Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Non-Curative ER for SESCC
2.1. Non-Curative ER in the Guidelines
2.2. LNM and Metastatic Recurrence in Non-Curative ER
2.3. Esophagectomy or CRT, the Preferable Optimal Treatment Option as an Additional Treatment following Non-Curative ER for SESCC
2.4. A Novel Treatment Method following Non-Curative ER
2.5. Prognosis and Prognostic Factors
3. Non-Curative ER for EGCs
3.1. Non-Curative ER in the Guidelines
3.2. LNM in Non-Curative ER
3.3. Metastatic Recurrence after Non-Curative ER without Additional Treatment
3.4. Metastatic Recurrence after Additional Gastrectomy
3.5. Prognosis and Prognostic Factors
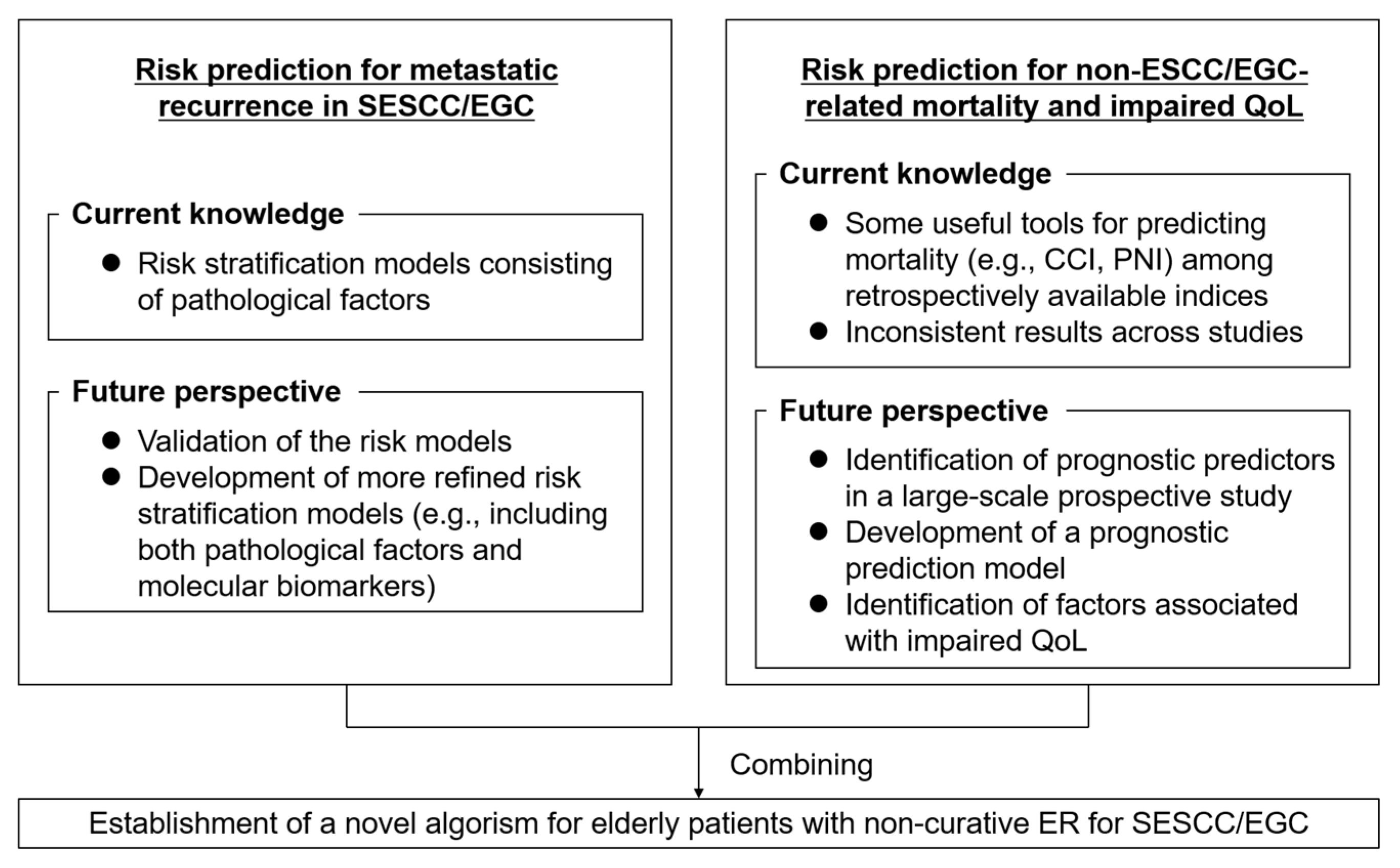
4. Current Issues and Future Perspective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muto, M.; Minashi, K.; Yano, T.; Saito, Y.; Oda, I.; Nonaka, S.; Omori, T.; Sugiura, H.; Goda, K.; Kaise, M.; et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J. Clin. Oncol. 2010, 28, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, W.; Koike, T.; Ogata, Y.; Kondo, Y.; Ara, N.; Uno, K.; Asano, N.; Imatani, A.; Masamune, A. Comparison of magnifying endoscopy with blue light imaging and narrow band imaging for determining the invasion depth of superficial esophageal squamous cell carcinoma by the Japanese Esophageal Society’s intrapapillary capillary loop classification. Diagnostics 2021, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Kawada, K.; Dohi, O.; Kitamura, S.; Koike, T.; Hori, S.; Kanzaki, H.; Murao, T.; Yagi, N.; Sasaki, F.; et al. Linked color imaging focused on neoplasm detection in the upper gastrointestinal tract: A randomized trial. Ann. Intern. Med. 2021, 174, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kakushima, N.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021, 70, 67–75. [Google Scholar] [CrossRef]
- Katada, C.; Yokoyama, T.; Yano, T.; Kaneko, K.; Oda, I.; Shimizu, Y.; Doyama, H.; Koike, T.; Takizawa, K.; Hirao, M.; et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology 2016, 151, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, W.; Gotoda, T.; Koike, T.; Masamune, A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig. Endosc. 2020, 32, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Probst, A.; Aust, D.; Markl, B.; Anthuber, M.; Messmann, H. Early esophageal cancer in Europe: Endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015, 47, 113–121. [Google Scholar] [CrossRef]
- Tsujii, Y.; Nishida, T.; Nishiyama, O.; Yamamoto, K.; Kawai, N.; Yamaguchi, S.; Yamada, T.; Yoshio, T.; Kitamura, S.; Nakamura, T.; et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: A multicenter retrospective cohort study. Endoscopy 2015, 47, 775–783. [Google Scholar] [CrossRef]
- Tanabe, S.; Ishido, K.; Matsumoto, T.; Kosaka, T.; Oda, I.; Suzuki, H.; Fujisaki, J.; Ono, H.; Kawata, N.; Oyama, T.; et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: A multicenter collaborative study. Gastric Cancer 2017, 20, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.C.; Kim, D.H.; Gong, E.J.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Ten-year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J. Intern. Med. 2016, 31, 1064–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.Q.; Zhang, Z.C.; Chen, W.F.; Xu, M.D.; Chen, S.Y.; Zhong, Y.S.; Zhang, Y.Q.; Hu, J.W.; Cai, M.Y.; Yao, L.Q.; et al. Repeat endoscopic submucosal dissection as salvage treatment for local recurrence of esophageal squamous cell carcinoma after initial endoscopic submucosal dissection. Gastrointest. Endosc. 2022, 96, 18–27.e1. [Google Scholar] [CrossRef]
