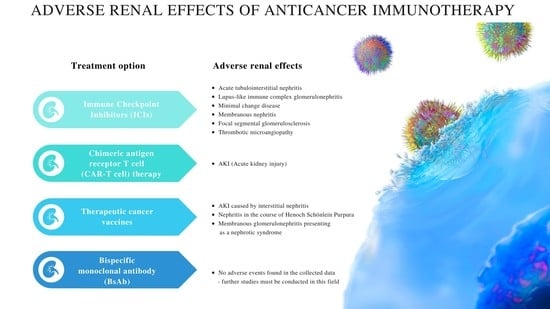
Adverse Renal Effects of Anticancer Immunotherapy: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Immune Checkpoint Inhibitors (ICIs)
3.1. Mechanism of Action
3.2. Possible Manifestations and Pathophysiology of Renal irAEs
3.3. Risk Factors
3.4. Occurrence and Specific Nephrotoxicities
3.5. Management and Outcomes
4. Tumor-Targeting Monoclonal Antibodies (TT-mAbs)
4.1. Mechanism of Action
4.2. Renal Adverse Effects
5. Chimeric Antigen Receptor T Cell (CAR-T Cell) Therapy
5.1. Mechanism of Action
5.2. Renal Adverse Effects and Their Pathomechanisms
5.3. Occurrence and Outcomes
5.4. