Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Scope
2.2. Search Strategy
2.3. Data Charting and Extraction Process
2.4. Synthesis, Reporting of Results, and Consultation
3. Results
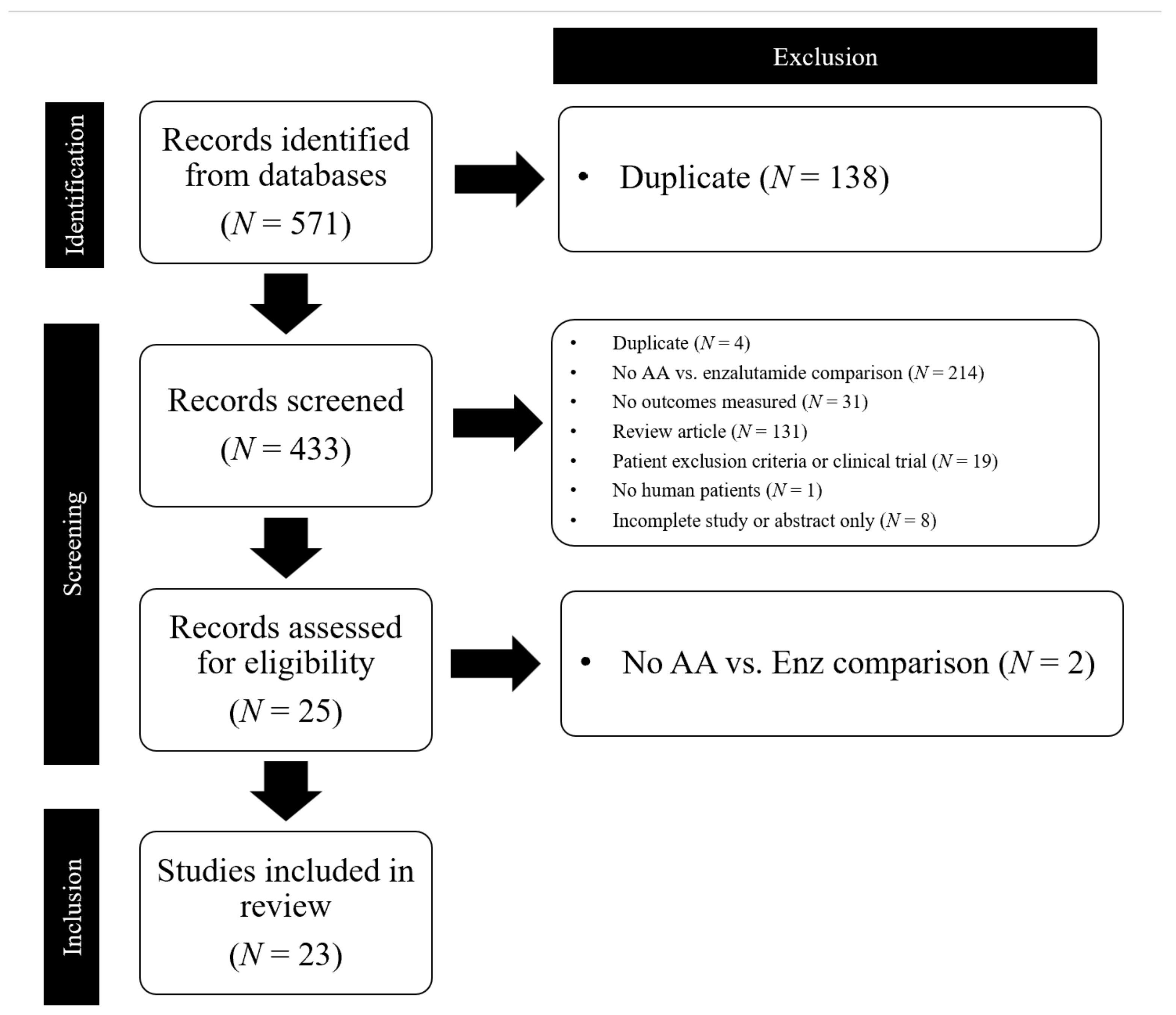
3.1. Selection of Sources
3.2. Characteristics of Included Sources
3.3. Comparison of Disease Progression and Survival
3.4. Comparison of Drug-Associated Toxicities
3.5. Comparison of Treatment Adherence, Dose Reduction, and Dose Modification
| Authors | Study Design | Population Characteristics | Outcomes Reported |
|---|---|---|---|
| Al-Ali, B. et al. (2018) [35] | Retrospective population-based database | CRPC patients (N = 457), mean age 74.4 y, AA N = 195, ENZ N = 139 | OS, MPR, treatment duration, length of hospital stay |
| Banna, G. et al. (2020) [36] | Observational prospective cohort | mCRPC patients (N = 58), median age 76 y, AA N = 22, ENZ N = 36 | Cancer response, OS, radiographic PFS, adherence |
| Behl, A. et al. (2017) [45] | Retrospective population-based database | mCRPC patients, AA N = 2591, ENZ N = 807 | OS, MPR, dose reduction |
| Caffo, O. et al. (2014) [29] | Observational prospective cohort | Progressive CRPC patients, AA N = 26, ENZ N = 31 | Cancer response, cancer progression, toxicities |
| Chang, L. et al. (2019) [37] | Retrospective single-institutional cohort | mCRPC patients with prior docetaxel treatment, AA N = 64, ENZ N = 13 | Cancer response, OS, PFS, toxicities |
| Chowdhury, S. et al. (2020) [38] | Retrospective population-based database | mCRPC patients, AA N = 754, ENZ N = 227 | Time to progression, OS, treatment duration |
| Cindolo, L. et al. (2019) [44] | Retrospective population-based database | mCRPC patients, AA N = 109, ENZ N = 14 | Drug persistence, adherence |
| Crombag, M. et al. (2019) [39] | Retrospective single-institutional cohort | CRPC patients, AA N = 71, ENZ N = 64 | Drug exposure by co-morbidity |
| Demirci, A. et al. (2021) [34] | Retrospective multi-institutional cohort | mCRPC patients (N = 250) | Treatment response, radiographic PFS, OS |
| Freedland, S. et al. (2021) [46] | Retrospective population-based database | mCRPC patients (N = 6069) | Dose reduction |
| George, G. et al. (2021) [40] | Retrospective population-based database | CRPC patients, AA N = 1310, ENZ N = 3579 | Toxicities (cardiovascular) |
| Hu, J. et al. (2021) [15] | Retrospective population-based database | mCRPC patients (N = 2183), AA N = 1773, ENZ N = 410 | Hospitalizations, toxicities (cardiovascular) |
| Jarimba, R. et al. (2021) [2] | Retrospective single-institutional cohort | mCRPC patients (N = 91), AA N = 56, ENZ N = 35 | Treatment response, PFS, toxicities |
| Lu-Yao, G. et al. (2019) [18] | Retrospective population-based database | CRPC patients, AA N = 2845, ENZ N = 1031 | Mortality, hospitalizations, toxicities (cardiovascular) |
| Miyake, H. et al. (2017) [30] | Retrospective single-institutional cohort | mCRPC patients (N = 280), AA N = 113, ENZ N = 167 | Treatment response, cancer progression, toxicities |
| Pilon, D. et al. (2017) [31] | Retrospective population-based database | mCRPC patients, N = 3398, AA N = 2591, ENZ N = 807 | Treatment discontinuation, treatment duration |
| Ramaswamy, K. et al. (2020) [48] | Retrospective population-based database | mCRPC patients (N = 3174), AA N = 1945, ENZ N = 1229 | HRU, costs |
| Salem, S. et al. (2017) [42] | Retrospective single-institutional cohort | mCRPC patients (N = 189), AA N = 76, ENZ N = 113 | Treatment duration, dose reduction, toxicities |
| Scailteux, L. et al. (2020) [33] | Retrospective population-based database | CRPC patients (N = 10,308), AA N = 6585, ENZ N = 3723 | OS |
| Schultz, N. et al. (2018) [47] | Retrospective population-based database | mCRPC patients, AA N = 2310, ENZ N = 920 | Treatment duration, hospitalizations, HRU, costs |
| Shore, N. et al. (2019) [41] | Prospective Phase IV surveillance study | mCRPC patients with exclusion of those with prior chemotherapy, seizure disorder, dementia, or substance abuse, N = 92, AA N = 46, ENZ N = 46 | Dose reduction, toxicities |
| Soleimani, M. et al. (2021) [28] | Retrospective single-institutional cohort | mCRPC patients aged ≥ 80 years (N = 278), AA N = 153, ENZ N = 125 | Treatment response, cancer progression, dose reduction |
| Tagawa, S. et al. (2021) [32] | Retrospective population-based database | mCRPC patients, AA N = 1229, ENZ N = 1945 | OS, treatment duration, toxicities |
3.6. Comparison of Resource Utilization, Hospitalization, and Cost
4. Discussion
4.1. Consideration of Treatment Toxicities
4.2. Differences in Systemic Healthcare Quality Metrics
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, D.; Lee Chuy, K.; Yang, J.C.; Bates, M.; Lombardo, M.; Steingart, R.M. Cardiovascular and Metabolic Effects of Androgen-Deprivation Therapy for Prostate Cancer. J. Oncol. Pract. 2018, 14, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Jarimba, R.S.; Eliseu, M.N.; Pedroso Lima, J.; Quaresma, V.; Moreira, P.; Coelho Nunes, P.; Tavares da Silva, E.; Figueiredo, A.J. Novel hormonal agents for metastatic Castration-Resistant Prostate Cancer: Comparing outcomes. A single-center retrospective study. Arch. Ital. Urol. Androl. 2021, 93, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, T.Y.; Chen, Q.; Shi, X.L.; Xiao, G.A.; Zhao, L.; Xu, C.L.; Zhou, T.; Sun, Y.H. Indirect comparison between abiraterone acetate and enzalutamide for the treatment of metastatic castration-resistant prostate cancer: A systematic review. Asian J. Androl. 2017, 19, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Zhang, X.; Zhao, J.; Ni, Y.; Zhu, S.; He, B.; Dai, J.; Wang, Z.; Wang, Z.; et al. Comparison of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer After Docetaxel Failure: A Systematic Review and Network Meta-analysis. Front. Pharmacol. 2021, 12, 789319. [Google Scholar] [CrossRef] [PubMed]
- Woon, D.T.S.; Finelli, A.; Cheung, D.C.; Martin, L.J.; Alibhai, S.; Wallis, C.J.D.; Diong, C.; Saskin, R.; Kulkarni, G.; Fleshner, N. A Population-based Study Comparing Outcomes for Patients with Metastatic Castrate Resistant Prostate Cancer Treated by Urologists or Medical Oncologists with First Line Abiraterone Acetate or Enzalutamide. Urology 2021, 153, 147–155. [Google Scholar] [CrossRef]
- Shore, N.D.; Ionescu-Ittu, R.; Laliberté, F.; Yang, L.; Lejeune, D.; Yu, L.; Duh, M.S.; Mahendran, M.; Kim, J.; Ghate, S.R. Beyond Frontline Therapy with Abiraterone and Enzalutamide in Metastatic Castration-Resistant Prostate Cancer: A Real-World US Study. Clin. Genitourin Cancer. 2021, 19, 480–490. [Google Scholar] [CrossRef]
- Semenas, J.; Dizeyi, N.; Persson, J.L. Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer. Drug Des. Devel. Ther. 2013, 7, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Ingrosso, G.; Detti, B.; Scartoni, D.; Lancia, A.; Giacomelli, I.; Baki, M.; Carta, G.; Livi, L.; Santoni, R. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin. Oncol. 2018, 45, 303–315. [Google Scholar] [CrossRef]
- Francini, E.; Yip, S.; Ahmed, S.; Li, H.; Ardolino, L.; Evan, C.P.; Kaymakcalan, M.; Shaw, G.K.; Kantoff, P.W.; Taplin, M.E.; et al. Clinical Outcomes of First-line Abiraterone Acetate or Enzalutamide for Metastatic Castration-resistant Prostate Cancer After Androgen Deprivation Therapy + Docetaxel or ADT Alone for Metastatic Hormone-sensitive Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, 130–134. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Scailteux, L.M.; Despas, F.; Balusson, F.; Campillo-Gimenez, B.; Mathieu, R.; Vincendeau, S.; Happe, A.; Nowak, E.; Kerbrat, S.; Oger, E. Hospitalization for adverse events under abiraterone or enzalutamide exposure in real-world setting: A French population-based study on prostate cancer patients. Br. J. Clin. Pharmacol. 2021, 88, 336–346. [Google Scholar] [CrossRef]
- Serrano Domingo, J.J.; Alonso Gordoa, T.; Lorca Alvaro, J.; Molina-Cerrillo, J.; Barquin Garcia, A.; Martinez Saez, O.; Burgos Revilla, J.; Carrato, A.; Alvarez Rodriguez, S. The effect of medical and urologic disorders on the survival of patients with metastatic castration resistant prostate cancer treated with abiraterone or enzalutamide. Ther. Adv. Urol. 2021, 13, 17562872211043341. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef]
