Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Detection and Diagnosis of HER2
2.1. HERmark ™
2.2. Real-Time PCR
2.3. Multiplex Ligation-Dependent Probe Amplification
2.4. Time Resolved Fluorescence Resonance Energy Transfer
3. Therapy
3.1. Targeting HER2 Receptors on The Cell Surface
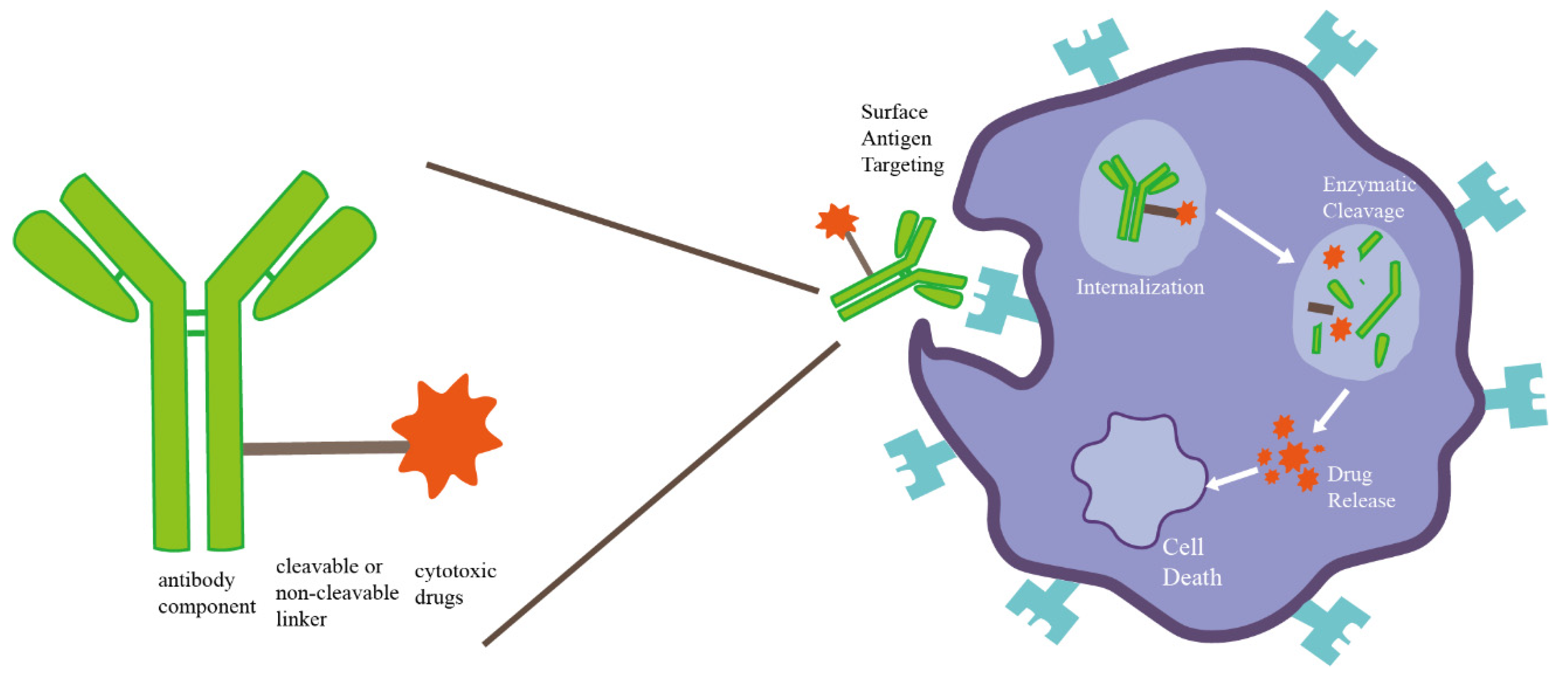
3.1.1. ADC
3.1.2. Monoclonal Antibodies
3.1.3. Bispecific Antibodies
3.1.4. Trispecific Antibody
3.1.5. Tyrosine Kinase Inhibitors
3.2. Targeting HER2-Related Intracellular Signaling Pathways
Targeting PI3K/AKT/mTOR
3.3. Targeting the Immune Microenvironment
3.3.1. HER2-Derived Peptide Vaccine
3.3.2. Immune Checkpoint Inhibitor
4. Other Therapies
4.1. Endocrine Therapy
4.2. Chemotherapy
5. Discussion and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, S.; Padilla, M.; Kurosumi, M.; Matsumoto, H.; Inoue, K.; Horiguchi, J.; Takeyoshi, I.; Oyama, T.; Ranger-Moore, J.; Allred, D.C.; et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res. Treat. 2016, 158, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchiò, C.; Dell’Orto, P.; Annaratone, L.; Geyer, F.C.; Venesio, T.; Berrino, E.; Di Cantogno, L.V.; Garofoli, A.; Rangel, N.; Casorzo, L.; et al. The dilemma of HER2 double-equivocal breast carcinomas: Genomic profiling and implications for treatment. Am. J. Surg. Pathol. 2018, 42, 1190. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E.; Forde, C.; McArt, D.G.; Boyle, D.P.; Mullan, P.B.; James, J.A.; Maxwell, P.; McQuaid, S.; Salto-Tellez, M. Quantification of HER2 heterogeneity in breast cancer–implications for identification of sub-dominant clones for personalised treatment. Sci Rep. 2016, 6, 23383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, V.; Sarotto, I.; Maggiorotto, F.; Berchialla, P.; Kubatzki, F.; Tomasi, N.; Redana, S.; Martinello, R.; Valabrega, G.; Aglietta, M.; et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 2012, 17, 1418–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-low breast cancer: Molecular characteristics and prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, M.Z.; Woodward, W.A.; Nicolas, M.M.; Corley, L.J.; Fuller, G.N.; Esteva, F.J.; Tucker, S.L.; Buchholz, T.A. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am. J. Surg. Pathol. 2009, 33, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggemann, H.; Ignatov, T.; Burger, E.; Kantelhardt, E.J.; Fettke, F.; Thomssen, C.; Costa, S.D.; Ignatov, A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Breast Cancer Res. Treat. 2015, 22, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E.; Rastogi, P., Jr.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Lladó, A.; Bianchi, G.; Cortes, J.; Kellokumpu-Lehtinen, P.L.; Cameron, D.A.; Miles, D.; Salvagni, S.; Wardley, A.; Goeminne, J.C.; et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1131–1137. [Google Scholar] [PubMed] [Green Version]
- Rosso, C.; Voutsadakis, I.A. Characteristics, Clinical Differences and Outcomes of Breast Cancer Patients with Negative or Low HER2 Expression. Clin. Breast Cancer 2022, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- de Moura Leite, L.; Cesca, M.G.; Tavares, M.C.; Santana, D.M.; Saldanha, E.F.; Guimarães, P.T.; Sá, D.; Simões, M.; Viana, R.L.; Rocha, F.G.; et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat. 2021, 190, 155–163. [Google Scholar] [CrossRef]
- Koleva-Kolarova, R.G.; Oktora, M.P.; Robijn, A.L.; Greuter, M.J.W.; Reyners, A.K.L.; Buskens, E.; de Bock, G.H. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 55, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Takegawa, N.; Tsurutani, J.; Kawakami, H.; Yonesaka, K.; Kato, R.; Haratani, K.; Hayashi, H.; Takeda, M.; Nonagase, Y.; Maenishi, O.; et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int. J. Cancer 2019, 145, 3414–3424. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; et al. Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13,864 women in seven randomised trials. Lancet Oncol. 2021, 21, 1139–1150. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 142, 1364–1382. [Google Scholar]
- Torlakovic, E.E.; Nielsen, S.; Francis, G.; Garratt, J.; Gilks, B.; Goldsmith, J.D.; Hornick, J.L.; Hyjek, E.; Ibrahim, M.; Miller, K.; et al. Standardization of positive controls in diagnostic immunohistochemistry: Recommendations from the International Ad Hoc Expert Committee. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Furrer, D.; Sanschagrin, F.; Jacob, S.; Diorio, C. Advantages and disadvantages of technologies for HER2 testing in breast cancer specimens. Am. J. Clin. Pathol. 2015, 144, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Nuciforo, P.; Bradbury, I.; Sperinde, J.; Agbor-Tarh, D.; Campbell, C.; Chenna, A.; Winslow, J.; Serra, V.; Parra, J.L.; et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin. Cancer Res. 2015, 21, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.S.; Goodman, L.J.; Tan, Y.; Defazio-Eli, L.; Paquet, A.C.; Cook, J.W.; Rivera, A.; Frankson, K.; Bose, J.; Chen, L.; et al. Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark®) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Pathol. Res. Int. 2010, 2010, 814176. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Suman, V.J.; Davidson, N.E.; Martino, S.; Kaufman, P.A.; Lingle, W.L.; Flynn, P.J.; Ingle, J.N.; Visscher, D.; Jenkins, R.B. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J. Clin. Oncol. 2006, 24, 3032–3038. [Google Scholar] [CrossRef]
