The Association between a Decrease in On-Treatment Neutrophil-to-Eosinophil Ratio (NER) at Week 6 after Ipilimumab Plus Nivolumab Initiation and Improved Clinical Outcomes in Metastatic Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Variables and Endpoints of Interests
2.3. Statistical Analysis
3. Results
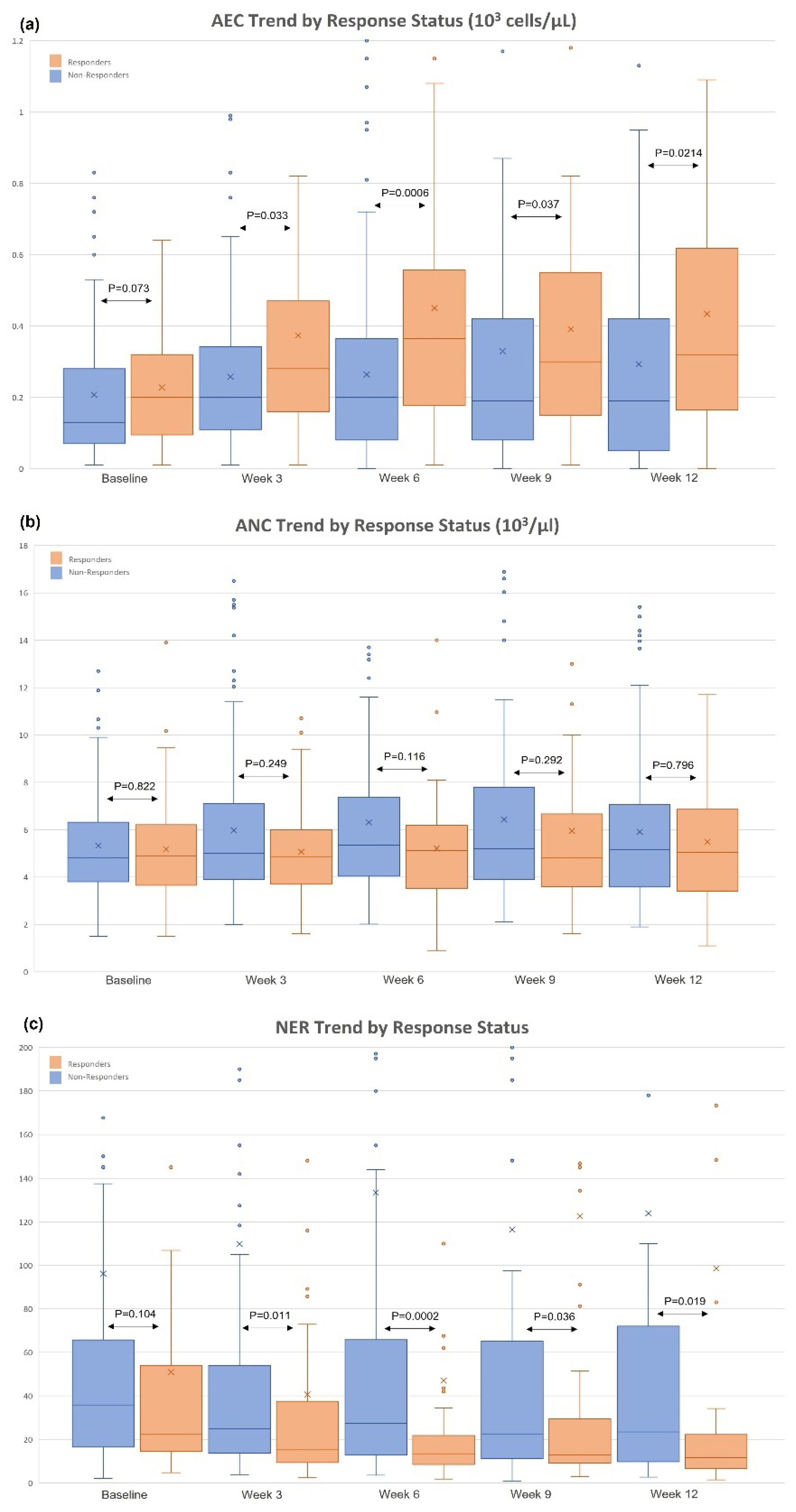
3.1. Changes in AEC/ANC/NER and Patient Baseline Characteristics
3.2. Association between Decreased NER at Week 6 and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Aroldi, F.; Middleton, M.R. Long-Term Outcomes of Immune Checkpoint Inhibition in Metastatic Melanoma. Am. J. Clin. Dermatol. 2022, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sham, N.O.; Zhao, L.; Zhu, Z.; Roy, T.M.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. Immunotherapy for Non-small Cell Lung Cancer: Current Agents and Potential Molecular Targets. Anticancer Res. 2022, 42, 3275–3284. [Google Scholar] [CrossRef]
- Houssiau, H.; Seront, E. The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Cancers 2022, 14, 1640. [Google Scholar] [CrossRef]
- Abd El Aziz, M.A.; Facciorusso, A.; Nayfeh, T.; Saadi, S.; Elnaggar, M.; Cotsoglou, C.; Sacco, R. Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma. Vaccines 2020, 8, 616. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Plimack, E.R.; Porta, C.G.; George, S.; Powles, T.B.; et al. Conditional survival and 5-year follow-up in CheckMate 214: First-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Ann. Oncol. 2021, 32 (Suppl. 5), S678–S724. [Google Scholar] [CrossRef]
- Xin, J.X.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar] [CrossRef] [Green Version]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gerard, C.L.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Linette, G.P.; Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malig. Rep. 2019, 14, 286–291. [Google Scholar] [CrossRef]
- Liu, X.; Hogg, G.D.; DeNardo, D.G. Rethinking immune checkpoint blockade: ‘Beyond the T cell’. J. Immunother. Cancer 2021, 9, e001460. [Google Scholar] [CrossRef]
- Hussain, K.; Cragg, M.S.; Beers, S.A. Remodeling the Tumor Myeloid Landscape to Enhance Antitumor Antibody Immunotherapies. Cancers 2021, 13, 4904. [Google Scholar] [CrossRef]
- Nakamura, K.; Smyth, M.J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell. Mol. Immunol. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Dulberg, S.; Beck, L.; Zhang, C.; Itan, M.; Hediyeh-Zadeh, S.; Caldwell, J.; Rozenberg, P.; Dolitzky, A.; Avlas, S.; et al. Metastasis-Entrained Eosinophils Enhance Lymphocyte-Mediated Antitumor Immunity. Cancer Res. 2021, 81, 5555–5571. [Google Scholar] [CrossRef]
- Martens, A.; Wistuba-Hamprecht, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, P.F.; Gandini, S.; Cocorocchio, E.; Pala, L.; Baldini, F.; Mosconi, M.; Antonini Cappellini, G.C.; Albertazzi, E.; Martinoli, C. Baseline relative eosinophil count as a predictive biomarker for ipilimumab treatment in advanced melanoma. Oncotarget 2017, 8, 79809–79815. [Google Scholar] [CrossRef] [Green Version]