- Kim, G.H.; Choi, K.D.; Ko, Y.; Park, T.; Kim, K.W.; Park, S.Y.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; et al. Impact of comorbidities, sarcopenia, and nutritional status on the long-term outcomes after endoscopic submucosal dissection for early gastric cancer in elderly patients aged ≥ 80 years. Cancers 2021, 13, 3598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Soyfoo, M.D.; Cao, J.L.; Sang, H.M.; Xu, S.F.; Jiang, J.X. Histopathological characteristics and therapeutic outcomes of endoscopic submucosal dissection for gastric high-grade intraepithelial neoplasia. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Koike, T.; Abe, H.; Ogata, Y.; Saito, M.; Jin, X.; Kanno, T.; Uno, K.; Asano, N.; Imatani, A.; et al. Recent approach for preventing complications in upper gastrointestinal endoscopic submucosal dissection. DEN Open 2021, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Rahmi, G.; Perrod, G.; Pioche, M.; Canard, J.M.; Cesbron-Metivier, E.; Boursier, J.; Samaha, E.; Vienne, A.; Lepilliez, V.; et al. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: A multicenter Western study. Endoscopy 2019, 51, 298–306. [Google Scholar] [CrossRef]
- Fleischmann, C.; Probst, A.; Ebigbo, A.; Faiss, S.; Schumacher, B.; Allgaier, H.P.; Dumoulin, F.L.; Steinbrueck, I.; Anzinger, M.; Marienhagen, J.; et al. Endoscopic submucosal dissection in Europe: Results of 1000 neoplastic lesions from the German endoscopic submucosal dissection registry. Gastroenterology 2021, 161, 1168–1178. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libanio, D.; Bastiaansen, B.A.J.; Bhandari, P.; Bisschops, R.; Bourke, M.J.; Esposito, G.; Lemmers, A.; Maselli, R.; Messmann, H.; et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2022. Endoscopy 2022, 54, 591–622. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus 2019, 16, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus 2019, 16, 25–43. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Kanno, T.; Yuan, Y.; Koike, T.; Moayyedi, P.; Masamune, A. Prevalence and risk factors for lymph node metastasis after non-curative endoscopic resection for early gastric cancer: A systematic review and meta-analysis. J. Gastroenterol. 2020, 55, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.B.; Li, S.B.; Yang, Z.H.; Tong, Q. Prediction of lymph node metastasis in superficial esophageal squamous cell carcinoma in Asia: A systematic review and meta-analysis. Dis. Esophagus 2020, 33, doaa032. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Zhang, X.; Su, Y.; Hao, S.; Teng, H.; Guo, X.; Yang, Y.; Sun, Y.; Mao, T.; Li, Z. The possibility of endoscopic treatment of cN0 submucosal esophageal cancer: Results from a surgical cohort. Surg. Endosc. 2021, 35, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Hatta, W.; Koike, T.; Takahashi, S.; Shimada, T.; Hikichi, T.; Toya, Y.; Tanaka, I.; Onozato, Y.; Hamada, K.; Fukushi, D.; et al. Risk of metastatic recurrence after endoscopic resection for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa: A multicenter retrospective study. J. Gastroenterol. 2021, 56, 620–632. [Google Scholar] [CrossRef]
- Tajima, Y.; Nakanishi, Y.; Tachimori, Y.; Kato, H.; Watanabe, H.; Yamaguchi, H.; Yoshimura, K.; Kusano, M.; Shimoda, T. Significance of involvement by squamous cell carcinoma of the ducts of esophageal submucosal glands. Analysis of 201 surgically resected superficial squamous cell carcinomas. Cancer 2000, 89, 248–254. [Google Scholar] [CrossRef]
- Eguchi, T.; Nakanishi, Y.; Shimoda, T.; Iwasaki, M.; Igaki, H.; Tachimori, Y.; Kato, H.; Yamaguchi, H.; Saito, D.; Umemura, S. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: Analysis of 464 surgically resected cases. Mod. Pathol. 2006, 19, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Bollschweiler, E.; Baldus, S.E.; Schroder, W.; Prenzel, K.; Gutschow, C.; Schneider, P.M.; Holscher, A.H. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006, 38, 149–156. [Google Scholar] [CrossRef]
- Akutsu, Y.; Uesato, M.; Shuto, K.; Kono, T.; Hoshino, I.; Horibe, D.; Sazuka, T.; Takeshita, N.; Maruyama, T.; Isozaki, Y.; et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: A retrospective analysis of 295 patients. Ann. Surg. 2013, 257, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Katada, C.; Muto, M.; Momma, K.; Arima, M.; Tajiri, H.; Kanamaru, C.; Ooyanagi, H.; Endo, H.; Michida, T.; Hasuike, N.; et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae—A multicenter retrospective cohort study. Endoscopy 2007, 39, 779–783. [Google Scholar] [CrossRef]
