Simple Summary
The lymph node ratio (LNR) is an emerging predictive marker for recurrence in papillary thyroid carcinoma (PTC). The purpose of this study was to investigate the association between LNR and disease-free survival (DFS) in patients with N1b PTC. Unlike that in the lateral or whole neck, LNR in the central compartment (CLNR) was found to have prognostic significance. The high-CLNR group (CLNR ≥ 0.7) had worse DFS and was 4.5 times more likely to experience recurrence in patients with N1b PTC.
Abstract
The lymph node ratio (LNR) indicates the number of metastatic lymph nodes (LNs) to the total number of LNs. The prognostic value of LNR in papillary thyroid carcinoma (PTC) and other solid tumors is known. This study aimed to investigate the relationship between LNR and disease-free survival (DFS) in patients with PTC with lateral LN metastases (N1b PTC). A total of 307 patients with N1b PTC who underwent total thyroidectomy and therapeutic central and lateral LN dissection were retrospectively analyzed. The DFS and recurrence risk in the patients with LNR, central-compartment LNR (CLNR), and lateral-compartment LNR (LLNR) were compared. The mean follow-up duration was 93.6 ± 19.9 months. Eleven (3.6%) patients experienced recurrence. Neither LNR nor LLNR affected the recurrence rate in our analysis (p = 0.058, p = 0.106, respectively). However, there was a significant difference in the recurrence rates between the patients with low and high CLNR (2.1% vs. 8.8%, p = 0.017). In the multivariate analysis, CLNR ≥ 0.7 and perineural invasion were independent predictors of tumor recurrence. High CLNR was associated with an increased risk of recurrence, and was shown to be a significant predictor of prognosis in patients with N1b PTC.
1. Introduction
Papillary thyroid carcinoma (PTC) is the most common pathological type and accounts for approximately 90% of thyroid cancer [1]. Among all types of malignancies, PTC has a relatively good prognosis, with an estimated 10-year disease-specific survival of >96% [2,3]. In most cases, surgical treatment is sufficient. Depending on the risk of recurrence, such as that associated with size [4], extrathyroidal extension (ETE) [5], vascular invasion [6], or lymph node (LN) metastasis [7], either thyroid lobectomy or total thyroidectomy is performed. Approximately 30–80% of patients with PTC develop cervical LN metastasis [8]. PTC progresses from the thyroid gland to the adjacent central compartment and then to the ipsilateral and contralateral lateral neck compartments [9].
In the eighth version of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) tumor–node–metastasis (TNM) staging system, patients with PTC with lateral neck LN metastasis are classified as N1b. Whether PTC invades the LNs outside the carotid artery differentiates N1b from N1a, regardless of the number or size. The definition of N1b included superior mediastinal node metastasis (level VII) in the past, which was reclassified from N1b to N1a in the current edition. The surgical extent of patients diagnosed with N1b PTC includes therapeutic central and lateral LN dissection, as well as bilateral thyroid glands [10,11,12,13]. In the seventh version of the AJCC/UICC TNM staging system, patients with PTC with LN metastasis of 45 years or older were classified as stage III or Iva, depending on the location of metastasis (N1a or N1b). In the eighth version, however, they were classified into the same stage, as long as the tumor is limited to LNs in the neck [14]. Thus, the N category in the AJCC/UICC TNM staging is insufficient to evaluate the risk of recurrence in patients with node-positive PTC.
The American Thyroid Association (ATA) proposed a risk-stratification system with three categories: low, intermediate, and high, to predict the risk of recurrence [15]. Although the system recommends that the number and size of metastatic LNs be considered as risk factors to predict recurrence, the system lacks evidence to provide appropriate information for following up with patients with N1b PTC after surgical treatment.
The lymph node ratio (LNR), which is defined as the number of positive LNs divided by the total number of LNs harvested, is used to evaluate oncological prognosis in solid tumors, such as those in lung, gastric, and colon cancer [16,17,18]. In terms of PTC, David et al. reported, after analyzing 10,955 cases, that LNR ≥ 0.42 was associated with disease-specific mortality [19]. Recently, it has been suggested that LNR is a predictor of recurrence in patients with N1b PTC. Lee et al. suggested that LNR > 0.25 in the lateral compartment is an independent prognostic factor affecting recurrence [20]. Another study demonstrated that lateral LNR > 0.3 had a significant effect on cancer-specific mortality [21]. To the best of our knowledge, only a few studies on patients with N1b PTC have investigated the relationship between LNR and tumor recurrence.