- Mota, J.M.; Teo, M.Y.; Whiting, K.; Li, H.A.; Regazzi, A.M.; Lee, C.H.; Funt, S.A.; Bajorin, D.; Ostrovnaya, I.; Iyer, G.; et al. Pretreatment Eosinophil Counts in Patients With Advanced or Metastatic Urothelial Carcinoma Treated With Anti-PD-1/PD-L1 Checkpoint Inhibitors. J. Immunother. 2021, 44, 248–253. [Google Scholar] [CrossRef]
- Minohara, K.; Matoba, T.; Kawakita, D.; Takano, G.; Oguri, K.; Murashima, A.; Nakai, K.; Iwaki, S.; Hojo, W.; Matsumura, A.; et al. Novel Prognostic Score for recurrent or metastatic head and neck cancer patients treated with Nivolumab. Sci. Rep. 2021, 11, 16992. [Google Scholar] [CrossRef]
- Sibille, A.; Henket, M.; Corhay, J.L.; Alfieri, R.; Louis, R.; Duysinx, B. White Blood Cells in Patients Treated with Programmed Cell Death-1 Inhibitors for Non-small Cell Lung Cancer. Lung 2021, 199, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Dias, M.; Campainha, S.; Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 2021, 13, 2716–2727. [Google Scholar] [CrossRef]
- Simon, S.C.S.; Hu, X.; Panten, J.; Grees, M.; Renders, S.; Thomas, D.; Weber, R.; Schulze, T.J.; Utikal, J.; Umansky, V. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 2020, 9, 1727116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, T.; Ginzac, A.; Molnar, I.; Bailly, S.; Durando, X.; Mahammedi, H. Eosinophil counts as a relevant prognostic marker for response to nivolumab in the management of renal cell carcinoma: A retrospective study. Cancer Med. 2021, 10, 6705–6713. [Google Scholar] [CrossRef]
- Tucker, M.D.; Brown, L.C.; Chen, Y.W.; Kao, C.; Hirshman, N.; Kinsey, E.N.; Ancell, K.K.; Beckermann, K.E.; Davis, N.B.; McAlister, R.; et al. Association of baseline neutrophil-to-eosinophil ratio with response to nivolumab plus ipilimumab in patients with metastatic renal cell carcinoma. Biomark. Res. 2021, 9, 80. [Google Scholar] [CrossRef]
- Gil, L.; Alves, F.R.; Silva, D.; Fernandes, I.; Fontes-Sousa, M.; Alves, M.; Papoila, A.; Da Luz, R. Prognostic Impact of Baseline Neutrophil-to-Eosinophil Ratio in Patients With Metastatic Renal Cell Carcinoma Treated With Nivolumab Therapy in Second or Later Lines. Cureus 2022, 14, e22224. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.L.; Rini, B.I.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T.; et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Rodgers, S.; Rees, R.C.; Hancock, B.W. Changes in the phenotypic characteristics of eosinophils from patients receiving recombinant human interleukin-2 (rhIL-2) therapy. Br. J. Haematol. 1994, 86, 746–753. [Google Scholar] [CrossRef]
- McNeel, D.G.; Gardner, T.A.; Higano, C.S.; Kantoff, P.W.; Small, E.J.; Wener, M.H.; Sims, R.B.; DeVries, T.; Sheikh, N.A.; Dreicer, R. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol. Res. 2014, 2, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef] [Green Version]