- Cone, E.B.; Reese, S.; Marchese, M.; Nabi, J.; McKay, R.R.; Kilbridge, K.L.; Trinh, Q.D. Cardiovascular toxicities associated with abiraterone compared to enzalutamide-A pharmacovigilance study. EClinicalMedicine 2021, 36, 100887. [Google Scholar] [CrossRef]
- Hu, J.; Aprikian, A.G.; Vanhuyse, M.; Dragomir, A. Comparative Cardiovascular Safety of Novel Hormonal Agents in Metastatic Castration-Resistant Prostate Cancer Using Real-World Data. Clin. Genitourin. Cancer 2021, 20, 17–24. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, H.L.; Teoh, J.Y.; Chen, T.C.; Hao, S.Y.; Tsai, H.Y.; Huang, W.H.; Juan, Y.S.; Cheng, H.M.; Chang, H.M. Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: A systematic review with pairwise and network meta-analyses. Prostate Cancer Prostatic Dis. 2021, 24, 244–252. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, R.; Su, C.; Liang, D.; Wu, J.; Qiu, K.; Li, J. Toxicity profile characteristics of novel androgen-deprivation therapy agents in patients with prostate cancer: A meta-analysis. Expert Rev. Anticancer. Ther. 2018, 18, 193–198. [Google Scholar] [CrossRef]
- Lu-Yao, G.; Nikita, N.; Keith, S.W.; Nightingale, G.; Gandhi, K.; Hegarty, S.E.; Rebbeck, T.R.; Chapman, A.; Kantoff, P.W.; Cullen, J.; et al. Mortality and Hospitalization Risk Following Oral Androgen Signaling Inhibitors Among Men with Advanced Prostate Cancer by Pre-existing Cardiovascular Comorbidities. Eur. Urol. 2020, 77, 158–166. [Google Scholar] [CrossRef]
- Wilk, M.; Waśko-Grabowska, A.; Szmit, S. Cardiovascular Complications of Prostate Cancer Treatment. Review. Front. Pharmacol. 2020, 11, 11555475. [Google Scholar] [CrossRef]
- Peters, M.D. In no uncertain terms: The importance of a defined objective in scoping reviews. JBI Database Syst. Rev. Implement Rep. Feb 2016, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.D.J.; Marnie, C.; Colquhoun, H.; Garritty, C.M.; Hempel, S.; Horsley, T.; Langlois, E.V.; Lillie, E.; O’Brien, K.K.; Tunçalp, Ö.; et al. Scoping reviews: Reinforcing and advancing the methodology and application. Syst. Rev. 2021, 10, 263. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Arksey Hom, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med. 2018, 16, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Shah, Y.; Shaver, A.; Lu-Yao, G. Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review Protocol. Figshare. Available online: https://figshare.com/articles/online_resource/Outcomes_Following_Abiraterone_versus_Enzalutamide_for_Prostate_Cancer_A_Scoping_Review_Protocol/19149227 (accessed on 10 May 2022).