The study’s aim was to investigate the relationship between LNR and DFS, determine an optimal cutoff for LNR, and validate the clinical significance as a predictor of tumor recurrence in patients with N1b PTC after surgical treatment.
2. Materials and Methods
2.1. Patients
We retrospectively reviewed 312 patients who had been diagnosed with N1b PTC and had undergone a total thyroidectomy with central and lateral neck dissection between January 2012 and December 2017 at Seoul St. Mary’s Hospital (Seoul, Korea). After excluding two patients who had not undergone lateral neck dissection as an initial surgery and three with distant metastasis at initial presentation, a total of 307 patients were enrolled in this study. The mean follow-up duration was 93.6 ± 19.9 months (range: 52–123 months). This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Seoul St. Mary’s Hospital, The Catholic University of Korea (IRB No: KC22RISI0318). The requirement for informed consent was waived due to the retrospective nature of this study.
2.2. Postoperative Management and Follow-Up
All enrolled patients were diagnosed and treated according to the ATA management guidelines [15]. The patients had undergone a physical examination, neck ultrasound (US), and serum thyroid function testing at 3–6-month intervals and annually thereafter. All patients had been regularly followed up by physical examination, thyroid function testing, serum thyroglobulin and anti-thyroglobulin antibody concentration measurements, and neck US at 3–6-month intervals and annually thereafter. Radioactive iodine (RAI) ablation was performed at 6–8 weeks postoperatively, and whole-body scans were performed 5–7 days after RAI ablation. Patients with suspected recurrence after neck US were confirmed by cytological examination using US-guided fine-needle aspiration during the routine follow-up evaluations.
2.3. Statistical Analysis
Continuous variables are presented as the mean ± standard deviation and categorical variables are presented as the number with percentage. Student’s t-test and Pearson’s chi-square test or Fisher’s exact test were used to compare continuous and categorical variables, respectively. Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff value for LNR relevant to the disease-free survival (DFS). We calculated the area under the curve (AUC), sensitivity, specificity, and 95% confidence interval (CI). The same procedure was repeated for LNR in the central (CLNR) and lateral (LLNR) compartments. Univariate and multivariate Cox regression analyses were performed to validate the predictors of DFS, with the hazard ratio (HR) and CI presented. A p-value of <0.05 was accepted as indicative of statistical significance. IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA), was used to perform all statistical analyses.
3. Results
3.1. Optimal Cutoff Values Determined by ROC Curve Analysis
The results of ROC curve analysis for LNR, CLNR, and LLNR are shown in Figure 1. The cutoff values that we adopted as best predictors for tumor recurrence in patients with N1b PTC were LNR of 0.32 (AUC, 0.631; sensitivity, 0.545; specificity, 0.828; 95% CI, 0.438–0.823; and p = 0.141), CLNR of 0.7 (AUC, 0.680; sensitivity, 0.545; specificity, 0.791; 95% CI, 0.497–0.863; and p = 0.043), and LLNR of 0.16 (AUC, 0.620; sensitivity, 0.636; specificity, 0.625; 95% CI, 0.455–0.785; and p = 0.177), respectively.
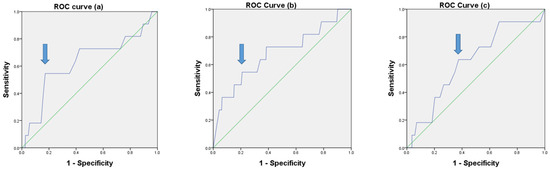
Figure 1.
ROC curves for LNR (a), CLNR (b), and LLNR (c) (p = 0.141, p = 0.043, and p = 0.177, respectively).
