Immunotherapy of Neuroendocrine Neoplasms: Any Role for the Chimeric Antigen Receptor T Cells?
Abstract
:Simple Summary
Abstract
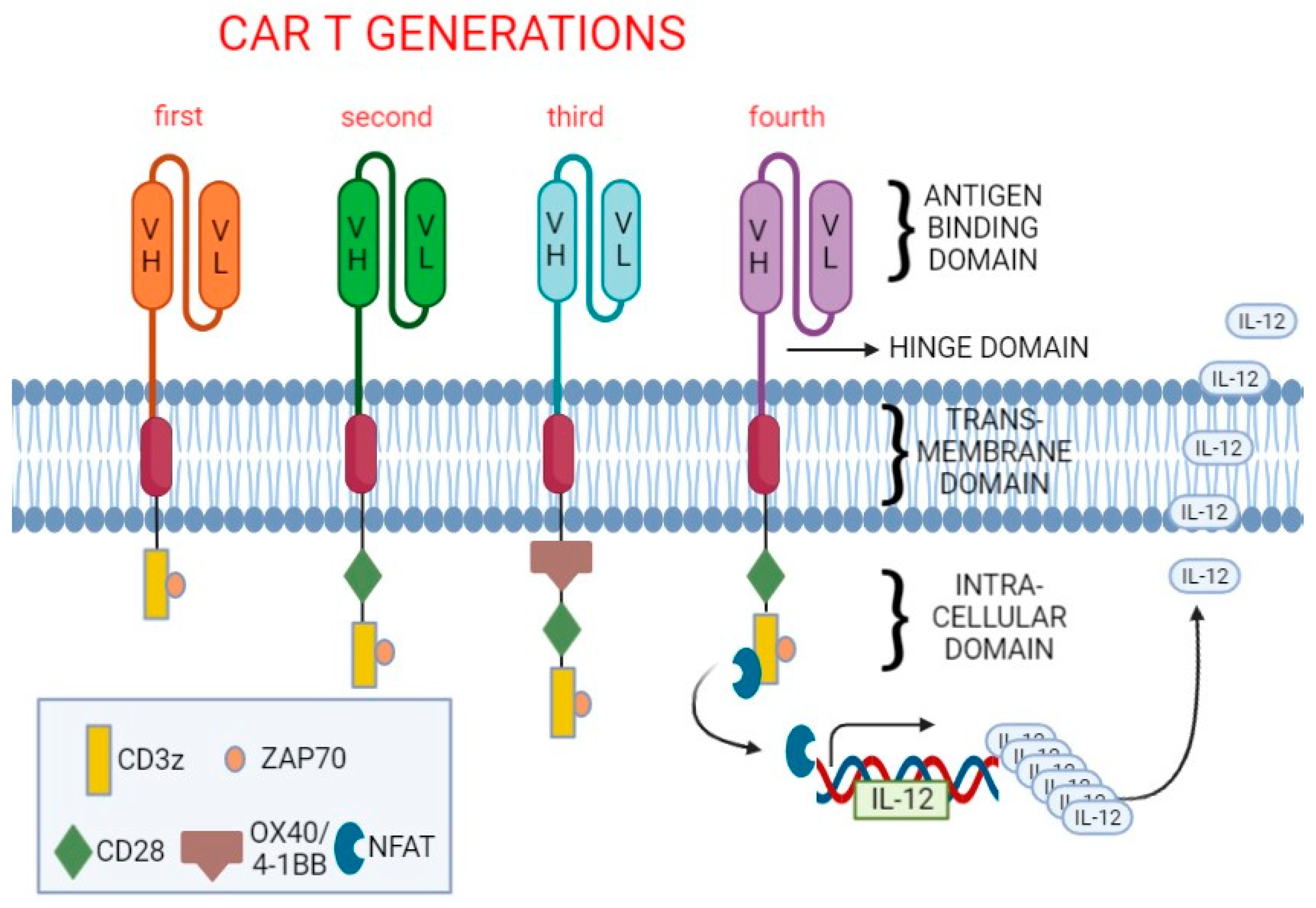
1. Introduction
2. Search Strategy
2.1. Medullary Thyroid Carcinoma
2.2. Small Cell Lung Carcinoma
2.3. Neuroendocrine Prostate Carcinoma
2.4. Pancreatic Neuroendocrine Neoplasms
2.5. Ileal and Lung Neuroendocrine Cells
2.6. Potential Future Applications
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faggiano, A.; Ferolla, P.; Grimaldi, F.; Campana, D.; Manzoni, M.; Davi, M.V.; Bianchi, A.; Valcavi, R.; Papini, E.; Giuffrida, D.; et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: The NET management study. J. Endocrinol. Investig. 2012, 35, 817–823. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Lo Calzo, F.; Pizza, G.; Modica, R.; Colao, A. The safety of available treatments options for neuroendocrine tumors. Expert Opin. Drug Saf. 2017, 16, 1149–1161. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Novel therapeutics for patients with well-differentiated gastroenteropancreatic neuroendocrine tumors. Ther. Adv. Med. Oncol. 2021, 13, 17588359211018047. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Faggiano, A.; Di Maio, S.; Mocerino, C.; Ottaviano, M.; De Divitiis, C.; Guarnotta, V.; Dolce, P.; Modica, R.; Puliafito, I.; Tozzi, L.; et al. Therapeutic sequences in patients with grade 1–2 neuroendocrine tumors (NET): An observational multicenter study from the ELIOS group. Endocrine 2019, 66, 417–424. [Google Scholar] [CrossRef]
- Lamberti, G.; Faggiano, A.; Brighi, N.; Tafuto, S.; Ibrahim, T.; Brizzi, M.P.; Pusceddu, S.; Albertelli, M.; Massironi, S.; Panzuto, F.; et al. Nonconventional Doses of Somatostatin Analogs in Patients With Progressing Well-Differentiated Neuroendocrine Tumor. J. Clin. Endocrinol. Metab. 2020, 105, 194–200. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Cives, M.; Strosberg, J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl. Gastroenterol. Hepatol. 2020, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Albertelli, M.; Dotto, A.; Nista, F.; Veresani, A.; Patti, L.; Gay, S.; Sciallero, S.; Boschetti, M.; Ferone, D. Present and future of immunotherapy in Neuroendocrine Tumors. Rev. Endocr. Metab. Disord. 2021, 22, 615–636. [Google Scholar] [CrossRef]
- Fazio, N.; Abdel-Rahman, O. Immunotherapy in Neuroendocrine Neoplasms: Where Are We Now? Curr. Treat. Options Oncol. 2021, 22, 19. [Google Scholar] [CrossRef]
- Gallo, M.; Guarnotta, V.; De Cicco, F.; Rubino, M.; Faggiano, A.; Colao, A.; Group, N. Immune checkpoint blockade for Merkel cell carcinoma: Actual findings and unanswered questions. J. Cancer Res. Clin. Oncol. 2019, 145, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, G.; Di Molfetta, S.; Dotto, A.; Florio, T.; Feola, T.; Rubino, M.; de Cicco, F.; Colao, A.; Faggiano, A.; Nike, G. Emerging Therapies in Pheochromocytoma and Paraganglioma: Immune Checkpoint Inhibitors in the Starting Blocks. J. Clin. Med. 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Di Molfetta, S.; Feola, T.; Fanciulli, G.; Florio, T.; Colao, A.; Faggiano, A.; Nike, G. Immune Checkpoint Blockade in Lung Carcinoids with Aggressive Behaviour: One More Arrow in Our Quiver? J. Clin. Med. 2022, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Di Molfetta, S.; Dotto, A.; Fanciulli, G.; Florio, T.; Feola, T.; Colao, A.; Faggiano, A. Immune Checkpoint Inhibitors: New Weapons Against Medullary Thyroid Cancer? Front. Endocrinol. 2021, 12, 667784. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Yakoub-Agha, I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr. Res. Transl. Med. 2017, 65, 93–102. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Ball, D.W.; Byrd, D.; Daniels, G.H.; Dilawari, R.A.; Doherty, G.M.; Duh, Q.Y.; Ehya, H.; Farrar, W.B.; Haddad, R.I.; et al. Medullary carcinoma. J. Natl. Compr. Cancer Netw. 2010, 8, 512–530. [Google Scholar] [CrossRef]
- Viola, D.; Elisei, R. Management of Medullary Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 285–301. [Google Scholar] [CrossRef]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017, 161, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Sippel, R.S.; Kunnimalaiyaan, M.; Chen, H. Current management of medullary thyroid cancer. Oncologist 2008, 13, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Chen, H.; Sippel, R.S. Current understanding and management of medullary thyroid cancer. Oncologist 2013, 18, 1093–1100. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Faggiano, A.; Modica, R.; Severino, R.; Camera, L.; Fonti, R.; Del Prete, M.; Chiofalo, M.G.; Aria, M.; Ferolla, P.; Vitale, G.; et al. The antiproliferative effect of pasireotide LAR alone and in combination with everolimus in patients with medullary thyroid cancer: A single-center, open-label, phase II, proof-of-concept study. Endocrine 2018, 62, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Florio, T.; Ferrau, F.; Kara, E.; Fanciulli, G.; Faggiano, A.; Colao, A.; Group, N. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr. Relat. Cancer 2018, 25, R453–R466. [Google Scholar] [CrossRef] [PubMed]
- Okafor, C.; Hogan, J.; Raygada, M.; Thomas, B.J.; Akshintala, S.; Glod, J.W.; Del Rivero, J. Update on Targeted Therapy in Medullary Thyroid Cancer. Front. Endocrinol. 2021, 12, 708949. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.Y.; Ehya, H.; et al. NCCN clinical practice guidelines in oncology: Thyroid carcinoma., V 2.2022 ed. J. Natl. Compr. Cancer Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.H. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinol. Metab. 2021, 36, 514–524. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumors 2021: Thoracic Tumors; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Dingemans, A.C.; Fruh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(). Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.; Gonzalez-Cao, M.; Domine, M.; De Castro, J.; Cobo, M.; Bernabe, R.; Navarro, A.; Sullivan, I.; Trigo, J.M.; Mosquera, J.; et al. Addition of Immune Checkpoint Inhibitors to Chemotherapy vs Chemotherapy Alone as First-Line Treatment in Extensive-Stage Small-Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. Oncol. Ther. 2022, 10, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csoszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Huang, L.L.; Hu, X.S.; Wang, Y.; Li, J.L.; Wang, H.Y.; Liu, P.; Xu, J.P.; He, X.H.; Hao, X.Z.; Jiang, P.D.; et al. Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients. Thorac. Cancer 2021, 12, 1943–1951. [Google Scholar] [CrossRef]
- Taromi, S.; Firat, E.; Simonis, A.; Braun, L.M.; Apostolova, P.; Elze, M.; Passlick, B.; Schumacher, A.; Lagies, S.; Frey, A.; et al. RETRACTED: Enhanced AC133-specific CAR T cell therapy induces durable remissions in mice with metastatic small cell lung cancer. Cancer Lett. 2021, 520, 385–399. [Google Scholar] [CrossRef]
- Sarvi, S.; Mackinnon, A.C.; Avlonitis, N.; Bradley, M.; Rintoul, R.C.; Rassl, D.M.; Wang, W.; Forbes, S.J.; Gregory, C.D.; Sethi, T. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res. 2014, 74, 1554–1565. [Google Scholar] [CrossRef]
- Reppel, L.; Tsahouridis, O.; Akulian, J.; Davis, I.J.; Lee, H.; Fuca, G.; Weiss, J.; Dotti, G.; Pecot, C.V.; Savoldo, B. Targeting disialoganglioside GD2 with chimeric antigen receptor-redirected T cells in lung cancer. J. Immunother. Cancer 2022, 10, e003897. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, C.; Landoni, E.; Metelitsa, L.; Dotti, G.; Savoldo, B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clin. Cancer Res. 2019, 25, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Crossland, D.L.; Denning, W.L.; Ang, S.; Olivares, S.; Mi, T.; Switzer, K.; Singh, H.; Huls, H.; Gold, K.S.; Glisson, B.S.; et al. Antitumor activity of CD56-chimeric antigen receptor T cells in neuroblastoma and SCLC models. Oncogene 2018, 37, 3686–3697. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Lorigan, P.; O’Brien, M.E.; Fossella, F.V.; Moore, K.N.; Bhatia, S.; Kirby, M.; Woll, P.J. Phase I study of IMGN901, a CD56-targeting antibody-drug conjugate, in patients with CD56-positive solid tumors. Investig. New Drugs 2016, 34, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Facchinetti, F.; Minari, R.; Cortellini, A.; Rolfo, C.D.; Giovannetti, E.; Tiseo, M. Notch pathway in small-cell lung cancer: From preclinical evidence to therapeutic challenges. Cell Oncol. 2019, 42, 261–273. [Google Scholar] [CrossRef]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef]