- Yamashina, T.; Ishihara, R.; Nagai, K.; Matsuura, N.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; Hanaoka, N.; Takeuchi, Y.; et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2013, 108, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakamura, K.; Hirano, M.; Esaki, M.; et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multicenter retrospective study in Japan. J. Gastroenterol. 2017, 52, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, K.; Hatta, W.; Gotoda, T.; Kawata, N.; Nakagawa, M.; Takahashi, A.; Esaki, M.; Mitoro, A.; Yamada, S.; Tanaka, K.; et al. Recurrence patterns and outcomes of salvage surgery in cases of non-curative endoscopic submucosal dissection without additional radical surgery for early gastric cancer. Digestion 2019, 99, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Hoshi, N.; Yoshizaki, T.; Fujishima, Y.; Ishida, T.; Morita, Y.; Ejima, Y.; Toyonaga, T.; Kakechi, Y.; Yokosaki, H.; et al. Endoscopic submucosal dissection (ESD) with additional therapy for superficial esophageal cancer with submucosal invasion. Intern. Med. 2015, 54, 2803–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koterazawa, Y.; Nakamura, T.; Oshikiri, T.; Kanaji, S.; Tanaka, S.; Ishida, T.; Yamashita, K.; Matsuda, T.; Morita, Y.; Suzuki, S.; et al. A comparison of the clinical outcomes of esophagectomy and chemoradiotherapy after non-curative endoscopic submucosal dissection for esophageal squamous cell carcinoma. Surg. Today 2018, 48, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, G.; Yamazaki, H.; Aibe, N.; Masui, K.; Sasaki, N.; Shimizu, D.; Kimoto, T.; Shiozaki, A.; Dohi, O.; Fujiwara, H.; et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: Choice of new approach. Radiat. Oncol. 2018, 13, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanie, Y.; Okamura, A.; Asari, T.; Maruyama, S.; Sakamoto, K.; Fujiwara, D.; Kanamori, J.; Imamura, Y.; Ishiyama, A.; Yoshio, T.; et al. Additional treatment following non-curative endoscopic resection for esophageal squamous cell carcinoma: A comparison of outcomes between esophagectomy and chemoradiotherapy. Ann. Surg. Oncol. 2021, 28, 8428–8435. [Google Scholar] [CrossRef]
- Miyata, H.; Sugimura, K.; Kanemura, T.; Takeoka, T.; Yamamoto, M.; Shinno, N.; Hara, H.; Omori, T.; Yamamoto, S.; Ishihara, R.; et al. Clinical outcome of additional esophagectomy after endoscopic treatment for superficial esophageal cancer. Ann. Surg. Oncol. 2021, 28, 7230–7239. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Sato, D.; Inaba, A.; Nishihara, K.; Takashima, K.; Nakajo, K.; Yukami, H.; Mishima, S.; Sawada, K.; Kotani, D.; et al. Long-term clinical outcomes of patients diagnosed with pT1a-muscularis mucosae with lymphovascular invasion or pT1b after endoscopic resection for cT1N0M0 esophageal squamous cell carcinoma. Esophagus 2022, 19, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Su, Y.; Zhang, X.; Liu, J.; Zhang, H.; Li, B.; Hua, R.; Tan, L.; Chen, H.; Li, Z. Esophagectomy versus definitive chemoradiotherapy for patients with clinical stage N0 and pathological stage T1b esophageal squamous cell carcinoma after endoscopic submucosal dissection: Study protocol for a multicenter randomized controlled trial (Ad-ESD Trial). Trials 2020, 21, 603. [Google Scholar]
- Kato, H.; Sato, A.; Fukuda, H.; Kagami, Y.; Udagawa, H.; Togo, A.; Ando, N.; Tanaka, O.; Shinoda, M.; Yamana, H.; et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn. J. Clin. Oncol. 2009, 39, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, G.; Sasamoto, R.; Abe, E.; Ohta, A.; Sato, H.; Tanaka, K.; Maruyama, K.; Kaizu, M.; Ayukawa, F.; Yamana, N.; et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat. Oncol. 2015, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Yoshimizu, S.; Yoshio, T.; Ishiyama, A.; Tsuchida, T.; Horiuchi, Y.; Omae, M.; Hirasawa, T.; Asari, T.; Chin, K.; Fujisaki, J. Long-term outcomes of combined endoscopic resection and chemoradiotherapy for esophageal squamous cell carcinoma with submucosal invasion. Dig. Liver. Dis. 2018, 50, 833–838. [Google Scholar] [CrossRef]