- Yardley, D.A.; Kaufman, P.A.; Huang, W.; Krekow, L.; Savin, M.; Lawler, W.E.; Zrada, S.; Starr, A.; Einhorn, H.; Schwartzberg, L.S.; et al. Quantitative measurement of HER2 expression in breast cancers: Comparison with ‘real-world’routine HER2 testing in a multicenter Collaborative Biomarker Study and cor.rrelation with overall survival. Breast Cancer Res. 2015, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Reinholz, M.; Weidler, J.; Yolanda, L.; Paquet, A.; Whitcomb, J.; Lingle, W.; Jenkins, R.B.; Chen, B.; Larson, J.S.; et al. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. Am. J. Clin. Pathol. 2010, 134, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Susini, T.; Bussani, C.; Marini, G.; Nori, J.; Olivieri, S.; Molino, C.; Bianchi, S.; Vezzosi, V.; Paglierani, M.; Giachi, M.; et al. Preoperative assessment of HER-2/neu status in breast carcinoma: The role of quantitative real-time PCR on core-biopsy specimens. Gynecol. Oncol. 2010, 116, 234–239. [Google Scholar] [CrossRef]
- Koudelakova, V.; Berkovcova, J.; Trojanec, R.; Vrbkova, J.; Radova, L.; Ehrmann, J.; Kolar, Z.; Melichar, B.; Hajduch, M. Evaluation of HER2 gene status in breast cancer samples with indeterminate fluorescence in situ hybridization by quantitative real-time PCR. J. Mol. Diagn 2015, 17, 446–455. [Google Scholar] [CrossRef]
- Cronin, M.; Sangli, C.; Liu, M.L.; Pho, M.; Dutta, D.; Nguyen, A.; Jeong, J.; Wu, J.; Langone, K.C.; Watson, D. Analytical Validation of the Onco type DX Genomic Diagnostic Test for Recurrence Prognosis and Therapeutic Response Prediction in Node-Negative, Estrogen Receptor–Positive Breast Cancer. Clin. Chem. 2007, 53, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caselli, E.; Pelliccia, C.; Teti, V.; Bellea, G.; Mandarano, M.; Ferri, I.; Hartmann, K.; Laible, M.; Sahin, U.; Varga, Z.; et al. Looking for more reliable biomarkers in breast cancer: Comparison between routine methods and RT-qPCR. PLoS ONE 2021, 16, e0255580. [Google Scholar] [CrossRef] [PubMed]
- Benöhr, P.; Henkel, V.; Speer, R.; Vogel, U.; Sotlar, K.; Aydeniz, B.; Reiser, A.; Neubauer, H.; Tabiti, K.; Wallwiener, D.; et al. Her-2/neu expression in breast cancer-a comparison of different diagnostic methods. Anticancer Res. 2005, 25, 1895–1900. [Google Scholar]
- Jacquemier, J.; Spyratos, F.; Esterni, B.; Mozziconacci, M.J.; Antoine, M.; Arnould, L.; Lizard, S.; Bertheau, P.; Lehmann-Che, J.; Fournier, C.B.; et al. SISH/CISH or qPCR as alternative techniques to FISH for determination of HER2 amplification status on breast tumors core needle biopsies: A multicenter experience based on 840 cases. BMC Cancer 2013, 13, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabbs, D.J.; Klein, M.E.; Mohsin, S.K.; Tubbs, R.R.; Shuai, Y.; Bhargava, R. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: An independent quality assurance study. J. Clin. Oncol. 2011, 29, 4279–4285. [Google Scholar] [CrossRef] [PubMed]
- Purnomosari, D.; Aryandono, T.; Setiaji, K.; Nugraha, S.B.; Pals, G.; van Diest, P.J. Comparison of multiplex ligation dependent probe amplification to immunohistochemistry for assessing HER-2/neu amplification in invasive breast cancer. Biotech. Histochem. 2006, 81, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth facto.or receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 131, 18–43. [Google Scholar]
- Sapino, A.; Goia, M.; Recupero, D.; Marchiò, C. Current challenges for HER2 testing in diagnostic pathology: State of the art and controversial issues. Front. Oncol. 2013, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Ho-Pun-Cheung, A.; Bazin, H.; Boissière-Michot, F.; Mollevi, C.; Simony-Lafontaine, J.; Landas, E.; Bleuse, J.P.; Chardès, T.; Prost, J.F.; Pèlegrin, A.; et al. Quantification of HER1, HER2 and HER3 by time-resolved Förster resonance energy transfer in FFPE triple-negative breast cancer samples. Br. J. Cancer 2020, 122, 397–404. [Google Scholar] [CrossRef]