3.2. Comparison of Baseline Clinicopathological Characteristics According to LNR
The results for LNR with a cutoff of 0.23 are presented in Table 1. The patients in the high-LNR group were significantly younger (40.6 ± 14.1 vs. 44.8 ± 12.6 years, and p = 0.006) and had larger tumors (1.8 ± 1.1 cm vs. 1.5 ± 1.0 cm, and p = 0.004) than those in the low-LNR group. Higher rates of lymphatic (69.7% vs. 85.3%, p = 0.002) and vascular (4.5% vs. 12.4%, p = 0.016) invasion were observed in the high-LNR group than in the low-LNR group. There were no significant differences in sex, multifocality, bilaterality, gross extrathyroid extension (ETE), perineural invasion, T category, and TNM stages. The number of positive LNs was significantly higher (17.9 ± 9.3 vs. 8.0 ± 4.7, p < 0.001) in patients with high LNR, but the number of harvested LNs was higher in the low-LNR group (59.3 ± 22.6 vs. 53.7 ± 22.9, p = 0.033). Three (1.7%) patients in the low-LNR group and eight (6.2%) in the high-LNR group had tumor recurrence. There was no significant difference in recurrence between the two groups (p = 0.058).

Table 1.
Comparison of baseline clinicopathological characteristics according to LNR (whole).
3.3. Comparison of Baseline Clinicopathological Characteristics According to CLNR
Table 2 shows a comparison of the baseline clinicopathological characteristics according to the CLNR. The rate of recurrence was higher in the high-CLNR group than that in the low-CLNR group (8.8% vs. 2.1%, p = 0.017). The cutoff value was 0.7.

Table 2.
Comparison of baseline clinicopathological characteristics according to CLNR.
There were fewer female patients in the high-CLNR group than in the low-CLNR group (48.5% vs. 71.1%, p = 0.001). The tumor size was larger in the high-CLNR group than in the low-CLNR group (2.0 ± 1.2 cm vs. 1.5 ± 1.0 cm, p = 0.001). There were no significant differences in the multifocality, bilaterality, gross ETE, BRAF positivity, lymphovascular invasion, perineural invasion, number of harvested LNs, T category, and TNM stages. The number of positive LNs was higher in the high-CLNR group than in the low-CLNR group, regardless of the neck compartment (18.6 ± 10.7 vs. 10.3 ± 6.7, p < 0.001; 10.6 ± 6.9 vs. 4.6 ± 4.1, p < 0.001; and 8.1 ± 5.8 vs. 5.7 ± 4.3, p < 0.001, respectively).
3.4. Comparison of Baseline Clinicopathological Characteristics According to LLNR
The baseline clinicopathological characteristics depending on the LNR in the lateral compartment are described in Table 3. A LLNR of 0.16 was adopted as the cutoff value to compare the factors related to tumor and node characteristics. There were no significant differences in age, sex, tumor size, gross ETE, perineural invasion, BRAF mutation, number of harvested LNs from the central neck, T category, and TNM stages. Patients in the high-LLNR group had more multifocal (67.9% vs. 52.3%, p = 0.008) and bilateral tumors (45.5% vs. 28.7%, p = 0.004) than the patients in the low-LLNR group. Between the low- and high-CNLR groups, the rates of lymphatic invasion (70.8% vs. 85.7%, p = 0.003) and vascular invasion (5.1% vs. 12.5%, p = 0.027) significantly differed. There was no significant difference in recurrence according to LLNR (2.1% vs. 6.3%, p = 0.106).

Table 3.
Comparison of the baseline clinicopathological characteristics according to LLNR.
3.5. Univariate and Multivariate Analyses of the Risk Factors for Recurrence
Table 4 presents the analysis results of univariate and multivariate Cox regression to identify risk factors related to tumor recurrence. The tumor size (HR, 1.707; p = 0.005), vascular invasion (HR, 4.320; p = 0.031), perineural invasion (HR, 5.588; p = 0.011), number of positive LNs in the whole neck (HR, 1.048; p = 0.017), number of positive LNs in the lateral compartment (HR, 1.095; p = 0.037), high CLNR (HR, 11.026, p = 0.031), and CLNR ≥ 0.7 (HR, 4.238; p = 0.017) were found to be significant predictors of recurrence in univariate analysis. In multivariate analysis, perineural invasion (HR, 6.045; p = 0.008) and higher CLNR with a cutoff of 0.7 (HR, 4.451; p = 0.014) were independent factors for predicting tumor recurrence. In Kaplan–Meier analysis, there was a statistically significant difference in DFS between the high- and low-CLNR groups (log-rank p = 0.009; Figure 2).

