.; Suzuki, C.; Sakurai, Y.; Numata, Y.; et al. CD109 attenuates TGF-beta1 signaling and enhances EGF signaling in SK-MG-1 human glioblastoma cells. Biochem. Biophys. Res. Commun. 2015, 459, 252–258. [Google Scholar] [CrossRef]
- Mo, X.T.; Leung, T.H.; Tang, H.W.; Siu, M.K.; Wan, P.K.; Chan, K.K.; Cheung, A.N.; Ngan, H.Y. CD109 mediates tumorigenicity and cancer aggressiveness via regulation of EGFR and STAT3 signalling in cervical squamous cell carcinoma. Br. J. Cancer 2020, 123, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Zorn, J.A.; Huang, Y.; Barros, T.; Kuriyan, J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu. Rev. Biochem. 2015, 84, 739–764. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J.; Ferguson, K.M. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2014, 6, a020768. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Shan, Y.; Eastwood, M.P.; Zhang, X.; Kim, E.T.; Arkhipov, A.; Dror, R.O.; Jumper, J.; Kuriyan, J.; Shaw, D.E. Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 2012, 149, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Grandis, J.R.; Sok, J.C. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol. Ther. 2004, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGFR family and its ligands in human cancer: Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37, 3–8. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1849. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Hagikura, M.; Murakumo, Y.; Hasegawa, M.; Jijiwa, M.; Hagiwara, S.; Mii, S.; Hagikura, S.; Matsukawa, Y.; Yoshino, Y.; Hattori, R.; et al. Correlation of pathological grade and tumor stage of urothelial carcinomas with CD109 expression. Pathol. Int. 2010, 60, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Maeda, T.; Suzuki, A.; Takada, S.; Fujii, M.; Kato, Y. Deguelin Potentiates Apoptotic Activity of an EGFR Tyrosine Kinase Inhibitor (AG1478) in PIK3CA-Mutated Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Zhou, Y.-S.; Yang, J.-X.; Zhao, Y.-L.; Liu, Q.-Q.; Yuan, C.; Wang, F.-Z. MK-2206 induces cell cycle arrest and apoptosis in HepG2 cells and sensitizes TRAIL-mediated cell death. Mol. Cell. Biochem. 2013, 382, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.; Passaniti, A.; Lapidus, R.G.; Liu, X.; Cullen, K.J.; Dan, H.C. A positive feedback loop involving EGFR/Akt/mTORC1 and IKK/NF-κB regulates head and neck squamous cell carcinoma proliferation. Oncotarget 2016, 7, 31892–31906. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Liu, F.; Yan, S.; Liu, X.; Jiang, Z.; Liu, J. Elevated Expression of CD109 in Esophageal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2015, 21, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Kuo, T.-C.; Chou, C.-M.; Hsu, W.-J.; Lee, W.-C.; Dai, J.-Z.; Wu, S.-M.; Lin, C.-W. Upregulation of CD109 Promotes the Epithelial-to-Mesenchymal Transition and Stemness Properties of Lung Adenocarcinomas via Activation of the Hippo-YAP Signaling. Cells 2021, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Shueng, P.W.; Chou, C.M.; Lin, B.X.; Lin, M.H.; Kuo, D.Y.; Tsai, I.L.; Wu, S.M.; Lin, C.W. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci. 2020, 111, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT factors and metabolic pathways in cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Senthebane, D.A.; Ganz, C.; Thomford, N.E.; Wonkam, A.; Dandara, C. Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: An updated review. Cells 2020, 9, 1896. [Google Scholar] [CrossRef] [PubMed]
- Emori, M.; Tsukahara, T.; Murase, M.; Kano, M.; Murata, K.; Takahashi, A.; Kubo, T.; Asanuma, H.; Yasuda, K.; Kochin, V.; et al. High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancer-initiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS ONE 2013, 8, e84187. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, H.; Li, Q.; Yang, Y. CD109 is a potential target for triple-negative breast cancer. Tumor Biol. 2014, 35, 12083–12090. [Google Scholar] [CrossRef] [PubMed]





| human GAPDH: | forward primer | 5′-GACAACTTTGGTATCGTGGAAGG-3′ |
| reverse primer | 5′-AGGGATGATGTTCTGGAGAGCC-3′ | |
| human EGFR: | forward primer | 5′-AAACCGGACTGAAGGAGCTG-3′ |
| reverse primer | 5′-CCCATTGGGACAGCTTGGAT-3′ | |
| human CD109: | forward primer | 5′-CTGGAACACTGCCCTTCACA-3′ |
| reverse primer | 5′-GTCCGGTTACACGTAGCTCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Hassan, A.; Kungyal, T.; Tabariès, S.; Luna, J.L.R.G.; Siegel, P.M.; Philip, A. CD109 Is a Critical Determinant of EGFR Expression and Signaling, and Tumorigenicity in Squamous Cell Carcinoma Cells. Cancers 2022, 14, 3672. https://doi.org/10.3390/cancers14153672
Zhou S, Hassan A, Kungyal T, Tabariès S, Luna JLRG, Siegel PM, Philip A. CD109 Is a Critical Determinant of EGFR Expression and Signaling, and Tumorigenicity in Squamous Cell Carcinoma Cells. Cancers. 2022; 14(15):3672. https://doi.org/10.3390/cancers14153672
Chicago/Turabian StyleZhou, Shufeng, Amani Hassan, Tenzin Kungyal, Sebastien Tabariès, José Luis Ramírez García Luna, Peter M. Siegel, and Anie Philip. 2022. "CD109 Is a Critical Determinant of EGFR Expression and Signaling, and Tumorigenicity in Squamous Cell Carcinoma Cells" Cancers 14, no. 15: 3672. https://doi.org/10.3390/cancers14153672
APA StyleZhou, S., Hassan, A., Kungyal, T., Tabariès, S., Luna, J. L. R. G., Siegel, P. M., & Philip, A. (2022). CD109 Is a Critical Determinant of EGFR Expression and Signaling, and Tumorigenicity in Squamous Cell Carcinoma Cells. Cancers, 14(15), 3672. https://doi.org/10.3390/cancers14153672