- Wesley, N.A.; Skrajna, A.; Simmons, H.C.; Budziszewski, G.R.; Azzam, D.N.; Cesmat, A.P.; McGinty, R.K. Time Resolved-Fluorescence Resonance Energy Transfer platform for quantitative nucleosome binding and footprinting. Protein Sci. 2022, 31, e4339. [Google Scholar] [CrossRef]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, demonstrates a Promising Antitumor Efficacy with Differentiation fr.rom T-DM1Preclinical Efficacy of DS-8201a, a Novel HER2-Targeting ADC. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 2020, 38, 1887. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Camidge, D.R.; Gemma, A.; Kusumoto, M.; Baba, T.; Kuwano, K.; Bankier, A.; Kiura, K.; Tamura, K.; Modi, S.; et al. Abstract P6-17-06: Characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: Analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with a topoisomerase I inhibitor payload. Cancer Res. 2019, 79 (Suppl. S4), P6-17. [Google Scholar]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Barrios, C.; Dent, R.; Hu, X.; Óshaughnessy, J.; Yonemori, K.; Darilay, A.; Boston, S.; Liu, Y.; Patel, G.; et al. Abstract OT-03-09: Trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: A randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021, 81 (Suppl. S4), OT-03. [Google Scholar]
- Jhaveri, K.; Hamilton, E.; Loi, S.; Schmid, P.; Darilay, A.; Gao, C.; Patel, G.; Wrona, M.; Andre, F. Abstract OT-03-05: Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with other anticancer agents in patients with HER2-low metastatic breast cancer: A phase 1b, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast08). Cancer Res. 2021, 81 (Suppl. S4), OT-03. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; Ma, F.; Luo, Y.; et al. Abstract PD4-06: Early clinical development of RC48-ADC in patients with HER2 positive metastatic breast cancer. Cancer Res. 2020, 80 (Suppl. S4), PD4-06. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J. Clin. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lian, W.; Zhao, X.; Qi, W.; Xu, J.; Xiao, L.; Qing, Y.; Xue, T.; Wang, J. A phase I study of safety and pharmacokinetics of A166, a novel selective inhibitor of human epidermal growth factor receptor-2 in Chinese patients with advanced solid tumors. J. Clin. Oncol. 2020, 38, e13007. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Liu, R.; Gao, S.; Qing, Y.; Yi, S.; Yuan, J.; Chen, H.; Fan, B.; Zheng, H.; et al. Phase I study of A166 in patients with HER2-expressing locally advanced or metastatic solid tumors. J. Clin. Oncol. 2021, 39, 1024. [Google Scholar] [CrossRef]
- Oganesyan, V.; Peng, L.; Bee, J.S.; Li, J.; Perry, S.R.; Comer, F.; Xu, L.; Cook, K.; Senthil, K.; Clarke, L.; et al. Structural insights into the mechanism of action of a biparatopic anti-HER2 antibody. J. Biol. Chem. 2018, 293, 8439–8448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokter, W.; Ubink, R.; van der Lee, M.; van der Vleuten, M.; van Achterberg, T.; Jacobs, D.; Loosveld, E.; van den Dobbelsteen, D.; Egging, D.; Mattaar, E. Preclinical Profile of the HER2-Targeting ADC SYD983/SYD985: Introduction of a New Duocarmycin-Based Linker-Drug PlatformPreclinical Profile of SYD983/SYD985. Mol. Cancer Ther. 2014, 13, 2618–2629. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Riaz, K.M.; Gill, M.S.; Patnaik, A.; Ulahannan, S.V.; Wang, J.S.; Gombos, D.S.; Ang, Q.; Cicic, D.; Bergonio, G.R.; et al. Reversible HER2 antibody-drug conjugate–induced ocular toxicity. Can. J. Ophthalmol. 2022, 57, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef] [Green Version]
- Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Pegram, M.D.; Rugo, H.S.; Brown-Glaberman, U.; Yardley, D.A.; Kim, S.-B.; de Boer , M.; et al. Abstract PS10-12: Integrated safety summary of single agent and combination margetuximab in phase 1, 2, and 3 studies of HER2-positive advanced cancers and metastatic breast cancer (MBC). Cancer Res. 2021, 81 (Suppl. S4), PS10-12. [Google Scholar]
- Hayes, D.F. HER2 and breast cancer—A phenomenal success story. N. Engl. J. Med. 2019, 381, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific antibodies: From research to clinical application. Front. Immunol. 2021, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khalili, S.; Baradaran, B.; Bidar, N.; Shahbazi, M.A.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Hamblin, M.R. Bispecific monoclonal antibodies for targeted immunotherapy of solid tumors: Recent advances and clinical trials. Int. J. Biol. Macromol. 2021, 167, 1030–1047. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Gampenrieder, S.P.; Greil, R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Pegram, M.D.; Hamilton, E.P.; Tan, A.R.; Storniolo, A.M.; Balic, K.; Rosenbaum, A.I.; Liang, M.; He, P.; Marshall, S.; Scheuber, A.; et al. First-in-human, phase 1 dose-escalation study of biparatopic anti-HER2 antibody–drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol. Cancer Ther. 2021, 20, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Geuijen, C.A.W.; De Nardis, C.; Maussang, D.; Rovers, E.; Gallenne, T.; Hendriks, L.J.A.; Visser, T.; Nijhuis, R.; Logtenberg, T.; de Kruif, J.; et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell 2018, 33, 922–936. [Google Scholar] [CrossRef] [Green Version]
