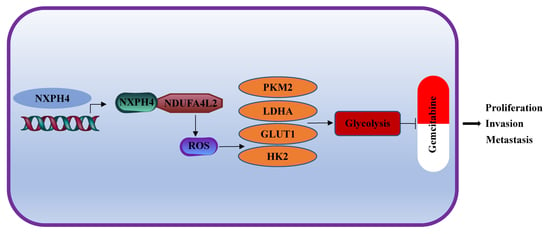
NXPH4 Promotes Gemcitabine Resistance in Bladder Cancer by Enhancing Reactive Oxygen Species and Glycolysis Activation through Modulating NDUFA4L2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Data Collection and Processing
2.3. The Baseline Data of Patients with Bladder Cancer from TCGA
2.4. The Exploration of the Expression Levels of NXPH4 in Patients with Different Status
2.5. The Prognostic Value of NXPH4, Gene Set Enrichment Analysis, and the Infiltration Level of Immune Cells
2.6. Cell Culture and Establishment of Resistant Cell Line
2.7. Glycolytic Activity Assay
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot Assay and Co-Immunoprecipitation
2.10. Wound Healing and Transwell Invasion Assays
2.11. CCK-8 Assay
2.12. Plate Clone Formation Assay
2.13. Transfection
2.14. Immunohistochemical Staining
2.15. Intracellular ROS Levels
2.16. Cell Proliferation
2.17. Xenograft Tumor Model
2.18. Statistical Analyses
3. Results
3.1. The Expression Patterns of NXPH4 across 33 Kinds of Cancers from TCGA and Its Expression in Patients with Bladder Cancer from Different Groups
3.2. High Level of NXPH4 Is Associated with Worse Prognosis in Bladder Cancer
3.3. NXPH4 Was Upregulated in BC Tissues and Cells, Promoting the Proliferation, Invasion, and Migration of BC Cells in Vitro
3.4. The Expression of NXPH4 Is Elevated in Acquired Gemcitabine-Resistant Bladder Cancer Cell Lines and Mediates Gemcitabine Resistance through Enhancing Both Intracellular Reactive Oxygen Species and Glycolysis
3.5. NDUFA4L2 Is a Downstream Target of NXPH4
3.6. NXPH4 Promotes ROS Production and Glycolysis-Dependent Gemcitabine Resistance by Regulating NDUFA4L2
3.7. NXPH4-NDUFA4L2 Pathway Is Essential for Gemcitabine Resistance of Bladder Cancer Cells In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bladder cancer |
| NB | Normal bladder |
| N | Normal |
| T | Tumor |
| TCGA | The Cancer Genome Atlas; |
| OS | Overall survival |
| DSS | Disease Specific Survival |
| PFI | Progression Free Interval |
| CR | Complete Response; |
| PR | Partial Response; |
| SD | Stable Disease; |
| PD | Progressive Disease; |
| GSEA | Gene Set Enrichment Analysis; |
| KM analysis | Kaplan-Meier analysis; |
| GEM | Gemcitabine; |
| T24-GEM-R | Gemcitabine resistant T24; |
| 5637GEM-R | Gemcitabine resistant 5637 |
| IC50 | Half maximal inhibitory concentration. |
| ROS | Reactive oxygen species |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Modlich, O. Identifying superficial, muscle-invasive, and metastasizing transitional cell carcinoma of the bladder: Use of cDNA array analysis of gene expression profiles. Clin. Cancer Res. 2004, 10, 3410–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markus, M.; Südhof, T. Neurexophilins Form a Conserved Family of Neuropeptide-Like Glycoproteins. J. Neurosci. 1998, 18, 3630–3638. [Google Scholar]
- Petrenko, A.; Ullrich, B.; Missler, M.; Krasnoperov, V.; Rosahl, T.W.; Südhof, T.C. Structure and Evolution of Neurexophilin. J. Neurosci. 1996, 16, 4360–4369. [Google Scholar] [CrossRef]
- Meng, X.; McGraw, C.M.; Wang, W.; Jing, J.; Yeh, S.-Y.; Wang, L.; Lopez, J.; Brown, A.M.; Lin, T.; Chen, W.; et al. Neurexophilin4 is a selectively expressed α-neurexin ligand that modulates specific cerebellar synapses and motor functions. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, B.; Qiao, A.; Yang, P.; Chen, W.; Zhen, D.; Qiu, X. A novel EZH2/NXPH4/CDKN2A axis is involved in regulating the proliferation and migration of non-small cell lung cancer cells. Biosci. Biotechnol. Biochem. 2021, 86, 340–350. [Google Scholar] [CrossRef]
- An, Q.; Zhou, L.; Xu, N. Long noncoding RNA FOXD2-AS1 accelerates the gemcitabine-resistance of bladder cancer by sponging miR-143. Biomed. Pharmacother. 2018, 103, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Bhatia, R.; Rauth, S.; Kisling, A.; Atri, P.; Thompson, C.; Vengoji, R.; Krishn, S.R.; Shinde, D.; Thomas, V.; et al. Mucin 5AC Serves as the Nexus for β-Catenin/c-Myc Interplay to Promote Glutamine Dependency during Pancreatic Cancer Chemoresistance. Gastroenterology 2022, 162, 253–268.e13. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol Can Reverse the 5-Fu Resistance of Colorectal Cancer Cells by Inhibiting PKM2-Mediated Glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef] [PubMed]