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Soleimani, M.; Zou, K.; Sunderland, K.; Struss, W.; Eigl, B.J.; Nappi, L.; Kollmannsberger, C.K.; Finch, D.; Noonan, K.; Vergidis, J.; et al. Effectiveness of first-line abiraterone versus enzalutamide among patients ≥80 years of age with metastatic castration-resistant prostate cancer: A retrospective propensity score-weighted comparative cohort study. Eur. J. Cancer 2021, 152, 215–222. [Google Scholar] [CrossRef]
- Caffo, O.; Veccia, A.; Maines, F.; Bonetta, A.; Spizzo, G.; Galligioni, E. Potential value of rapid prostate-specific antigen decline in identifying primary resistance to abiraterone acetate and enzalutamide. Future Oncol. 2014, 10, 985–993. [Google Scholar] [CrossRef]
- Miyake, H.; Hara, T.; Terakawa, T.; Ozono, S.; Fujisawa, M. Comparative Assessment of Clinical Outcomes Between Abiraterone Acetate and Enzalutamide in Patients with Docetaxel-Naive Metastatic Castration-Resistant Prostate Cancer: Experience in Real-World Clinical Practice in Japan. Clin. Genitourin. Cancer 2017, 15, 313–319. [Google Scholar] [CrossRef]
- Pilon, D.; Behl, A.S.; Ellis, L.A.; Emond, B.; Lefebvre, P.; Dawson, N.A. Duration of Treatment in Prostate Cancer Patients Treated with Abiraterone Acetate or Enzalutamide. J. Manag. Care Spec. Pharm. 2017, 23, 225–235. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Ramaswamy, K.; Huang, A.; Mardekian, J.; Schultz, N.M.; Wang, L.; Sandin, R.; Lechpammer, S.; George, D.J. Survival outcomes in patients with chemotherapy-naive metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate. Article. Prostate Cancer Prostatic Dis. 2021, 24, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Scailteux, L.M.; Campillo-Gimenez, B.; Kerbrat, S.; Despas, F.; Mathieu, R.; Vincendeau, S.; Balusson, F.; Happe, A.; Nowak, E.; Oger, E. Overall Survival Among Chemotherapy-Naive Patients with Castration-Resistant Prostate Cancer Under Abiraterone Versus Enzalutamide: A Direct Comparison Based on a 2014-2018 French Population Study (the SPEAR Cohort). Am. J. Epidemiol. 2021, 190, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.; Bilir, C.; Gülbağcı, B.; Hacıbekiroğlu, İ.; Bayoğlu, İ.V.; Bilgetekin, İ.; Koca, S.; Çınkır, H.Y.; Akdeniz, N.; Gül, D.; et al. Comparison of real-life data of abiraterone acetate and enzalutamide in metastatic castration-resistant prostate cancer. Sci. Rep. 2021, 11, 14131. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, B.M.; Eredics, K.; Madersbacher, S.; Schauer, I. Abiraterone acetate, enzalutamide and their sequence for castration-resistant prostate cancer: Adherence, survival and hospitalization analysis of a medical claims database. Wien. Klin. Wochenschr. 2018, 130, 659–664. [Google Scholar] [CrossRef]
- Banna, G.L.; Urzia, V.; Benanti, C.; Pitrè, A.; Lipari, H.; Di Quattro, R.; De Giorgi, U.; Schepisi, G.; Basso, U.; Bimbatti, D.; et al. Adherence to abiraterone or enzalutamide in elderly metastatic castration-resistant prostate cancer. Article. Supportive Care Cancer 2020, 28, 4687–4695. [Google Scholar] [CrossRef]
- Chang, L.W.; Hung, S.C.; Wang, S.S.; Li, J.R.; Yang, C.K.; Chen, C.S.; Ho, H.C.; Cheng, C.L.; Ou, Y.C.; Chiu, K.Y. Abiraterone Acetate and Enzalutamide: Similar Efficacy in Treating Post Docetaxel Metastatic Castration-resistant Prostate Cancer: Single Center Experience. Anticancer. Res. 2019, 39, 3901–3908. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bjartell, A.; Lumen, N.; Maroto, P.; Paiss, T.; Gomez-Veiga, F.; Birtle, A.; Kramer, G.; Kalinka, E.; Spaëth, D.; et al. Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry. Target Oncol. 2020, 15, 301–315. [Google Scholar] [CrossRef]
- Crombag, M.B.S.; van Nuland, M.; Bergman, A.M.; Rosing, H.; Schellens, J.H.M.; Huitema, A.D.R.; Beijnen, J.H. Impact of age on exposure to oral antiandrogen therapies in clinical practice. Prostate Cancer Prostatic Dis. 2019, 22, 168–175. [Google Scholar] [CrossRef]
- George, G.; Vikman, H.; Gedeborg, R.; Lissbrant, I.F.; Garmo, H.; Styrke, J.; Van Hemelrijck, M.; Stattin, P. Risk of cardiovascular events in men on abiraterone or enzalutamide combined with GnRH agonists: Nation-wide, population-based cohort study in Sweden. Acta. Oncol. 2021, 60, 459–465. [Google Scholar] [CrossRef]
- Shore, N.D.; Saltzstein, D.; Sieber, P.; Mehlhaff, B.; Gervasi, L.; Phillips, J.; Wong, Y.N.; Pei, H.; McGowan, T. Results of a Real-world Study of Enzalutamide and Abiraterone Acetate with Prednisone Tolerability (REAAcT). Clin. Genitourin. Cancer 2019, 17, 457–463.e6. [Google Scholar] [CrossRef]
- Salem, S.; Komisarenko, M.; Timilshina, N.; Martin, L.; Grewal, R.; Alibhai, S.; Finelli, A. Impact of Abiraterone Acetate and Enzalutamide on Symptom Burden of Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer. Clin. Oncol. 2017, 29, 601–608. [Google Scholar] [CrossRef]
- Iacorossi, L.; Gambalunga, F.; De Domenico, R.; Serra, V.; Marzo, C.; Carlini, P. Qualitative study of patients with metastatic prostate cancer to adherence of hormone therapy. Eur. J. Oncol. Nurs. 2019, 38, 8–12. [Google Scholar] [CrossRef]
- Cindolo, L.; de Francesco, P.; Petragnani, N.; Simiele, F.; Marchioni, M.; Logreco, A.; Di Fabio, C.; de Tursi, M.; Tinari, N.; Schips, L. Persistence and adherence to androgen deprivation therapy in men with prostate cancer: An administrative database study. Article. Minerva Urol. Nefrol. 2020, 72, 615–621. [Google Scholar] [CrossRef]
- Behl, A.S.; Ellis, L.A.; Pilon, D.; Xiao, Y.; Lefebvre, P. Medication adherence, treatment patterns, and dose reduction in patients with metastatic castration-resistant prostate cancer receiving abiraterone acetate or enzalutamide. Article. Am. Health Drug Benefits. 2017, 10, 296–302. [Google Scholar]
- Freedland, S.J.; Li, S.; Pilon, D.; Bhak, R.H.; Narkhede, S.; Lefebvre, P.; Young-Xu, Y. Medication patterns of abiraterone acetate plus prednisone or enzalutamide and PSA progression in veterans with metastatic castration-resistant prostate cancer. Curr. Med. Res. Opin. 2021, 37, 635–642. [Google Scholar] [CrossRef]
- Schultz, N.M.; Flanders, S.C.; Wilson, S.; Brown, B.A.; Song, Y.; Yang, H.; Lechpammer, S.; Kassabian, V. Treatment Duration, Healthcare Resource Utilization, and Costs Among Chemotherapy-Naïve Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide or Abiraterone Acetate: A Retrospective Claims Analysis. Adv. Ther. 2018, 35, 1639–1655. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, K.; Lechpammer, S.; Mardekian, J.; Huang, A.; Schultz, N.M.; Sandin, R.; Wang, L.; Baser, O.; George, D.J. Economic Outcomes in Patients with Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide or Abiraterone Acetate Plus Prednisone. Article. Adv. Ther. 2020, 37, 2083–2097. [Google Scholar] [CrossRef] [Green Version]
- Sathianathen, N.J.; Koschel, S.; Thangasamy, I.A.; Teh, J.; Alghazo, O.; Butcher, G.; Howard, H.; Kapoor, J.; Lawrentschuk, N.; Siva, S.; et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2020, 77, 365–372. [Google Scholar] [CrossRef]
- Poorthuis, M.H.F.; Vernooij, R.W.M.; van Moorselaar, R.J.A.; de Reijke, T.M. Second-line therapy in patients with metastatic castration-resistant prostate cancer with progression after or under docetaxel: A systematic review of nine randomized controlled trials. Semin. Oncol. 2017, 44, 358–371. [Google Scholar] [CrossRef]