- De Nardis, C.; Hendriks, L.J.A.; Poirier, E.; Arvinte, T.; Gros, P.; Bakker, A.B.H.; de Kruif, J. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. Lancet Oncol. 2017, 292, 14706–14717. [Google Scholar] [CrossRef] [Green Version]
- Alsina, M.; Boni, V.; Schellens, J.H.; Moreno, V.; Bol, K.; Westendorp, M.; Sirulnik, L.A.; Tabernero, J.; Calvo, E. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: Final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). J. Clin. Oncol. 2017, 35 (Suppl. S15), 2522. [Google Scholar] [CrossRef]
- Collins, D.; Jacob, W.; Cejalvo, J.M.; Ceppi, M.; James, I.; Hasmann, M.; Crown, J.; Cervantes, A.; Weisser, M.; Bossenmaier, B. Direct estrogen receptor (ER)/HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS ONE 2017, 12, e0177331. [Google Scholar] [CrossRef]
- Pistilli, B.; Wildiers, H.; Hamilton, E.P.; Ferreira, A.A.; Dalenc, F.; Vidal, M.; Gavila, J.; Goncalves, A.; Murias, C.; Fournier, C.B.; et al. Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low, non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i). J. Clin. Oncol 2020, 38 (Suppl. S15), 1037. [Google Scholar] [CrossRef]
- Wenwen, S.; Sri, V.; Zhili, S.; Edward, S.; Zhen, X.; Liqing, C.; Virna, C.R.; Sukhvinder, S.; Dinesh, B.; Wu, L.; et al. SAR443216, a novel trispecific T cell engager with potent T cell-dependent cytotoxicity for HER2-low tumors. Cancer Res. 2021, 81 (Suppl. S13), 1825. [Google Scholar]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules 2014, 19, 15196–15212. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Luci, C.; Díaz-Rodríguez, E.; Gandullo-Sánchez, L.; Díaz-Gil, L.; Ocaña, A.; Pandiella, A. Adaptive resistance to trastuzumab impairs response to neratinib and lapatinib through deregulation of cell death mechanisms. Cancer Lett. 2020, 470, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Verma, C.; Guzman, M.; Jimenez, J.; Parra, J.L.; Pedersen, K.; Smith, D.J.; Landolfi, S.; Cajal, S.R.Y.; Arribas, J.; et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009, 28, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, H.L.; Doval, D.C.; Chavez, M.A.; Ang, P.C.; Aziz, Z.; Nag, S.; Ng, C.; Franco, S.X.; Chow, L.W.; Arbushites, M.C.; et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol. 2008, 26, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Trudeau, M.; Kaufman, B.; Boussen, H.; Blackwell, K.; LoRusso, P.; Lombardi, D.P.; Ben, A.S.; Citrin, D.L.; DeSilvio, M.L.; et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J. Clin. Oncol. 2008, 26, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.; Sledge, G.; Koehler, M.; Ellis, C.; Casey, M.; Vukelja, S.; Bischoff, J.; et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1124–1130. [Google Scholar] [CrossRef]
- Maruyama, T.; Mimura, K.; Izawa, S.; Inoue, A.; Shiba, S.; Watanabe, M.; Kawaguchi, Y.; Inoue, M.; Nogata, H.; Inoue, S.; et al. Lapatinib enhances herceptin-mediated antibody-dependent cellular cytotoxicity by up-regulation of cell surface HER2 expression. Anticancer Res. 2011, 31, 2999–3005. [Google Scholar] [PubMed]
- Cocco, E.; Javier, C.F.; Razavi, P.; Won, H.H.; Cai, Y.; Rossi, V.; Chan, C.; Cownie, J.; Soong, J.; Toska, E.; et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci. Signal. 2018, 11, eaat9773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Liu, M.C.; Yee, D.; Yau, C.; van’t Veer, L.J.; Symmans, W.F.; Paoloni, M.; Perlmutter, J.; Hylton, N.M.; Hogarth, M.; et al. Adaptive randomization of neratinib in early breast cancer. N. Engl. J. Med. 2016, 375, 11–22. [Google Scholar] [CrossRef]
- Collins, D.M.; Conlon, N.T.; Kannan, S.; Verma, C.S.; Eli, L.D.; Lalani, A.S.; Crown, J. Preclinical characteristics of the irreversible pan-HER kinase inhibitor neratinib compared with lapatinib: Implications for the treatment of HER2-positive and HER2-mutated breast cancer. Cancers 2019, 11, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, D.M.; Gately, K.; Hughes, C.; Edwards, C.; Davies, A.; Madden, S.F.; O’Byrne, K.J.; O’Donovan, N.; Crown, J. Tyrosine kinase inhibitors as modulators of trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity in breast cancer cell lines. Cell Immunol. 2017, 319, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Connell, C.M.; Doherty, G.J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2017, 2, e000279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Park, K.; Im, S.A.; Jung, K.H.; Sohn, J.; Lee, K.S.; Kim, J.H.; Yang, Y.; Park, Y.H. Clinical implications of HER2 mRNA expression and intrinsic subtype in refractory HER2-positive metastatic breast cancer treated with pan-HER inhibitor, poziotinib. Breast Cancer Res. Treat. 2020, 184, 743–753. [Google Scholar] [CrossRef]