- Tsou, Y.K.; Lee, C.H.; Le, P.H.; Chen, B.H. Adjuvant therapy for pT1a-m3/pT1b esophageal squamous cell carcinoma after endoscopic resection: Esophagectomy or chemoradiotherapy? A critical review. Crit. Rev. Oncol. Hematol. 2020, 147, 102883. [Google Scholar] [CrossRef]
- Minashi, K.; Nihei, K.; Mizusawa, J.; Takizawa, K.; Yano, T.; Ezoe, Y.; Tsuchida, T.; Ono, H.; Iizuka, T.; Hanaoka, N.; et al. Efficacy of endoscopic resection and selective chemoradiotherapy for stage I esophageal squamous cell carcinoma. Gastroenterology 2019, 157, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, Y.; Hatta, W.; Koike, T.; Saito, M.; Jin, X.; Nakagawa, K.; Kanno, T.; Uno, K.; Asano, N.; Imatani, A.; et al. Predictors of early and late mortality after endoscopic resection for esophageal squamous cell carcinoma. Tohoku J. Exp. Med. 2021, 253, 29–39. [Google Scholar] [CrossRef]
- Nakajo, K.; Abe, S.; Oda, I.; Ishihara, R.; Tanaka, M.; Yoshio, T.; Katada, C.; Yano, T. Impact of the Charlson Comorbidity Index on the treatment strategy and survival in elderly patients after non-curative endoscopic submucosal dissection for esophageal squamous cell carcinoma: A multicenter retrospective study. J. Gastroenterol. 2019, 54, 871–880. [Google Scholar] [CrossRef]
- Suzuki, T.; Furukawa, K.; Funasaka, K.; Ishikawa, E.; Sawada, T.; Maeda, K.; Yamamura, T.; Ishikawa, T.; Ohno, E.; Nakamura, M.; et al. Long-term prognostic predictors of esophageal squamous cell carcinoma potentially indicated for endoscopic submucosal dissection. Digestion 2021, 102, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Dohi, O.; Yamada, S.; Harusato, A.; Horie, R.; Yasuda, T.; Yamada, N.; Horii, Y.; Majima, A.; Zen, K.; et al. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: A multicenter cohort study. Surg. Endosc. 2022, 36, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Nagami, Y.; Yamamura, M.; Tanoue, K.; Sakai, T.; Maruyama, H.; Ominami, M.; Nadatani, Y.; Fukunaga, S.; Otani, K.; et al. Evaluation of long-term survival in patients with severe comorbidities after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Surg. Endosc. 2022, 36, 5011–5022. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hatta, W.; Takahashi, S.; Koike, T.; Ohira, T.; Hikichi, T.; Toya, Y.; Tanaka, I.; Onozato, Y.; Hamada, K.; et al. A combined assessment of clinical and pathological prognostic factors for deciding treatment strategies for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa after endoscopic submucosal dissection. Dig. Endosc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Jung, D.H.; Bae, Y.S.; Yoon, S.O.; Lee, Y.C.; Kim, H.; Noh, S.H.; Park, H.; Choi, S.H.; Kim, J.H.; Kim, H. Poorly differentiated carcinoma component in submucosal layer should be considered as an additional criterion for curative endoscopic resection of early gastric cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S772–S777. [Google Scholar] [CrossRef]
- Miyahara, K.; Hatta, W.; Nakagawa, M.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Hirano, M.; Esaki, M.; et al. The role of an undifferentiated component in submucosal invasion and submucosal invasion depth after endoscopic submucosal dissection for early gastric cancer. Digestion 2018, 98, 161–168. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Koike, T.; Masamune, A. A recent argument for the use of endoscopic submucosal dissection for early gastric cancers. Gut Liver 2020, 14, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Takizawa, K.; Hirasawa, T.; Takeuchi, Y.; Ishido, K.; Hoteya, S.; Yano, T.; Tanaka, S.; Endo, M.; Nakagawa, M.; et al. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: ‘Real-world evidence’ in Japan. Dig. Endosc. 2019, 31, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, M.; Hatta, W.; Shimosegawa, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Oka, S.; Hoteya, S.; Nakagawa, M.; Hirano, M.; et al. Age affects clinical management after non-curative endoscopic submucosal dissection for early gastric cancer. Dig. Dis. 2019, 37, 423–433. [Google Scholar] [CrossRef]
- Probst, A.; Schneider, A.; Schaller, T.; Anthuber, M.; Ebigbo, A.; Messmann, H. Endoscopic submucosal dissection for early gastric cancer: Are expanded resection criteria safe for Western patients? Endoscopy 2017, 49, 855–865. [Google Scholar] [CrossRef]
- Fujita, J.; Uyama, I.; Sugioka, A.; Komori, Y.; Matsui, H.; Hasumi, A. Laparoscopic right hemicolectomy with radical lymph node dissection using the no-touch isolation technique for advanced colon cancer. Surg. Today 2001, 31, 93–96. [Google Scholar] [CrossRef]
- Gall, T.M.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014, 149, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hatta, W.; Hirano, M.; et al. Long-term oncological outcomes of submucosal manipulation during non-curative endoscopic submucosal dissection for submucosal invasive gastric cancer: A multicenter retrospective study in Japan. Surg. Endosc. 2018, 32, 196–203. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A scoring system to stratify curability after endoscopic submucosal dissection for early gastric cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef]
- For iOS. Available online: https://apps.apple.com/app/ecura/id1490245005 (accessed on 28 July 2022).
- For Android. Available online: https://play.google.com/store/apps/details?id=hatta.eCura (accessed on 28 July 2022).
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirasawa, T.; Gotoda, T.; Miyata, S.; Kato, Y.; Shimoda, T.; Taniguchi, H.; Fujisaki, J.; Sano, T.; Yamaguchi, T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009, 12, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lee, J.H. Expanding indications of endoscopic submucosal dissection for early gastric cancer: Hope or hype? Gut Liver 2015, 9, 135–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. Is the eCura system useful for selecting patients who require radical surgery after non-curative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018, 21, 481–489. [Google Scholar] [CrossRef]
- Yamada, S.; Hatta, W.; Shimosegawa, T.; Takizawa, K.; Oyama, T.; Kawata, N.; Takahashi, A.; Oka, S.; Hoteya, S.; Nakagawa, M.; et al. Different risk factors between early and late cancer recurrences in patients without additional surgery after non-curative endoscopic submucosal dissection for early gastric cancer. Gastrointest. Endosc. 2019, 89, 950–960. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Oka, S.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. Is additional surgery always sufficient for preventing recurrence after endoscopic submucosal dissection with curability C-2 for early gastric cancer? Ann. Surg. Oncol. 2019, 26, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Bentrem, D.J.; Besh, S.; D’Amico, T.A.; Das, P.; Denlinger, C.; Fakih, M.G.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; et al. Gastric cancer, version 2.2013: Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2013, 11, 531–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.F.; Kim, S.; Kim, K.; Li, C.; Oh, S.J.; Hyung, W.J.; Rha, S.Y.; Chung, H.C.; Choi, S.H.; Wang, L.B.; et al. Prediction of recurrence of early gastric cancer after curative resection. Ann. Surg. Oncol. 2009, 16, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Saku, M.; Kishihara, F.; Maehara, Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. Br. J. Surg. 2005, 92, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Folli, S.; Morgagni, P.; Roviello, F.; De Manzoni, G.; Marrelli, D.; Saragoni, L.; Leo, A.D.; Gaudio, M.; Nanni, O.; Carli, A.; et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn. J. Clin. Oncol. 2001, 31, 495–499. [Google Scholar] [CrossRef]
- Shin, H.B.; An, J.Y.; Lee, S.H.; Choi, Y.Y.; Kim, J.W.; Sohn, S.S.; Noh, S.H. Is adjuvant chemotherapy necessary in pT1N1 gastric cancer? BMC Cancer 2017, 17, 287. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; An, J.Y.; Lee, J.; Sohn, T.S.; Kim, S. Adjuvant chemotherapy versus chemoradiotherapy versus surgery alone for early gastric cancer with one or two lymph node metastasis. Ann. Surg. Oncol. 2018, 25, 1616–1624. [Google Scholar] [CrossRef]
- Xie, Y.; Du, D.; Song, X.; Li, X.; Ni, Z.; Huang, H. The role of chemotherapy in patients with stage IB gastric adenocarcinoma: A real-world competing risk analysis. World J. Surg. Oncol. 2022, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Lee, H.; Min, B.H.; Lee, J.H.; Rhee, P.L.; Kim, J.J.; Kim, K.M.; Kim, S. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br. J. Surg. 2015, 102, 1394–1401. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, S.G.; Lim, J.H.; Choi, J.; Im, J.P.; Kim, J.S.; Kim, W.H.; Jung, H.C. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg. Endosc. 2015, 29, 1145–1155. [Google Scholar] [CrossRef]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Nonaka, S.; Yoshinaga, S.; Saito, Y.; Fukagawa, T.; Katai, H. Clinical outcomes of early gastric cancer patients after non-curative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer 2017, 20, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawata, N.; Kakushima, N.; Takizawa, K.; Tanaka, M.; Makuuchi, R.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Sugino, T.; et al. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg. Endosc. 2017, 31, 1607–1616. [Google Scholar] [CrossRef]
- Kikuchi, S.; Kuroda, S.; Nishizaki, M.; Kagawa, T.; Kanzaki, H.; Kawahara, Y.; Kagawa, S.; Tanaka, T.; Okada, H.; Fujiwara, T. Management of early gastric cancer that meet the indication for radical lymph node dissection following endoscopic resection: A retrospective cohort analysis. BMC Surg. 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, T.; Ishido, K.; Tanabe, S.; Wada, T.; Azuma, M.; Kawanishi, N.; Yamane, S.; Watanabe, A.; Katada, C.; Koizumi, W. Long-term outcomes of patients with early gastric cancer found to have lesions for which endoscopic treatment is not indicated on histopathological evaluation after endoscopic submucosal dissection. Surg. Endosc. 2018, 32, 1314–1323. [Google Scholar] [CrossRef]
- Dohi, O.; Hatta, W.; Gotoda, T.; Naito, Y.; Oyama, T.; Kawata, N.; Takahashi, A.; Oka, S.; Hoteya, S.; Nakagawa, M.; et al. Long-term outcomes after non-curative endoscopic submucosal dissection for early gastric cancer according to hospital volumes in Japan: A multicenter propensity-matched analysis. Surg. Endosc. 2019, 33, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. (In Japanese) [Google Scholar] [PubMed]
- Yoshifuku, Y.; Oka, S.; Tanaka, S.; Sanomura, Y.; Miwata, T.; Numata, N.; Hiyama, T.; Chayama, K. Long-term prognosis after endoscopic submucosal dissection for early gastric cancer in super-elderly patients. Surg. Endosc. 2016, 30, 4321–4329. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Oda, I.; Suzuki, H.; Abe, S.; Nonaka, S.; Yoshinaga, S.; Taniguchi, H.; Sekine, S.; Saito, Y. Clinical outcomes and prognostic factors in gastric cancer patients aged ≥85 years undergoing endoscopic submucosal dissection. Gastrointest. Endosc. 2017, 85, 963–972. [Google Scholar] [CrossRef]
- Iwai, N.; Dohi, O.; Naito, Y.; Inada, Y.; Fukui, A.; Takayama, S.; Ogita, K.; Terasaki, K.; Nakano, T.; Ueda, T.; et al. Impact of the Charlson comorbidity index and prognostic nutritional index on prognosis in patients with early gastric cancer after endoscopic submucosal dissection. Dig. Endosc. 2018, 30, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Toya, Y.; Endo, M.; Nakamura, S.; Akasaka, R.; Yanai, S.; Kawasaki, K.; Koeda, K.; Eizuka, M.; Fujita, Y.; Uesugi, N.; et al. Long-term outcomes and prognostic factors with non-curative endoscopic submucosal dissection for gastric cancer in elderly patients aged ≥ 75 years. Gastric Cancer 2019, 22, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Tanoue, K.; Fukunaga, S.; Nagami, Y.; Sakai, T.; Maruyama, H.; Ominami, M.; Otani, K.; Hosomi, S.; Tanaka, F.; Taira, K.; et al. Long-term outcome of endoscopic submucosal dissection for early gastric cancer in patients with severe comorbidities: A comparative propensity score analysis. Gastric Cancer 2019, 22, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Ogata, Y.; Hatta, W.; Ohara, Y.; Koike, T.; Abe, H.; Saito, M.; Jin, X.; Kanno, T.; Uno, K.; Asano, N.; et al. Predictors of early and late mortality after the treatment for early gastric cancers. Dig. Endosc. 