- Zhou, D.; Byers, L.; Sable, B.; Smit, M.; Sadraei, N.H.; Dutta, S.; Upreti, V. Clinical pharmacology characterization of AMG 119, a chimeric antigen receptor T (CAR-T) cell therapy targeting Delta-Like Ligand 3 (DLL3), in patients with relapsed patients with relapsed/refractory Small Cell Lung Cancer. In Clinical Pharmacology & Therapeutics; Wiley: New York, NY, USA, 2022; Volume 111. [Google Scholar]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Beltran, H.; Lotan, T.L.; Mosquera, J.M.; Reuter, V.E.; Robinson, B.D.; Troncoso, P.; Rubin, M.A. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 2014, 38, 756–767. [Google Scholar] [CrossRef]
- Kranitz, N.; Szepesvary, Z.; Kocsis, K.; Kullmann, T. Neuroendocrine Cancer of the Prostate. Pathol. Oncol. Res. 2020, 26, 1447–1450. [Google Scholar] [CrossRef]
- Alanee, S.; Moore, A.; Nutt, M.; Holland, B.; Dynda, D.; El-Zawahry, A.; McVary, K.T. Contemporary Incidence and Mortality Rates of Neuroendocrine Prostate Cancer. Anticancer Res. 2015, 35, 4145–4150. [Google Scholar]
- Bhagirath, D.; Liston, M.; Akoto, T.; Lui, B.; Bensing, B.A.; Sharma, A.; Saini, S. Novel, non-invasive markers for detecting therapy induced neuroendocrine differentiation in castration-resistant prostate cancer patients. Sci. Rep. 2021, 11, 8279. [Google Scholar] [CrossRef]
- Beltran, H.; Demichelis, F. Therapy considerations in neuroendocrine prostate cancer: What next? Endocr. Relat. Cancer 2021, 28, T67–T78. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Ci, X.; Choi, S.Y.C.; Crea, F.; Lin, D.; Wang, Y. Molecular events in neuroendocrine prostate cancer development. Nat. Rev. Urol. 2021, 18, 581–596. [Google Scholar] [CrossRef]
- Lee, J.K.; Bangayan, N.J.; Chai, T.; Smith, B.A.; Pariva, T.E.; Yun, S.; Vashisht, A.; Zhang, Q.; Park, J.W.; Corey, E.; et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E4473–E4482. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.S.; Kim, Y.J.; Vergara, S.; Conard, A.; Adams, C.; Calero, G.; Ishima, R.; Mellors, J.W.; Dimitrov, D.S. A highly-specific fully-human antibody and CAR-T cells targeting CD66e/CEACAM5 are cytotoxic for CD66e-expressing cancer cells in vitro and in vivo. Cancer Lett. 2022, 525, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Su, M.C.; Yuan, R.H.; Lin, C.Y.; Jeng, Y.M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod. Pathol. 2008, 21, 1379–1386. [Google Scholar] [CrossRef]
- Snow, A.N.; Mangray, S.; Lu, S.; Clubwala, R.; Li, J.; Resnick, M.B.; Yakirevich, E. Expression of cadherin 17 in well-differentiated neuroendocrine tumours. Histopathology 2015, 66, 1010–1021. [Google Scholar] [CrossRef]
- Barbieri, F.; Albertelli, M.; Grillo, F.; Mohamed, A.; Saveanu, A.; Barlier, A.; Ferone, D.; Florio, T. Neuroendocrine tumors: Insights into innovative therapeutic options and rational development of targeted therapies. Drug Discov. Today 2014, 19, 458–468. [Google Scholar] [CrossRef]