- Reichman, H.; Karo-Atar, D.; Munitz, A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer 2016, 2, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S. Novel Therapies for Eosinophilic Disorders. Immunol. Allergy Clin. North Am. 2015, 35, 577–598. [Google Scholar] [CrossRef] [Green Version]
- Arnold, I.C.; Artola-Boran, M.; Gurtner, A.; Bertram, K.; Bauer, M.; Frangez, Z.; Becher, B.; Kopf, M.; Yousefi, S.; Simon, H.U.; et al. The GM-CSF-IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. J. Exp. Med. 2020, 217, e20190706. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faget, J.; Peters, S.; Quantin, X.; Meylan, E.; Bonnefoy, N. Neutrophils in the era of immune checkpoint blockade. J. Immunother. Cancer 2021, 9, e002242. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [Green Version]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Miranda, K.; Tucker, M.D.; Chen, Y.W.; Beckermann, K.E.; Rini, B.I. Concurrent Immunotherapy And Dipeptidyl Peptidase-4 Inhibition Among Patients With Solid Tumors. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Costin, D.; O’Neill, V.J.; Corsi-Travali, S.; Adurthi, S.; Adedoyin, A.; Healey, D.I.; De Bono, J.S.; Monk, P.; Zhang, J.; et al. Phase 1b study of BXCL701, a novel small molecule inhibitor of dipeptidyl peptidases (DPP), combined with pembrolizumab (pembro), in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, e17581. [Google Scholar] [CrossRef]
- Monk, P.; Zhang, J.; Costin, D.; Petrylak, D.P.; Tagawa, S.T.; Karsh, L.I.; Zhu, X.; Karsh, L.I.; Zhu, X.; Linch, M.; et al. BXCL701-1st-in-class oral activator of systemic innate immunity-combined with pembrolizumab, in men with metastatic castration-resistant prostate cancer (mCRPC): Phase II results. Ann. Oncol. 2021, 5, S651. [Google Scholar] [CrossRef]
- Osawa, H.; Shiozawa, T.; Okauchi, S.; Miyazaki, K.; Kodama, T.; Kagohashi, K.; Nakamura, R.; Satoh, H.; Hizawa, N. Association between time to treatment failure and peripheral eosinophils in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Pol. Arch. Intern. Med. 2021, 131, 16049. [Google Scholar] [CrossRef]
- Takayasu, S.; Mizushiri, S.; Watanuki, Y.; Yamagata, S.; Usutani, M.; Nakada, Y.; Asari, Y.; Murasawa, S.; Kageyama, K.; Daimon, M. Eosinophil counts can be a predictive marker of immune checkpoint inhibitor-induced secondary adrenal insufficiency: A retrospective cohort study. Sci. Rep. 2022, 12, 1294. [Google Scholar] [CrossRef]
- Yamada, H.; Washino, S.; Suzuki, D.; Saikawa, R.; Tonezawa, S.; Hagiwara, R.; Funazaki, S.; Yoshida, M.; Konishi, T.; Saito, K.; et al. Hypereosinophilia is a predictive biomarker of immune checkpoint inhibitor-induced hypopituitarism in patients with renal cell carcinoma. BMC Endocr. Disord. 2022, 22, 110. [Google Scholar] [CrossRef]