- Thortzen, A.; Thim, S.; Røder, M.A.; Brasso, K. A single-center experience with abiraterone as treatment for metastatic castration-resistant prostate cancer. Article. Urol. Oncol. Semin. Orig. Invest. 2016, 34, 291.e1–291.e7. [Google Scholar] [CrossRef]
- Perletti, G.; Monti, E.; Marras, E.; Cleves, A.; Magri, V.; Trinchieri, A.; Rennie, P.S. Efficacy and safety of second-line agents for treatment of metastatic castration-resistant prostate cancer progressing after docetaxel. A systematic review and meta-analysis. Arch. Ital. Urol. Androl. 2015, 87, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, A.; Pfister, D.; Merseburger, A.; Bartsch, G. Modern management of castration-resistant prostate cancer. Eur. Oncol. Haematol. 2012, 9, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Marar, M.; Long, Q.; Mamtani, R.; Narayan, V.; Vapiwala, N.; Parikh, R.B. Outcomes Among African American and Non-Hispanic White Men With Metastatic Castration-Resistant Prostate Cancer With First-Line Abiraterone. JAMA Netw. Open. 2022, 5, e2142093. [Google Scholar] [CrossRef]
- Ramalingam, S.; Humeniuk, M.S.; Hu, R.; Rasmussen, J.; Healy, P.; Wu, Y.; Harrison, M.R.; Armstrong, A.J.; George, D.J.; Zhang, T. Prostate-specific antigen response in black and white patients treated with abiraterone acetate for metastatic castrate–resistant prostate cancer. Article. Urol. Oncol. Semin. Orig. Invest 2017, 35, 418–424. [Google Scholar] [CrossRef]
- Karantanos, T.; Karanika, S.; Gignac, G. Uncontrolled diabetes predicts poor response to novel antiandrogens. Endocr. Relat. Cancer 2016, 23, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.; Lin, C.C.; Chen, M.F.; Liu, K.L.; Lin, C.F.; Chen, T.H.; Wu, C.T. Risk of major adverse cardiovascular events among second-line hormonal therapy for metastatic castration-resistant prostate cancer: A real-world evidence study. Prostate 2021, 81, 194–201. [Google Scholar] [CrossRef]
- Poon, D.M.C.; Wong, K.C.W.; Chan, T.W.; Law, K.; Chan, K.; Lee, E.K.C.; Lee, C.; Chan, M. Hong Kong Society of U-O. Survival Outcomes, Prostate-specific Antigen Response, and Tolerance in First and Later Lines of Enzalutamide Treatment for Metastatic Castration-resistant Prostate Cancer: A Real-World Experience in Hong Kong. Clin. Genitourin. Cancer 2018, 16, 402–412.e1. [Google Scholar] [CrossRef] [Green Version]
- Pilon, D.; Queener, M.; Lefebvre, P.; Ellis, L.A. Cost per median overall survival month associated with abiraterone acetate and enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. J. Med. Econ. 2016, 19, 777–784. [Google Scholar] [CrossRef]
- Wu, B.; Li, S.S.; Song, J.; Pericone, C.D.; Behl, A.S.; Dawson, N.A. Total cost of care for castration-resistant prostate cancer in a commercially insured population and a medicare supplemental insured population. Article. J. Med. Econ. 2020, 23, 54–63. [Google Scholar] [CrossRef]
- Shah, A.; Shah, R.; Kebede, N.; Mohamed, A.; Botteman, M.; Waldeck, R.; Hussain, A. Real-world incidence and burden of adverse events among non-metastatic prostate cancer patients treated with secondary hormonal therapies following androgen deprivation therapy. J. Med. Econ. 2020, 23, 330–346. [Google Scholar] [CrossRef] [Green Version]
- Vignani, F.; Bertaglia, V.; Buttigliero, C.; Tucci, M.; Scagliotti, G.V.; Di Maio, M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Review. Cancer Treat. Rev. 2016, 44, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Wong, S.; Shapiro, J.; Weickhardt, A.; Azad, A.; Kwan, E.M.; Spain, L.; Gunjur, A.; Torres, J.; Parente, P.; et al. Real-world incidence of symptomatic skeletal events and bone-modifying agent use in castration-resistant prostate cancer—An Australian multi-centre observational study. Eur. J. Cancer 2021, 157, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ritch, C.; Cookson, M. Recent trends in the management of advanced prostate cancer. F1000Res 2018, 7, 1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Database | Search String | Result |