- Robichaux, J.P.; Elamin, Y.Y.; Vijayan, R.S.K.; Nilsson, M.B.; Hu, L.; He, J.; Zhang, F.; Pisegna, M.; Poteete, A.; Sun, H.; et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 2019, 36, 444–457. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, K.H.; Sohn, J.H.; Lee, K.S.; Jung, K.H.; Kim, J.H.; Lee, K.H.; Ahn, J.S.; Kim, T.Y.; Kim, G.M.; et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: Results of the NOV120101-203 trial. Int. J. Cancer 2018, 143, 3240–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, E.; Park, K.; Jung, H.H.; Park, W.Y.; Lee, K.H.; Sohn, J.; Lee, K.S.; Jung, K.H.; Kim, J.H.; et al. Molecular alterations and poziotinib efficacy, a pan-HER inhibitor, in human epidermal growth factor receptor 2 (HER2)-positive breast cancers: Combined exploratory biomarker analysis from a phase II clinical trial of poziotinib for refractory HER2-positive breast cancer patients. Int. J. Cancer 2019, 145, 1669–1678. [Google Scholar]
- Simmons, C.; Rayson, D.; Joy, A.A.; Henning, J.W.; Lemieux, J.; McArthur, H.; Card, P.B.; Dent, R.; Brezden-Masley, C. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066677. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Rong, G.; Guan, Y.; Li, J.; Chang, L.; Li, H.; Liu, B.; Wang, W.; Guan, X.; Ouyang, Q.; et al. Molecular landscape and efficacy of HER2-targeted therapy in patients with HER2-mutated metastatic breast cancer. NPJ Breast Cancer 2020, 6, 59. [Google Scholar] [CrossRef]
- Haque, M.M.; Desai, K.V. Pathways to endocrine therapy resistance in breast cancer. Front. Endocrinol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Hasson, S.P.; Rubinek, T.; Ryvo, L.; Wolf, I. Endocrine resistance in breast cancer: Focus on the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin signaling pathway. Breast Care 2013, 8, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, F.; Prazeres, H.; Mendes, F.; Martins, D.; Schmitt, F. Resistance to endocrine therapy in HR+ and/or HER2+ breast cancer: The most promising predictive biomarkers. Mol. Biol. Rep. 2022, 49, 717–733. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Vuylsteke, P.; Huizing, M.; Petrakova, K.; Roylance, R.; Laing, R.; Chan, S.; Abell, F.; Gendreau, S.; Rooney, I.; Apt, D.; et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016, 27, 2059–2066. [Google Scholar] [PubMed]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast CancerAlpelisib and Letrozole in ER+ Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef] [Green Version]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. Lancet Oncol. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.C.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB) Neoadjuvant Letrozole Plus Alpelisib for Breast Cancer. Clin. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef] [Green Version]
- Juric, D.; Janku, F.; Rodón, J.; Burris, H.A.; Mayer, I.A.; Schuler, M.; Seggewiss-Bernhardt, R.; Gil-Martin, M.; Middleton, M.R.; Baselga, J.; et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor–positive advanced breast cancer: A phase 1b clinical trial. JAMA Oncol. 2019, 5, e184475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Abramson, V.G.; O’Dea, A.; Nye, L.; Mayer, I.; Pathak, H.B.; Hoffmann, M.; Stecklein, S.R.; Elia, M.; Lewis, S.; et al. Clinical and biomarker results from Phase I/II study of PI3K inhibitor, BYL719 (Alpelisib) plus Nab-paclitaxel in HER2 negative metastatic breast cancer. Clin. Cancer Res. 2021, 27, 3896–3904. [Google Scholar] [CrossRef]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: An update regarding potential drugs and natural products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef]
- Prêtre, V.; Wicki, A. Inhibition of Akt and other AGC kinases: A target for clinical cancer therapy? Semin. Cancer Biol. 2018, 48, 70–77. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sangai, T.; Akcakanat, A.; Chen, H.; Tarco, E.; Wu, Y.; Do, K.A.; Miller, T.W.; Arteaga, C.L.; Mills, G.B.; Gonzalez-Angulo, A.M.; et al. Biomarkers of Response to Akt Inhibitor MK-2206 in Breast CancerAntitumor Activity of MK-2206. Clin. Cancer Res. 2012, 18, 5816–5828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Lin, N.U.; Maurer, M.A.; Chen, H.; Mahvash, A.; Sahin, A.; Akcakanat, A.; Li, Y.; Abramson, V.; Litton, J.; et al. Phase II trial of AKT inhibitor MK-2206 in pat.tients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, B.R.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J.; et al. Preclinical Pharmacology of AZD5363, an Inhibitor of AKT: Pharmacodynamics, Antitumor Activity, and Correlation of Monotherapy Activity with Genetic BackgroundAZD5363, an Oral Inhibitor of AKT. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C.; Alarcón, E.; Armstrong, A.C.; Philco, M.; Chuken, Y.; Sablin, M.P.; Tamura, K.; Villanueva, A.; Perez-Fidalgo, J.A.; Cheung, S.Y.A.; et al. BEECH: A dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann. Oncol. 2019, 30, 774–780. [Google Scholar]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: The PAKT trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef]