- Bottlaender, L.; Amini-Adle, M.; Maucort-Boulch, D.; Robinson, P.; Thomas, L.; Dalle, S. Cutaneous adverse events: A predictor of tumour response under anti-PD-1 therapy for metastatic melanoma, a cohort analysis of 189 patients. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 2096–2105. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Wu, F.; Ren, S.; et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar] [CrossRef]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Tiwary, V.; Sunil, K.; Suresh, D.; Shetty, S.; Muthukaliannan, G.K.; Baxi, S.; Jayaraj, R. Prognostic Utility of Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio and Monocyte-Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers 2021, 13, 4166. [Google Scholar] [CrossRef] [PubMed]
- Booka, E.; Kikuchi, H.; Haneda, R.; Soneda, W.; Kawata, S.; Murakami, T.; Matsumoto, T.; Hiramatsu, Y.; Takeuchi, H. Neutrophil-to-Lymphocyte Ratio to Predict the Efficacy of Immune Checkpoint Inhibitor in Upper Gastrointestinal Cancer. Anticancer Res. 2022, 42, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Mori, K.; Katayama, S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Rajwa, P.; Motlagh, R.S.; Aydh, A.; König, F.; et al. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2022, 14, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Deng, Q.; Zhang, L.; He, S.; Rong, J.; Zheng, F. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with urological cancers: A meta-analysis. Pathol. Res. Pract. 2019, 215, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Serviddio, G.; Vendemiale, G.; Muscatiello, N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J. Gastroenterol. 2016, 22, 4211–4218. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Jin, X.; Wang, K.; Shao, X.; Huang, J. Prognostic implications of the peripheral platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in predicting pathologic complete response after neoadjuvant chemotherapy in breast cancer patients. Gland Surg. 2022, 11, 1057–1066. [Google Scholar] [CrossRef]
- Leng, J.; Wu, F.; Zhang, L. Prognostic Significance of Pretreatment Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, or Monocyte-to-Lymphocyte Ratio in Endometrial Neoplasms: A Systematic Review and Meta-analysis. Front. Oncol. 2022, 12, 734948. [Google Scholar] [CrossRef]


| Median (IQR) | |
|---|---|
| Baseline NER | 23.8 (15–57) |
| Week 6 NER | 19.8 (10.6–40.8) |
| N (%) | |
| Week 6 NER change | |
| Decrease > 50% | 44 (29) |
| Decrease ≤ 50% | 58 (39) |
| Increase | 48 (32) |
| Baseline NER | |
| High (≥median) | 75 (50) |
| Low (<median) | 75 (50) |
| Age | (Median: 62 (IQR: 53–70)) |
| <60 | 63 (42) |
| ≥60 | 87 (58) |
| Sex | |
| Male | 111 (74) |
| Female | 39 (26) |
| Race | |
| White | 120 (80) |
| Non-White | 24 (14) |
| Unknown | 9 (6) |
| Histology | |
| Clear cell | 117 (78) |
| Non-clear cell | 31 (21) |
| Unknown | 2 (1) |
| IMDC risk | |
| Favorable | 33 (22) |
| Intermediate | 94 (63) |
| Poor | 23 (15) |
| Nephrectomy | |
| Yes | 104 (69) |