Table 4.
Univariate and multivariate analyses of the risk factors for recurrence.

Figure 2.
Disease-free survival curves according to the central lymph node ratio (log-rank p = 0.009).
3.6. Recurrence Patterns for the Study Population
The recurrence patterns of the study cohort are summarized in Table 5. Three patients in the low-CLNR group experienced recurrence in the ipsilateral lateral compartment. Only one patient developed recurrence in the contralateral lateral compartment (level III LNs). In contrast, three out of six patients in the high-CLNR group developed recurrence in the contralateral lateral compartment. Patient No. 1 experienced recurrence in the left-level-5 LNs after bilateral lateral neck dissection diagnosed with bilateral metastasis. The largest tumor (5.0 cm) found during primary surgery was located in the left lobe of the thyroid gland. The recurrence of Patient No. 5 was found in the left-level-6 (central) LNs, which was ipsilateral to the largest tumor (2.7 cm) in the thyroid gland.

Table 5.
Recurrence patterns for the study population.
4. Discussion
Our results showed no significant difference in the recurrence rates between the low-LNR and high-LNR groups divided by an LNR cutoff value of 0.23 (1.7% vs. 6.2%, p = 0.058) among a total of 307 patients with N1b PTC. With respect to CLNR, however, the rate of tumor recurrence differed significantly between the high- and low-CLNR groups (8.8% vs. 2.1%, p = 0.017). CLNR ≥ 0.7 was an independent prognostic factor for recurrence in multivariate Cox regression analysis (HR, 4.451; 95% CI, 1.356–14.631; p = 0.014). This result is noteworthy in that few studies have linked CLNR with recurrence or DFS in patients with N1b PTC.
Cervical LN metastases are common in patients with PTC. If occult metastasis is included, cervical LN metastasis is reported in up to 90% of patients with PTC [22]. LN metastasis in patients with PTC matters because it is associated with a higher rate of recurrence after surgical treatment [23]. The ATA management guidelines published in 2015 classified patients with clinical N1 or >5 pathological N1 with all involved LNs < 3 cm in the largest dimension as having an intermediate risk of recurrence [15]. However, it is difficult to stratify patients with N1b PTC by risk using the existing criteria because the number of metastatic LNs depends on the extent of LN dissection [24].
The AJCC/UICC staging system is also insufficient to assess the risk of patients with N1b PTC; in the seventh edition, for patients > 45 years of age, N1a was classified as stage III and N1b as stage IV. In the revised version, all node-positive patients are classified as stage II, regardless of LN location in patients aged ≥55 years [12]. Although it had the effect of lowering the survival rate of high-stage patients [14], it is difficult to predict the prognosis in detail by observing patients with N1b PTC after treatment. To better predict prognosis in patients with N1b PTC, we introduced the concept of LNR, which is known to be a prognostic factor in various types of solid tumors [25,26,27].
LNR has attracted attention recently as a predictive marker for PTC recurrence that can complement the existing staging system or risk-stratification system. Yip et al. suggested that TNM nodal classification combined with LNR is a better predictor of recurrence than nodal classification alone in a retrospective cohort of 253 patients with PTC with LN metastasis [28]. Lee et al. reported that the eighth edition of the AJCC/UICC staging system or the 2015 ATA risk stratification had higher predictive power for recurrence in patients with PTC when combined with LNR [29]. Parvathareddy et al. retrospectively reviewed a cohort of 1407 patients with PTC and concluded that LNR predicted tumor recurrence better than the AJCC/UICC N stage (odds ratio, 1.96 vs. 1.30; p-value, 0.0184 vs. 0.3831) [30]. Kim et al. found, in a study with 745 patients with N1b PTC, that a lateral LNR > 0.3 was predictive of cancer-specific mortality [21]. If more evidence is accumulated, the LNR can be included in the staging system in the future.