- Mandriani, B.; Pelle, E.; Mannavola, F.; Palazzo, A.; Marsano, R.M.; Ingravallo, G.; Cazzato, G.; Ramello, M.C.; Porta, C.; Strosberg, J.; et al. Development of anti-somatostatin receptors CAR T cells for treatment of neuroendocrine tumors. J. Immunother. Cancer 2022, 10, e004854. [Google Scholar] [CrossRef]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Hardaway, J.; Prince, E.; Guha, P.; Cunetta, M.; Moody, A.; Wang, L.J.; Armenio, V.; Espat, N.J.; Junghans, R.P. HITM-SIR: Phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA(+) liver metastases. Cancer Gene Ther. 2020, 27, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Moody, A.E.; Guha, P.; Hardaway, J.C.; Prince, E.; LaPorte, J.; Stancu, M.; Slansky, J.E.; Jordan, K.R.; Schulick, R.D.; et al. HITM-SURE: Hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J. Immunother. Cancer 2020, 8, e001097. [Google Scholar] [CrossRef]
- Gold, P.; Freedman, S.O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 1965, 122, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Hamada, S. Association of medullary carcinoma of the thyroid with carcinoembryonic antigen. Br. J. Cancer 1976, 34, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Hamada, S. Localization of carcinoembryonic antigen in medullary thyroid carcinoma by immunofluorescent techniques. Br. J. Cancer 1977, 36, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Edington, H.D.; Watson, C.G.; Levine, G.; Tauxe, W.N.; Yousem, S.A.; Unger, M.; Kowal, C.D. Radioimmunoimaging of metastatic medullary carcinoma of the thyroid gland using an indium-111-labeled monoclonal antibody to CEA. Surgery 1988, 104, 1004–1010. [Google Scholar]
- De Labriolle-Vaylet, C.; Cattan, P.; Sarfati, E.; Wioland, M.; Billotey, C.; Brocheriou, C.; Rouvier, E.; de Roquancourt, A.; Rostene, W.; Askienazy, S.; et al. Successful surgical removal of occult metastases of medullary thyroid carcinoma recurrences with the help of immunoscintigraphy and radioimmunoguided surgery. Clin. Cancer Res. 2000, 6, 363–371. [Google Scholar]
- Bodet-Milin, C.; Faivre-Chauvet, A.; Carlier, T.; Rauscher, A.; Bourgeois, M.; Cerato, E.; Rohmer, V.; Couturier, O.; Drui, D.; Goldenberg, D.M.; et al. Immuno-PET Using Anticarcinoembryonic Antigen Bispecific Antibody and 68Ga-Labeled Peptide in Metastatic Medullary Thyroid Carcinoma: Clinical Optimization of the Pretargeting Parameters in a First-in-Human Trial. J. Nucl. Med. 2016, 57, 1505–1511. [Google Scholar] [CrossRef]
- Bodet-Milin, C.; Bailly, C.; Touchefeu, Y.; Frampas, E.; Bourgeois, M.; Rauscher, A.; Lacoeuille, F.; Drui, D.; Arlicot, N.; Goldenberg, D.M.; et al. Clinical Results in Medullary Thyroid Carcinoma Suggest High Potential of Pretargeted Immuno-PET for Tumor Imaging and Theranostic Approaches. Front. Med. 2019, 6, 124. [Google Scholar] [CrossRef]