| No | 46 (31) |
| Prior systemic therapy | |
| Yes | 49 (33) |
| No | 100 (67) |
| Unknown | 1 (1) |
| PFS | OS | |||
|---|---|---|---|---|
| AHR (95%) | p-Value | AHR (95%) | p-Value | |
| Continuous variable | ||||
| Baseline LnNER | 0.98 (0.78–1.23) | 0.84 | 0.82 (0.57–1.19) | 0.30 |
| Week 6 LnNER | 0.78 (0.66–0.93) | 0.005 | 0.67 (0.52–0.86) | 0.002 |
| Week 6 NER change | ||||
| All patients (N = 150) | ||||
| Decrease > 50% | 0.55 (0.31–0.95) | 0.03 | 0.37 (0.16–0.84) | 0.02 |
| Decrease ≤ 50% | 0.63 (0.38–1.05) | 0.07 | 0.49 (0.23–1.06) | 0.07 |
| Increase | Ref | Ref | ||
| Subgroup with high baseline NER (N = 75) | ||||
| Decrease > 50% | 0.46 (0.22–1.00) | 0.048 | 0.28 (0.11–0.74) | 0.01 |
| Decrease ≤ 50% | 0.59 (0.26–1.31) | 0.19 | 0.44 (0.16–1.23) | 0.12 |
| Increase | Ref | Ref | ||
| Subgroup with low baseline NER (N = 75) | ||||
| Decrease > 50% | 0.58 (0.22–1.48) | 0.25 | 0.60 (0.08–4.30) | 0.61 |
| Decrease ≤ 50% | 0.72 (0.34–1.53) | 0.39 | 1.08 (0.25–4.70) | 0.92 |
| Increase | Ref | Ref |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-W.; Tucker, M.D.; Brown, L.C.; Yasin, H.A.; Ancell, K.K.; Armstrong, A.J.; Beckermann, K.E.; Davis, N.B.; Harrison, M.R.; Kaiser, E.G.; et al. The Association between a Decrease in On-Treatment Neutrophil-to-Eosinophil Ratio (NER) at Week 6 after Ipilimumab Plus Nivolumab Initiation and Improved Clinical Outcomes in Metastatic Renal Cell Carcinoma. Cancers 2022, 14, 3830. https://doi.org/10.3390/cancers14153830
Chen Y-W, Tucker MD, Brown LC, Yasin HA, Ancell KK, Armstrong AJ, Beckermann KE, Davis NB, Harrison MR, Kaiser EG, et al. The Association between a Decrease in On-Treatment Neutrophil-to-Eosinophil Ratio (NER) at Week 6 after Ipilimumab Plus Nivolumab Initiation and Improved Clinical Outcomes in Metastatic Renal Cell Carcinoma. Cancers. 2022; 14(15):3830. https://doi.org/10.3390/cancers14153830
Chicago/Turabian StyleChen, Yu-Wei, Matthew D. Tucker, Landon C. Brown, Hesham A. Yasin, Kristin K. Ancell, Andrew J. Armstrong, Kathryn E. Beckermann, Nancy B. Davis, Michael R. Harrison, Elizabeth G. Kaiser, and et al. 2022. "The Association between a Decrease in On-Treatment Neutrophil-to-Eosinophil Ratio (NER) at Week 6 after Ipilimumab Plus Nivolumab Initiation and Improved Clinical Outcomes in Metastatic Renal Cell Carcinoma" Cancers 14, no. 15: 3830. https://doi.org/10.3390/cancers14153830
APA StyleChen, Y.-W., Tucker, M. D., Brown, L. C., Yasin, H. A., Ancell, K. K., Armstrong, A. J., Beckermann, K. E., Davis, N. B., Harrison, M. R., Kaiser, E. G., McAlister, R. K., Schaffer, K. R., Wallace, D. E., George, D. J., Rathmell, W. K., Rini, B. I., & Zhang, T. (2022). The Association between a Decrease in On-Treatment Neutrophil-to-Eosinophil Ratio (NER) at Week 6 after Ipilimumab Plus Nivolumab Initiation and Improved Clinical Outcomes in Metastatic Renal Cell Carcinoma. Cancers, 14(15), 3830. https://doi.org/10.3390/cancers14153830