2022, 34, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Ishida, M.; Kono, Y.; Hirata, T.; Obayashi, Y.; Gotoda, T.; Ninomiya, Y.; Moritou, Y.; Kunihiro, M.; Kubota, T.; et al. Prognosis after curative resection for stage IA gastric cancer in elderly patients: Endoscopic submucosal dissection versus surgery. Surg. Today 2022. [Google Scholar] [CrossRef]
- Waki, K.; Shichijo, S.; Uedo, N.; Takeuchi, Y.; Maekawa, A.; Kanesaka, T.; Takeuchi, Y.; Higashino, K.; Ishihara, R.; Tanaka, Y.; et al. Long-term outcomes after endoscopic resection for late-elderly patients with early gastric cancer. Gastrointest. Endosc. 2022, 95, 873–883. [Google Scholar] [CrossRef]
- Toya, Y.; Shimada, T.; Hamada, K.; Watanabe, K.; Nakamura, J.; Fukushi, D.; Hatta, W.; Shinkai, H.; Ito, H.; Matsuhashi, T.; et al. Prediction model of 3-year survival after endoscopic submucosal dissection for early gastric cancer in elderly patients aged ≥ 85 years: EGC-2 model. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Shimada, M.; Murano, T.; Takamaru, H.; Morine, Y.; Ikemoto, T.; Saito, Y.; Balaguer, F.; Bujanda, L.; Pellise, M.; et al. A liquid biopsy assay for noninvasive identification of lymph node metastases in T1 colorectal cancer. Gastroenterology 2021, 161, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Koike, T.; Masamune, A. Management following endoscopic resection in elderly patients with early-stage upper gastrointestinal neoplasia. Dig. Endosc. 2020, 32, 861–873. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Koike, T.; Uno, K.; Asano, N.; Imatani, A.; Masamune, A. Is additional gastrectomy required for elderly patients after endoscopic submucosal dissection with endoscopic curability C-2 for early gastric cancer? Digestion 2022, 103, 83–91. [Google Scholar] [CrossRef]
- Scotte, F.; Bossi, P.; Carola, E.; Cudennec, T.; Dielenseger, P.; Gomes, F.; Knox, S.; Strasser, F. Addressing the quality of life needs of older patients with cancer: A SIOG consensus paper and practical guide. Ann. Oncol. 2018, 29, 1718–1726. [Google Scholar] [CrossRef]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, R.; Matsuura, N.; Hanaoka, N.; Yamamoto, S.; Akasaka, T.; Takeuchi, Y.; Higashino, K.; Uedo, N.; Iishi, H. Endoscopic imaging modalities for diagnosing invasion depth of superficial esophageal squamous cell carcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, R.; Mizusawa, J.; Kushima, R.; Matsuura, N.; Yano, T.; Kataoka, T.; Fukuda, H.; Hanaoka, N.; Yoshio, T.; Abe, S.; et al. Assessment of the diagnostic performance of endoscopic ultrasonography after conventional endoscopy for the evaluation of esophageal squamous cell carcinoma invasion depth. JAMA Netw. Open 2021, 4, e2125317. [Google Scholar] [CrossRef]
- Kato, M.; Uedo, N.; Nagahama, T.; Yao, K.; Doyama, H.; Tsuji, S.; Gotoda, T.; Kawamura, T.; Ebi, M.; Yamamoto, K.; et al. Self-study of the non-extension sign in an e-learning program improves diagnostic accuracy of invasion depth of early gastric cancer. Endosc. Int. Open 2019, 7, E871–E882. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhu, M.; Wang, Y.; Niu, Y.; Ji, M.; Li, P.; Zhang, S. Diagnostic efficacy and decision-making role of preoperative endoscopic ultrasonography in early gastric cancer. Front. Med. 2021, 8, 761295. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, K.; Oka, S.; Tanaka, S.; Yorita, N.; Hata, K.; Kotachi, T.; Boda, T.; Arihiro, K.; Chayama, K. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer 2021, 24, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.C.; Xu, M.D.; Zhang, Z.; Cheng, J.; Zhong, Y.S.; Zhang, Y.Q.; Chen, W.F.; Yao, L.Q.; Zhou, P.H.; et al. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest. Endosc. 2019, 89, 806–815. [Google Scholar] [CrossRef] [PubMed]