Several studies have investigated cutoff values of LNR to verify its prognostic ability in patients with N1b PTC. Yuksel et al. reported that a cutoff of 0.21 for LNR was a predictor of DFS in patients with N1b PTC [31]. Lee et al. revealed that a cutoff of 0.218 for LNR was a predictor of recurrence in patients with N1b PTC [32]. Park et al. reported that an LNR > 0.22 significantly reduced loco-regional recurrence-free survival in patients with N1b PTC [33]. In this study, we found that the incidence of recurrence tended to be higher in patients with LNR ≥ 0.23 than in those with LNR < 0.23, but the difference was not significant (6.2% vs. 1.7%, p = 0.058).
Only a few studies have reported that CLNR in patients with N1b PTC had clinical significance. In a study of 324 patients with N1b PTC, a CLNR > 0.42 was an independent prognostic factor predicting loco-regional recurrence [34]. Ryu et al. described, in another study, that CLNR > 0.44 was associated with worse prognosis in patients with N1b PTC [35]. In our analysis, CLNR of 0.7 as a cutoff was statistically significant for predicting DFS. The differences in cutoff values of CLNR between studies may result from differences in the proportion of recurrent patients. Eleven (3.6%) patients developed recurrence in our study, whereas 14.5% and 21.5% of patients, respectively, developed recurrence in the other two studies mentioned above. Another reason why the cutoff values varied from study to study was the difference in the extent of LN dissection. The two studies cited above showed a smaller number of harvested LNs in the central compartment than in the present study. The number of harvested LNs in the central compartment was 11.0 ± 6.0 in one of those two previous studies [34] and 7.4 ± 6.0 in the other [35]. In contrast, our data demonstrated that 13.6 ± 7.6 LNs were harvested and examined in the central compartment. The mean values of LNs harvested in the central compartment were 13.2 in the low-LNR group and 14.3 in the high-LNR group. In addition, the number of positive central LNs was 9.2 ± 6.3 in the high-LNR group. These results show that more central LNs were harvested from the patients enrolled in this study compared with other studies.
In addition to CLNR, our multivariate Cox regression analysis results showed that perineural invasion was an independent prognostic factor for DFS. Perineural invasion refers to tumor cells circumferentially surrounding a nerve and is associated with an increased rate of recurrence and decreased survival, especially in head and neck cancers [36]. Rowe et al. reported that the perineural invasion of PTC was associated with extrathyroidal invasion [37]. To the best of our knowledge, no clinical study has revealed the relationship between perineural invasion and recurrence in thyroid cancer. A multicenter study in a large cohort should be performed to confirm the role of perineural invasion as a prognostic factor.
There were some limitations in this retrospective study that should be considered. This was a single-center study, and selection bias could have occurred, so our results may not be applicable to the broader population. Our results are difficult to generalize to patients with PTC because we only included pathologic N1b PTC. Other LN-related factors, such as extra-nodal extension or the maximal diameter of metastatic LNs, were not considered in our analysis, although they were reported to have prognostic value in previous studies [38,39]. We have a plan to perform a prospective study dealing with more LN-related factors in the future to overcome the limitations.
Our study has several strengths. All patients were diagnosed, treated, and followed up with according to a single standardized protocol. The relatively long period of postoperative follow-up also differentiated this study from others [34,35]. In addition, the metastatic and harvested LNs were evaluated and counted by a single pathology team, which increased the reliability of the data.
5. Conclusions
Our analysis indicated that CLNR was associated with recurrence in patients undergoing surgery after diagnosis with N1b PTC. CLNR ≥ 0.7 and perineural invasion were independent predictors of worse DFS. Notably, neither LNR in the whole neck nor LLNR was significant. This indicates that CLNR might be important in predicting recurrence with both N1a and N1b PTC. We expect this analysis to shed light on future investigations and to contribute to a better staging system or guidelines. In addition, this study provides evidence for which patients are more likely to experience recurrence after surgery. We recommend that attention should be given to patients with CLNR ≥ 0.7 after surgery for N1b PTC.