- Zhang, Q.; Ping, J.; Huang, Z.; Zhang, X.; Zhou, J.; Wang, G.; Liu, S.; Ma, J. CAR-T Cell Therapy in Cancer: Tribulations and Road Ahead. J. Immunol. Res. 2020, 2020, 1924379. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Muffly, L.S.; Spinner, M.A.; Barnes, J.I.; Owens, D.K.; Goldhaber-Fiebert, J.D. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 2105–2119. [Google Scholar] [CrossRef] [PubMed]

| Brand Name | Generic Name | Target | Indications | FDA Approval (Year) | EMA Approval (Year) |
|---|---|---|---|---|---|
| Abecma | idecabtagene vicleucel | B-cell maturation antigen | relapsed/refractory multiple myeloma | 2021 | 2021 (conditional) |
| Breyanzi | lisocabtagene maraleucel | CD19 | relapsed/refractory diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, follicular lymphoma grade 3B, after two or more lines of systemic therapy | 2021 | 2022 (initial orphan drug approval 2017) |
| Carvykti | ciltacabtagene autoleucel | CD38 | Relapsed/refractory multiple myeloma | 2022 | 2022 (orphan) |
| Kymriah | tisagenlecleucel | CD19 | B-cell acute lymphoblastic leukemia, relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma | 2017 | 2018 (initial orphan drug approval 2016) |
| Tecartus | brexucabtagene autoleucel | CD19 | relapsed/refractory mantle cell lymphoma | 2020 | 2019 (conditional) |
| Yescarta | axicabtagene ciloleucel | CD19 | diffuse large B-cell lymphoma, transformed follicular lymphoma, primary mediastinal B-cell lymphoma | 2017 | 2018 (initial orphan drug approval 2016) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanciulli, G.; Modica, R.; La Salvia, A.; Campolo, F.; Florio, T.; Mikovic, N.; Plebani, A.; Di Vito, V.; Colao, A.; Faggiano, A., on behalf of NIKE Group . Immunotherapy of Neuroendocrine Neoplasms: Any Role for the Chimeric Antigen Receptor T Cells? Cancers 2022, 14, 3991. https://doi.org/10.3390/cancers14163991
Fanciulli G, Modica R, La Salvia A, Campolo F, Florio T, Mikovic N, Plebani A, Di Vito V, Colao A, Faggiano A on behalf of NIKE Group . Immunotherapy of Neuroendocrine Neoplasms: Any Role for the Chimeric Antigen Receptor T Cells? Cancers. 2022; 14(16):3991. https://doi.org/10.3390/cancers14163991
Chicago/Turabian StyleFanciulli, Giuseppe, Roberta Modica, Anna La Salvia, Federica Campolo, Tullio Florio, Nevena Mikovic, Alice Plebani, Valentina Di Vito, Annamaria Colao, and Antongiulio Faggiano on behalf of NIKE Group . 2022. "Immunotherapy of Neuroendocrine Neoplasms: Any Role for the Chimeric Antigen Receptor T Cells?" Cancers 14, no. 16: 3991. https://doi.org/10.3390/cancers14163991
APA StyleFanciulli, G., Modica, R., La Salvia, A., Campolo, F., Florio, T., Mikovic, N., Plebani, A., Di Vito, V., Colao, A., & Faggiano, A., on behalf of NIKE Group . (2022). Immunotherapy of Neuroendocrine Neoplasms: Any Role for the Chimeric Antigen Receptor T Cells? Cancers, 14(16), 3991. https://doi.org/10.3390/cancers14163991