- Oliveira, M.; Saura, C.; Nuciforo, P.; Calvo, I.; Andersen, J.; Passos-Coelho, J.L.; Gil, G.M.; Bermejo, B.; Patt, D.A.; Ciruelos, E.; et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 2019, 30, 1289–1297. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Robson, M.; Iwata, H.; Hegg, R.; Verma, S.; Nechaeva, M.; Xu, B.; Haddad, V.; Imedio, E.R.; et al. Abstract OT2-08-02: Capivasertib and paclitaxel in first-line treatment of patients with metastatic triple-negative breast cancer: A phase III trial (CAPItello-290). Cancer Res. 2020, 80 (Suppl. S4), OT2-08. [Google Scholar] [CrossRef]
- Kim, S.B.; Dent, R.; Im, S.A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT inhibitors: New weapons in the fight against breast cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.D.; Payne, K.K.; Posey, A.D.; Hill, C.; Conejo-Garcia, J.; June, C.H.; Tchou, J. Immunotherapy for breast cancer: Current and future strategies. Curr. Surg. Rep. 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Awadhi, A.; Lee Murray, J.; Ibrahim, N. Developing anti-HER 2 vaccines: B reast cancer experience. Int. J. Cancer 2018, 143, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-positive breast cancer immunotherapy: A focus on vaccine development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef] [PubMed]
- Peoples, G.E.; Holmes, J.P.; Hueman, M.T.; Mittendorf, E.A.; Amin, A.; Khoo, S.; Dehqanzada, Z.A.; Gurney, J.M.; Woll, M.M.; Ryan, G.B.; et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin. Cancer Res. 2008, 14, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Patil, R.; Holmes, J.P.; Hueman, M.T.; Mittendorf, E.A.; Craig, D.; Stojadinovic, A.; Ponniah, S.; et al. The impact of HER2/neu expression level on response to the E75 vaccine: From US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin. Cancer Res. 2009, 15, 2895–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peoples, G.E.; Gurney, J.M.; Hueman, M.T.; Woll, M.M.; Ryan, G.B.; Storrer, C.E.; Fisher, C.; Shriver, C.D.; Ioannides, C.G.; Ponniah, S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol. 2005, 23, 7536–7545. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial Randomized Phase III Trial of Nelipepimut-S in Breast Cancer. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef] [Green Version]
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 2016, 7, 66192. [Google Scholar] [CrossRef] [Green Version]
- Clifton, G.T.; Hale, D.; Vreeland, T.J.; Hickerson, A.T.; Litton, J.K.; Alatrash, G.; Murthy, R.K.; Qiao, N.; Philips, A.V.; Lukas, J.J.; et al. Results of a Randomized Phase IIb Trial of Nelipepimut-S+ Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast CancerPhase IIb Breast Cancer Trial of Nelipepimut-S+ Trastuzumab. Clin. Cancer Res. 2020, 26, 2515–2523. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, P.M.; Clifton, G.T.; Vreeland, T.J.; Adams, A.M.; O’Shea, A.E.; Peoples, G.E. AE37: A HER2-targeted vaccine for the prevention of breast cancer recurrence. Exp. Opin. Investig. Drugs 2021, 30, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Ardavanis, A.; Symanowski, J.; Murray, J.L.; Shumway, N.M.; Litton, J.K.; Hale, D.F.; Perez, S.A.; Anastasopoulou, E.A.; Pistamaltzian, N.F.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 2016, 27, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Stephen, T.L.; Payne, K.K.; Chaurio, R.A.; Allegrezza, M.J.; Zhu, H.; Perez-Sanz, J.; Perales-Puchalt, A.; Nguyen, J.M.; Vara-Ailor, A.E.; Eruslanov, E.B.; et al. SATB1 expression governs epigenetic repression of PD-1 in tumor-reactive T cells. Immunity 2017, 46, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curdy, N.; Lanvin, O.; Laurent, C.; Fournié, J.J.; Franchini, D.M. Regulatory mechanisms of inhibitory immune checkpoint receptors expression. Trends Cell Biol. 2019, 29, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Bozeman, E.N.; He, S.; Shafizadeh, Y.; Selvaraj, P. Therapeutic efficacy of PD-L1 blockade in a breast cancer model is enhanced by cellular vaccines expressing B7-1 and glycolipid-anchored IL-12. Hum. Vaccin Immunother. 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Lee, S.H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 study. Clin. Cancer Res. 2017, 35, 4050–4056. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.P.; Pohlmann, P.R.; Tolaney, S.M.; Molinero, L.; He, X.; Waterkamp, D.; Funke, R.P.; Powderly, J.D. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J. Clin. Oncol. 2020, 34, 1009. [Google Scholar] [CrossRef]
- Adams, S.; Othus, M.; Patel, S.P.; Miller, K.D.; Chugh, R.; Schuetze, S.M.; Chamberlin, M.D.; Haley, B.J.; Storniolo, A.M.V.; Reddy, M.P.; et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART, SWOG S1609) Ipilimumab and Nivolumab in Rare Tumors S1609: Metaplastic. Clin. Cancer Res. 2022, 28, 271–278. [Google Scholar] [PubMed]
- Hamilton, E.; Shapiro, C.L.; Petrylak, D.; Boni, V.; Martin, M.; Conte, G.D.; Cortes, J.; Agarwal, L.; Arkenau, H.T.; Tan, A.R.; et al. Abstract PD3-07: Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: A 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021, 81 (Suppl. S4), PD3-07. [Google Scholar] [CrossRef]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients 860 with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Peter, S.; Seock-Ah, I.; Anne, A.; Yeon, H.P.; Wei-Pang, C.; Zbigniew, N.; Simon, L.; Piotr, J.W.; Yen-Shen, L.; Hannah, D.; et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+ paclitaxel (P), and arm 6, d+ trastuzumab deruxtecan (T-DXd). J. Clin. Oncol. 2020, 39, 15. [Google Scholar]
- Borghaei, H.; Besse, B.; Bardia, A.; Mazieres, J.; Popat, S.; Augustine, B.; D’amelio, A.M.; Barrios, D.; Rugo, H.S. Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with pembrolizumab in patients with advanced/metastatic breast or non-small cell lung cancer (NSCLC): A phase Ib, multicenter, study. J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Regan, M.M.; Lykkesfeldt, A.E.; Dell’Orto, P.; Del Curto, B.; Henriksen, K.L.; Mastropasqua, M.G.; Price, K.N.; Méry, E.; Lacroix-Triki, M.; et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: Supplementary results from the BIG 1-98 randomised trial. Lancet Oncol. 2008, 9, 23–28. [Google Scholar] [CrossRef]
- Bartlett, J.; Ahmed, I.; Regan, M.M.; Sestak, I.; Mallon, E.A.; Dell’Orto, P.; Thürlimann, B.; Seynaeve, C.; Putter, H.; Van de Velde, C.; et al. HER2 status predicts for upfront AI benefit: A TRANS-AIOG meta-analysis of 12, 129 patients from ATAC, BIG 1-98 and TEAM with centrally determined HER2. Eur. J. Cancer 2017, 79, 129–138. [Google Scholar] [CrossRef]
- Schiza, A.; Mauri, D.; Fredriksson, I.; Anna-Karin, W.; Valachis, A. Predictive role of HER2-status on the effectiveness of endocrine adjuvant treatment in postmenopausal breast cancer patients: A population-based cohort study. Breast Cancer Res. Treat. 2021, 186, 779–789. [Google Scholar] [CrossRef]
- Taskaynatan, H.; Kucukzeybek, Y.; Alacacioglu, A.; Yildiz, Y.; Salman, T.; Oflazoglu, U.; Varol, U.; Bolat, K.B.; Kemal, A.M.; Oktay, T.M. Is adjuvant chemotherapy necessary for Luminal A-like breast cancer. J. BUON 2018, 23, 877–882. [Google Scholar]
- Goel, S.; De Cristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 inhibition in cancer: Beyond cell cycle arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Lloyd, M.R.; Spring, L.M.; Bardia, A.; Wander, S.A. Mechanisms of Resistance to CDK4/6 Blockade in Advanced Hormone Receptor–positive, HER2-negative Breast Cancer and Emerging Therapeutic Opportunities. Clin. Cancer Res. 2022, 28, 821–830. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 26, 25–35. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast CancerPhase II Study of Abemaciclib in HR+/HER2- MBC. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [Green Version]
- Sledge, G.W.; Toi, M., Jr.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Lancet Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Wang, Q.; Cao, J.; Sun, J.; Zhu, Z. Mechanisms of resistance to estrogen receptor modulators in ER+/HER2-advanced breast cancer. Cell Mol. Life Sci. 2020, 77, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Sutanto, L.; Tse, S.; Man Cheung, K.; Chan, J. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor–positive, ERBB2-negative metastatic breast cancer. JAMA Netw. Open 2021, 4, e2133132. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Park-Simon, T.W.; Albanell, J.; Lassen, U.; Cortés, J.; Dieras, V.; May, M.; Schindler, C.; Marmé, F.; Cejalvo, J.M.; et al. Phase Ib study evaluating safety and cli.inical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investig. New Drugs 2018, 36, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries Schultink, A.; Doornbos, R.P.; Bakker, A.; Bol, K.; Throsby, M.; Geuijen, C.; Maussang, D.; Schellens, J.; Beijnen, J.H.; Huitema, A.D. Translational PK-PD modeling analysis of MCLA-128, a HER2/HER3 bispecific monoclonal antibody, to predict 918 clinical efficacious exposure and dose. Investig. New Drugs 2018, 36, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Gianni, L.; Bisagni, G.; Colleoni, M.; Del Mastro, L.; Zamagni, C.; Mansutti, M.; Zambetti, M.; Frassoldati, A.; De Fato, R.; Valagussa, P.; et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): An exploratory, open-label, phase 2 study. Lancet Oncol. 2018, 19, 249–256. [Google Scholar] [CrossRef]