Author Contributions
Conceptualization, K.K.; methodology, K.K., J.S.B. and J.S.K.; software, K.K., I.K.K. and J.S.B.; validation, K.K. and J.P.; formal analysis, K.K. and J.P.; investigation, K.K. and J.P.; resources, K.K., J.S.B. and J.S.K.; data curation, K.K. and I.K.K.; writing—original draft preparation, K.K. and I.K.K.; writing—review and editing, all authors; visualization, K.K. and J.P.; supervision, J.S.B. and J.S.K.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul St. Mary’s Hospital, The Catholic University of Korea (IRB No: KC22RISI0318 and date of approval: 23 May 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We would like to thank all the nurses who participated in the surgery and contributed to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rossi, E.D.; Pantanowitz, L.; Hornick, J.L. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021, 9, 193–194. [Google Scholar] [CrossRef]
- Ganly, I.; Nixon, I.J.; Wang, L.Y.; Palmer, F.L.; Migliacci, J.C.; Aniss, A.; Sywak, M.; Eskander, A.E.; Freeman, J.L.; Campbell, M.J. Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid 2015, 25, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Kim, W.G.; Kim, T.H.; Kim, H.K.; Kim, B.H.; Yi, H.-S.; Kim, E.S.; Kim, H.; Kim, Y.N.; Kim, E.H. Disease-specific mortality of differentiated thyroid cancer patients in Korea: A multicenter cohort study. Endocrinol. Metab. 2017, 32, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.H.; Elashoff, D.A.; Abemayor, E.; St John, M.A. Surgery for papillary thyroid carcinoma: Is lobectomy enough? Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Radowsky, J.S.; Howard, R.S.; Burch, H.B.; Stojadinovic, A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014, 24, 241–244. [Google Scholar] [CrossRef]
- Gardner, R.E.; Tuttle, R.M.; Burman, K.D.; Haddady, S.; Truman, C.; Sparling, Y.H.; Wartofsky, L.; Sessions, R.B.; Ringel, M.D. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 309–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, H.J.; Park, C.S.; Kim, B.W. Frozen section analysis of central lymph nodes in papillary thyroid cancer: The significance in determining the extent of surgery. Gland Surg. 2022, 11, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann. Surg. Oncol. 2015, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Yan, T.; Qiu, W.W.; Fan, Y.B.; Yang, Z.L. The prognosis of skip metastasis in papillary thyroid microcarcinoma is better than that of continuous metastasis. J. Clin. Endocrinol. Metab. 2022, 107, 1589–1598. [Google Scholar] [CrossRef]
- Shaha, A.R. TNM classification of thyroid carcinoma. World J. Surg. 2007, 31, 879–887. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, M.J.; Oh, H.S.; Park, S.; Song, D.E.; Sung, T.Y.; Kim, T.Y.; Chung, K.W.; Kim, W.B.; Shong, Y.K.; et al. Prognostic implication of N1b classification in the eighth edition of the tumor-node-metastasis staging system of differentiated thyroid cancer. Thyroid 2018, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Song, Y.; Xu, G.; Bai, Y.; Wang, T.; Fei, K.; Zhang, B. Level IIb neck dissection guided by fine-needle aspiration for N1b papillary thyroid carcinoma. Surg. Oncol. 2022, 40, 101705. [Google Scholar] [CrossRef]
- Nam, S.H.; Bae, M.R.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral. Oncol. 2018, 87, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann. Thorac. Surg. 2012, 93, 1614–1619; discussion 1619–1620. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Hosoda, K.; Ema, A.; Watanabe, M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur. J. Surg. Oncol. 2016, 42, 1253–1260. [Google Scholar] [CrossRef]