|---|---|---|
| PubMed | (“Prostatic Neoplasms”[MeSH Terms] OR “Prostatic Neoplasms”[All Fields] OR “prostate cancer”[All Fields]) AND (“Abiraterone Acetate”[MeSH Terms] OR “Abiraterone Acetate”[All Fields]) AND (“enzalutamide”[All Fields] OR “Androgen signaling inhibitor”[All Fields]) AND (“Drug-Related Side Effects and Adverse Reactions”[MeSH Terms] OR “Treatment Outcome”[MeSH Terms] OR “Hospitalization”[MeSH Terms] OR “Mortality”[MeSH Terms] OR “Comorbidity”[Mesh]) | 169 |
| Scopus | TITLE-ABS-KEY ((“Prostatic Neoplasms” OR “prostate cancer”) AND (“Abiraterone Acetate”) AND (enzalutamide OR “Androgen signaling inhibitor”) AND (“Drug-Related Side Effects and Adverse Reactions” OR “Treatment Outcome” OR hospitalization OR mortality OR comorbidity)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “no”)) AND (LIMIT-TO (LANGUAGE, “English”)) | 399 |
| CINAHL | ((MH “Prostatic Neoplasms+”) OR “Prostatic Neoplasms” OR “prostate cancer”) AND ((MH “Abiraterone Acetate+”) OR “Abiraterone Acetate”) AND (enzalutamide OR “Androgen signaling inhibitor”) AND ((MH “Drug-Related Side Effects and Adverse Reactions+”) OR (MH “Treatment Outcome+”) OR (MH Hospitalization+) OR (MH Mortality+) OR (MH Comorbidity+)) | 3 |
| Cochrane Reviews | ([mh “Prostatic Neoplasms”] OR “Prostatic Neoplasms” OR “prostate cancer”) AND ([mh “Abiraterone Acetate”] OR “Abiraterone Acetate”) AND (enzalutamide OR “Androgen signaling inhibitor”) AND ([mh “Drug-Related Side Effects and Adverse Reactions”] OR [mh “Treatment Outcome”] OR [mh Hospitalization] OR [mh Mortality] OR [mh Comorbidity]) | 0 |
| Outcomes | AA | ENZ |
|---|---|---|
| Disease progression and survival | ||
| Higher biochemical response rate [28,29,30,31] | X | |
| Improved biochemical progression-free survival [29,30,31] | X | |
| Improved OS or rPFS [33,34,35] | X | |
| Comparable OS or rPFS [36,37,38,39] | Equal | |
| Baseline inferior patient health [31,35,37,46] | X | |
| Drug-associated toxicities | ||
| Higher cardiovascular toxicities [15,18,41] | X | |
| Higher CNS toxicities [15,28,42] | X | |
| Increased fatigue [15,28,29,38,42,43] | X | |
| Increased hepatotoxicity [31,38] | X | |
| Treatment adherence, dose reduction, and dose modification | ||
| Adherence [36,37,44] | Equal | |
| Increased dose reduction [32,42,46] | X | |
| HRU, hospitalization, and cost | ||
| Increased length of hospital stay [36] | X | |
| Increased all-cause cost [49] | X | |
| Increased HRU, visits, or admissions [48,49] | X | |
| Increased pharmacy costs [48] | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, Y.B.; Shaver, A.L.; Beiriger, J.; Mehta, S.; Nikita, N.; Kelly, W.K.; Freedland, S.J.; Lu-Yao, G. Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review. Cancers 2022, 14, 3773. https://doi.org/10.3390/cancers14153773
Shah YB, Shaver AL, Beiriger J, Mehta S, Nikita N, Kelly WK, Freedland SJ, Lu-Yao G. Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review. Cancers. 2022; 14(15):3773. https://doi.org/10.3390/cancers14153773
Chicago/Turabian StyleShah, Yash B., Amy L. Shaver, Jacob Beiriger, Sagar Mehta, Nikita Nikita, William Kevin Kelly, Stephen J. Freedland, and Grace Lu-Yao. 2022. "Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review" Cancers 14, no. 15: 3773. https://doi.org/10.3390/cancers14153773
APA StyleShah, Y. B., Shaver, A. L., Beiriger, J., Mehta, S., Nikita, N., Kelly, W. K., Freedland, S. J., & Lu-Yao, G. (2022). Outcomes Following Abiraterone versus Enzalutamide for Prostate Cancer: A Scoping Review. Cancers, 14(15), 3773. https://doi.org/10.3390/cancers14153773