- Tantai, J.; Pan, X.; Chen, Y.; Shen, Y.; Ji, C. TRIM46 activates AKT/HK2 signaling by modifying PHLPP2 ubiquitylation to promote glycolysis and chemoresistance of lung cancer cells. Cell Death Dis. 2022, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, H.; Luo, D.; Gan, L.; Mo, S.; Dai, W.; Liang, L.; Yang, Y.; Xu, M.; Li, J.; et al. Lnc-RP11-536 K7.3/SOX2/HIF-1α signaling axis regulates oxaliplatin resistance in patient-derived colorectal cancer organoids. J. Exp. Clin. Cancer Res. 2021, 40, 238. [Google Scholar] [CrossRef]
- Liu, J.; Anurag, M.; Sekhar, R.; Andrew, W.M.; Gang, R.; Gabby, R. Structural Plasticity of Neurexin 1α: Implications for its Role as Synaptic Organizer. J. Mol. Biol. 2018, 430, 4325–4343. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigerup, C.; Påhlman, S.; Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016, 164, 152–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von der Maase, H. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.; Rosenberg, J.; Knowles, M. SnapShot: Bladder Cancer. Cancer Cell 2018, 34, 350–350.e1. [Google Scholar] [CrossRef] [PubMed]
- Von Der Maase, H.; Sengelov, L.; Roberts, J.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.; Zimmermann, A.; Arning, M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, with Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients with Bladder Cancer. J. Urol. 2006, 175, 482. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, S.; Wang, S.; Zhang, Y.; Chen, H.; Wang, Y.; Liu, R.L.; Niu, Y.J.; Xu, Y. EIF4A3-induced circARHGAP29 promotes aerobic glycolysis in docetaxel-resistant prostate cancer through IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 2022, 82, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, F.; Liang, S.; Wang, Q.; Wen, Y.; Wang, Q.; Zhang, J.; Li, M.; Fang, S.; Wei, T.; et al. lncRNA Linc00173 modulates glucosemetabolism and multidrug chemoresistancein SCLC: Potential molecular panel for targeted therapy. Mol. Ther. 2021, S1525-0016(21)00574-8. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Lin, C.; Lai, S.; Dai, H.; Wang, Z.; Dai, L.; Su, H.; Song, Y.; Zhang, N.; et al. LNCAROD enhances hepatocellular carcinoma malignancy by activating glycolysis through induction of pyruvate kinase isoform PKM2. J. Exp. Clin. Cancer Res. 2021, 40, 299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, R.; Zhou, C.; Yu, H.; Luo, W.; Zhu, J.; Liu, J.; Zhang, Z.; Xie, N.; Peng, X.; et al. ANGPTL4-Mediated Promotion of Glycolysis Facilitates the Colonization of Fusobacterium nucleatum in Colorectal Cancer. Cancer Res. 2021, 81, 6157–6170. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, X.; Lin, F.; Cheng, X.; Wang, Z.; Wang, X. Long non-coding RNA CTSLP8 mediates ovarian cancer progression and chemotherapy resistance by modulating cellular glycolysis and regulating c-Myc expression through PKM2. Cell Biol. Toxicol. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Wang, X.; Wei, X.; Wang, D.; Liu, X.; Xu, L.; Batu, W.; Li, Y.; Guo, B.; et al. RSL3 enhances the antitumor effect of cisplatin on prostate cancer cells via causing glycolysis dysfunction. Biochem. Pharmacol. 2021, 192, 114741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Lu, T.; Bi, G.; Li, M.; Liang, J.; Hu, Z.; Zheng, Y.; Yin, J.; Xi, J.; et al. HIF-1α switches the functionality of TGF-β signaling via changing the partners of smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 398. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.-L.; Huang, M.-L.; Zou, Y.; Yang, R.; Jiang, Y.; Sheng, J.-F.; Kong, Y.-G.; Tao, Z.-Z.; Feng, H.-Y.; Hua, Q.-Q.; et al. The IRF2/CENP-N/AKT signaling axis promotes proliferation, cell cycling and apoptosis resistance in nasopharyngeal carcinoma cells by increasing aerobic glycolysis. J. Exp. Clin. Cancer Res. 2021, 40, 390. [Google Scholar] [CrossRef]