| SESCC | EGC | |
|---|---|---|
| The rate of detection as locoregional recurrence among patients with metastatic recurrence | 65.2% | 21.4% |
| The rate of no further recurrence among patients undergoing salvage treatment for metastatic recurrence | 83.3% | 20.0% |
| The rate of patients with long-term survival and no further recurrence after salvage treatment among patients with metastatic recurrence | 47.8% | 3.7% |
| Authors, Year | No. of Cases | Recurrence | Treatment-Related Mortality | |
|---|---|---|---|---|
| Additional Esophagectomy | Additional CRT | |||
| Ikeda et al., 2015 [37] | 15 | 11 | 0 (0.0%) vs. 3 (27.2%) | 1 (6.6%) vs. 0 (0.0%) |
| Koterazawa et al., 2018 [38] | 28 | 31 | 0 (0.0%) vs. 5 (16.1%) | 2 (7.1%) vs. 0 (0.0%) |
| Suzuki et al., 2018 [39] | 16 | 16 | 0 (0.0%) vs. 1 (6.3%) | 0 (0.0%) vs. 0 (0.0%) |
| Kanie et al., 2021 [40] | 56 | 52 | 0 (0.0%) vs. 2 (3.8%) | 1 (1.8%) vs. 0 (0.0%) |
| Miyata et al., 2021 [41] | 37 | 123 | 2 (5.4%) vs. 16 (13.0%) | 0 (0.0%) vs. (0.0%) |
| Kadota et al., 2022 [42] | 18 | 50 | 2 (11.1%) vs. 2 (4.0%) | 0 (0.0%) vs. (0.0%) |
| Authors, Year | Study Population | No. of Subjects | Study Design | Prognostic Factors |
|---|---|---|---|---|
| Nakajo et al., 2019 [50] | 75 years | 360 | Multicenter, retrospective | CCI ≥ 2 |
| Ogata et al., 2021 [49] | All | 407 | Single-center, retrospective | Early mortality: ECOG-PS ≥ 2, CCI ≥ 2; Late mortality: ECOG-PS ≥ 2, CCI ≥ 2, age ≥ 80 years |
| Suzuki et al., 2021 [51] | pT1a-EP/LPM/MM or pT1b-SM1 | 286 | Single-center, retrospective | PNI < 45, CCI ≥ 3 |
| Iwai et al., 2021 [52] | All | 659 | Multicenter, retrospective | pT1a-MM/pT1b-SM1, pT1b-SM2, CCI ≥ 3, PNI ≤ 47.75 |
| Hirano et al., 2022 [53] | PS-matched cohort | 138 | Single-center, retrospective | ASA-PS = 3 |
| Shimada et al., 2022 [54] | pT1a-MM/pT1b-SM | 593 | Multicenter, retrospective | Male, CCI ≥ 3, ≥ 75 years, PNI < 45, pathological intermediate-/high-risk 1 |
| Authors, Year | Study Population | No. of Subjects | Study Design | Prognostic Factors |
|---|---|---|---|---|
| Yoshifuku et al., 2016 [91] | ≥85 years | 85 | Single-center, retrospective | ASA-PS ≥ 2 |
| Sekiguchi et al., 2017 [92] | ≥85 years | 108 | Single-center, retrospective | PNI < 44.6 |
| Iwai et al., 2018 [93] | All | 585 | Single-center, retrospective | CCI ≥ 3, ECOG-PS ≥ 2, PNI < 47.7 |
| Toya et al., 2019 [94] | ≥75 years, non-curative ER | 87 | Single-center, retrospective | CCI ≥ 3 |
| Tanoue et al., 2019 [95] | PS-matched cohort | 178 | Single-center, retrospective | ASA-PS = 3. |
| Ogata et al., 2022 [96] | All (including surgery) | 1439 | Single-center, retrospective | Early mortality: age ≥ 85 years, CCI ≥ 2, ASA-PS ≥ 3, ECOG-PS ≥ 2, CAR ≥ 0.028, eCuraC-2-intermediate/high 1, low PMI; Late mortality: age ≥ 75 years, CCI ≥ 2, ASA-PS ≥ 3, ECOG-PS ≥ 2, CAR ≥ 0.028 |
| Miyahara et al., 2022 [97] | ≥80 years (including surgery) | 535 | Single-center, retrospective | age > 80 years, male, ECOG-PS ≥ 2, CCI ≥ 2, BMI ≤ 21.875, PNI ≤ 46.7 |
| Waki et al., 2022 [98] | ≥75 years | 400 | Single-center, retrospective | ECOG-PS ≥ 2, PNI < 49.1, eCuraC-2 |
| Toya et al., 2022 [99] | ≥85 years | 740 | Multicenter, retrospective | GNRI, CCI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatta, W.; Koike, T.; Uno, K.; Asano, N.; Masamune, A. Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection. Cancers 2022, 14, 3757. https://doi.org/10.3390/cancers14153757
Hatta W, Koike T, Uno K, Asano N, Masamune A. Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection. Cancers. 2022; 14(15):3757. https://doi.org/10.3390/cancers14153757
Chicago/Turabian StyleHatta, Waku, Tomoyuki Koike, Kaname Uno, Naoki Asano, and Atsushi Masamune. 2022. "Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection" Cancers 14, no. 15: 3757. https://doi.org/10.3390/cancers14153757
APA StyleHatta, W., Koike, T., Uno, K., Asano, N., & Masamune, A. (2022). Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection. Cancers, 14(15), 3757. https://doi.org/10.3390/cancers14153757