- Sjo, O.H.; Merok, M.A.; Svindland, A.; Nesbakken, A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis. Colon Rectum 2012, 55, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.F.; Chen, H.; Sippel, R.S. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2013, 20, 1906–1911. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. Ann. Surg. 2019, 269, 966–971. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, K.; Park, S.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Ahn, H.S.; et al. Refining the eighth edition AJCC TNM classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral. Oncol. 2018, 78, 80–86. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.H.; Lee, C.H.; Chen, H.A.; Loh, E.W.; Tam, K.W. Prophylactic central neck dissection for papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes: A systematic review and meta-analysis. World J. Surg. 2018, 42, 2846–2857. [Google Scholar] [CrossRef]
- Liu, F.H.; Kuo, S.F.; Hsueh, C.; Chao, T.C.; Lin, J.D. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J. Surg. Oncol. 2015, 112, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; LiVolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [CrossRef] [Green Version]
- Sakin, A.; Aldemir, M.N. Lymph node ratio predicts long-term survival in lymph node-positive breast cancer. Eur. J. Breast Health 2020, 16, 270–275. [Google Scholar] [CrossRef]
- Macedo, F.; Sequeira, H.; Ladeira, K.; Bonito, N.; Viana, C.; Martins, S. Metastatic lymph node ratio as a better prognostic tool than the TNM system in colorectal cancer. Future Oncol. 2021, 17, 1519–1532. [Google Scholar] [CrossRef]
- Khan, J.; Ullah, A.; Matolo, N.; Waheed, A.; Nama, N.; Sharma, N.; Ballur, K.; Gilstrap, L.; Singh, S.G.; Ghleilib, I.; et al. Prognostic value of lymph node ratio in cutaneous melanoma: A systematic review. Cureus 2021, 13, e19117. [Google Scholar] [CrossRef]
- Yip, J.; Orlov, S.; Orlov, D.; Vaisman, A.; Hernandez, K.G.; Etarsky, D.; Kak, I.; Parvinnejad, N.; Freeman, J.L.; Walfish, P.G. Predictive value of metastatic cervical lymph node ratio in papillary thyroid carcinoma recurrence. Head Neck 2013, 35, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.G.; Kim, K.; Yim, S.H.; Ryu, H.; Lee, C.R.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. Clinical value of lymph node ratio integration with the 8(th) edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci. Rep. 2019, 9, 13361. [Google Scholar] [CrossRef]
- Parvathareddy, S.K.; Siraj, A.K.; Qadri, Z.; Ahmed, S.O.; DeVera, F.; Al-Sobhi, S.; Al-Dayel, F.; Al-Kuraya, K.S. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr. Connect. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, U.M.; Turanli, S.; Acar, Y.; Berberoglu, U. The prognostic factors for clinical N1b patients in thyroid papillary carcinoma. J. Cancer Res. Ther. 2019, 15, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.W.; Son, S.H.; Hong, C.M.; Jeong, J.H.; Jeong, S.Y.; Ahn, B.C.; Lee, J. Prognostic value of lymph node uptake on pretreatment F-18 fdg pet/ct in patients with N1b papillary thyroid carcinoma. Endocr. Pract. 2019, 25, 787–793. [Google Scholar] [CrossRef]
- Park, Y.M.; Wang, S.G.; Shin, D.H.; Kim, I.J.; Son, S.M.; Lee, B.J. Lymph node status of lateral neck compartment in patients with N1b papillary thyroid carcinoma. Acta Otolaryngol. 2016, 136, 319–324. [Google Scholar] [CrossRef]
- Lee, Y.M.; Sung, T.Y.; Kim, W.B.; Chung, K.W.; Yoon, J.H.; Hong, S.J. Risk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissection. Br. J. Surg. 2016, 103, 1020–1025. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Cho, J.S.; Yoon, J.H.; Park, M.H. Identifying risk factors for recurrence of papillary thyroid cancer in patients who underwent modified radical neck dissection. World J. Surg. Oncol. 2018, 16, 205. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Dill, T.; Griffin, N.; Jobling, P.; Faulkner, S.; Paul, J.W.; King, S.; Smith, R.; Hondermarck, H. Innervation of papillary thyroid cancer and its association with extra-thyroidal invasion. Sci. Rep. 2020, 10, 1539. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, M.; Ito, Y.; Hirokawa, M.; Miya, A.; Shimizu, K.; Miyauchi, A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J. Surg. 2010, 34, 3007–3014. [Google Scholar] [CrossRef]
- Ito, Y.; Kudo, T.; Takamura, Y.; Kobayashi, K.; Miya, A.; Miyauchi, A. Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J. Surg. 2012, 36, 593–597. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).