- Lu, S.; Han, L.; Hu, X.; Sun, T.; Xu, D.; Li, Y.; Chen, Q.; Yao, W.; He, M.; Wang, Z.; et al. N6-methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: Implication in colorectal cancer. J. Hematol. Oncol. 2021, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, S.; Yang, F.; Zhang, Y.; Xiong, L.; Zhao, J.; Huang, L.; Chen, P.; Ren, L.; Li, H.; et al. Rabeprazole suppresses cell proliferation in gastric epithelial cells by targeting STAT3-mediated glycolysis. Biochem. Pharmacol. 2021, 188, 114525. [Google Scholar] [CrossRef]
- Wu, F.; Gao, P.; Wu, W.; Wang, Z.; Yang, J.; Di, J.; Jiang, B.; Su, X. STK25-induced inhibition of aerobic glycolysis via GOLPH3-mTOR pathway suppresses cell proliferation in colorectal cancer. J. Exp. Clin. Cancer Res. 2018, 37, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Huang, H.; Han, Q.; Hu, Z.; Teng, X.-L.; Ding, R.; Ye, Y.; Yu, X.; Zhao, R.; Wang, Z.; et al. SENP7 senses oxidative stress to sustain metabolic fitness and antitumor functions of CD8+ T cells. J. Clin. Investig. 2022, 132, e155224. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yang, Q.; Luo, L.; Sun, Y.; Lv, W.; Wan, S.; Guan, Z.; Xiao, Z.; Liu, F.; Li, Z.; et al. The kinase PDK1 regulates regulatory T cell survival via controlling redox homeostasis. Theranostics 2021, 11, 9503–9518. [Google Scholar] [CrossRef]
- Dong, F.; Li, R.; Wang, J.; Zhang, Y.; Yao, J.; Jiang, S.-H.; Hu, X.; Feng, M.; Bao, Z. Hypoxia-dependent expression of MAP17 coordinates the Warburg effect to tumor growth in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 121. [Google Scholar] [CrossRef] [PubMed]
- Willson, J.A.; Arienti, S.; Sadiku, P.; Reyes, L.; Coelho, P.; Morrison, T.; Rinaldi, G.; Dockrell, D.H.; Whyte, M.K.B.; Walmsley, S.R. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood 2022, 139, 281–286. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; Cheung, E.C.; Blagih, J.; Domart, M.-C.; Vousden, K.H. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab. 2019, 30, 720–734.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-J.; Hoffman, N.E.; Shanmughapriya, S.; Bao, L.; Keefer, K.; Conrad, K.; Merali, S.; Takahashi, Y.; Abraham, T.; Hirschler-Laszkiewicz, I.; et al. A Splice Variant of the Human Ion Channel TRPM2 Modulates Neuroblastoma Tumor Growth through Hypoxia-inducible Factor (HIF)-1/2α. J. Biol. Chem. 2014, 289, 36284–36302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wang, Y.; Shi, Z.; Liu, J.; Sun, P.; Hou, X.; Zhang, J.; Zhao, S.; Zhou, B.P.; Mi, J. Metabolic Reprogramming of Cancer-Associated Fibroblasts by IDH3α Downregulation. Cell Rep. 2015, 10, 1335–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apanovich, N.; Apanovich, P.; Mansorunov, D.; Kuzevanova, A.; Matveev, V.; Karpukhin, A. The Choice of Candidates in Survival Markers Based on Coordinated Gene Expression in Renal Cancer. Front. Oncol. 2021, 11, 615787. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, H.; Zhuang, Y.; Wei, L.; Yu, J.; Zhang, Z.; Zhang, L.; Wang, L. NDUFA4L2 promotes trastuzumab resistance in HER2-positive breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211027836. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.B.; Chen, Q.; Khani, F.; Attarwala, N.; Gross, S.S.; Dow, L.; Nanus, D.M.; Gudas, L.J. Mitochondrial Ndufa4l2 Enhances Deposition of Lipids and Expression of Ca9 in the TRACK Model of Early Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 783856. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.-M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updates 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, P.; Liu, Z.; Xing', Y.; Xiao, Y. NXPH4 Promotes Gemcitabine Resistance in Bladder Cancer by Enhancing Reactive Oxygen Species and Glycolysis Activation through Modulating NDUFA4L2. Cancers 2022, 14, 3782. https://doi.org/10.3390/cancers14153782
Wang D, Zhang P, Liu Z, Xing' Y, Xiao Y. NXPH4 Promotes Gemcitabine Resistance in Bladder Cancer by Enhancing Reactive Oxygen Species and Glycolysis Activation through Modulating NDUFA4L2. Cancers. 2022; 14(15):3782. https://doi.org/10.3390/cancers14153782
Chicago/Turabian StyleWang, Decai, Pu Zhang, Zijian Liu, Yifei Xing', and Yajun Xiao. 2022. "NXPH4 Promotes Gemcitabine Resistance in Bladder Cancer by Enhancing Reactive Oxygen Species and Glycolysis Activation through Modulating NDUFA4L2" Cancers 14, no. 15: 3782. https://doi.org/10.3390/cancers14153782