- Gianni, L.; Bisagni, G.; Colleoni, M.; Del Mastro, L.; Zamagni, C.; Mansutti, M.; Zambetti, M.; Frassoldati, A.; Barlera, S.; Valagussa, P.; et al. Ki67 during and after neoadjuvant trastuzumab, pertuzumab and palbociclib plus or minus fulvestrant in HER2 and ER-positive breast cancer: The NA- CPHER2 Michelangelo study. J. Clin. Oncol. 2019, 37, 527. [Google Scholar] [CrossRef]
- O’Brien, N.A.; McDermott, M.; Conklin, D.; Luo, T.; Ayala, R.; Salgar, S.; Chau, K.; DiTomaso, E.; Babbar, N.; Su, F.; et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 2020, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Sartori, G.P.; Souza, A.A.; Pellegrini, R.; Rosa, M.L.; Rossatto, N.; Coelho, G.P.; Litvin, I.E.; Zerwes, F.; Millen, E.; et al. Abstract PS4-22: Prevalence of HER2-low and HER2-zero subgroups and correlation with response to neoadjuvant chemotherapy (NACT) in patients with HER2-negative breast cancer. Cancer Res. 2021, 81 (Suppl. S4), PS4-22. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, X.; Fei, X.; Lin, L.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; Li, Y.; et al. Can breast cancer patients with HER2 dual-equivocal tumours be managed as HER2-negative disease? Eur. J. Cancer 2018, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Rosie, B.; Jeremy, B.; Zulian, L.; Richard, P.; Lucy, D.; David, D.; Paul, M.; Hongchao, P.; Carolyn, T.; et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37298 women with early breast cancer in 26 randomised trials. Lancet 2019, 393, 1440–1452. [Google Scholar]
- Zambelli, A.; Simoncini, E.; Giordano, M.; La Verde, N.; Farina, G.; Torri, V.; Colombo, G.; Piacentini, G.; Fotia, V.; Vassalli, L.; et al. Prospective observational study on the impact of the 21-gene assay on treatment decisions and resources optimization in breast cancer patients in Lombardy: The BONDX study. Breast 2020, 52, 1–7. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C., Jr.; Perez, E.A.; Olson, J.A.; et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef] [PubMed]

| Drug | Target | Representative Clinical Trial | Patient Cohort | N | Treatment Arms | Main Efficacy Results | Toxity |
|---|---|---|---|---|---|---|---|
| T-DXd | HER2 | DS8201-A-J101 trial NCT02564900 | Metastatic HER2-low BC | 54 | T-DXd | ORR: 37.0% | Interstitial lung disease, anemia, diarrhea |
| RC48 | HER2 | C001CANCER phase I trial NCT02881138 | Advanced malignant solid tumors with HER2+ | 118 (HER2-low BC: 48) | RC48 | ORR: 39.6%; mPFS: 5.7 months | Hypoesthesia, fatigue |
| SYD985 | HER2 | SYD985.001 phase I trial NCT02277717 | Advanced BC or gastric, urothelial, or endometrial cancer with at least HER2 IHC 1+ | 146 (HER2-low BC: 47) | SYD985 | ORR in HR+/HER2-low BC: 28%; ORR in HR-/HER2-low BC: 40% | Atigue, conjunctivitis, dry eye |
| A166 | HER2 | KL166-I-01-CTP NCT05311397 | Solid tumors with HER2 expression | 57 (HER2-low BC: 6) | A166 | DCR: 75% | Keratitis, decreased appetite, dry eye, vision blurred |
| MEDI4276 | HER2 | D5760C00001 NCT02576548 | HER2 expressing BC or gastric/stomach cancers | 47 | MEDI4276 | NA | Nausea, fatigue, vomiting |
| MCLA128 | HER2, HER3 | MCLA-128-CL02 NCT03321981 | Metastatic BC | 106 (HER2-low BC: 48) | MCLA128 with ET | DCR: 45% | Fatigue, diarrhea, nausea |
| SAR443216 | HER2 | TED16925 trial NCT05013554 | Relapsed/refractory HER2 expressing solid tumors | NA | SAR443216 | NA | NA |
| ADC | HER2-Targeting Antibody | Linker | Cytotoxic Drug | Ongoing Clinical Trials with HER2-low BC |
|---|---|---|---|---|
| T-DXd | Trastuzumab | Cleavable | Topoisomerase I inhibitor | NCT04494425 NCT04556773 |
| RC48 [55] | Hertuzumab (anti-HER2 humanized Ab) | Cleavable | MMAE | NCT04400695 NCT04965519 |
| SYD985 [56] | Trastuzumab | Cleavable | Duocarmycin analogs | NCT04205630 NCT04602117 NCT04235101 |
| A166 [57] | Trastuzumab | Cleavable | Microtubule inhibitor | NCT03602079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.-Z.; Han, J.-R.; Fu, X.; Ren, Y.-F.; Li, Z.-H.; You, F.-M. Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers 2022, 14, 3774. https://doi.org/10.3390/cancers14153774
Lai H-Z, Han J-R, Fu X, Ren Y-F, Li Z-H, You F-M. Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers. 2022; 14(15):3774. https://doi.org/10.3390/cancers14153774
Chicago/Turabian StyleLai, Heng-Zhou, Jie-Rong Han, Xi Fu, Yi-Feng Ren, Zhuo-Hong Li, and Feng-Ming You. 2022. "Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions" Cancers 14, no. 15: 3774. https://doi.org/10.3390/cancers14153774
APA StyleLai, H.-Z., Han, J.-R., Fu, X., Ren, Y.-F., Li, Z.-H., & You, F.-M. (2022). Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers, 14(15), 3774. https://doi.org/10.3390/cancers14153774






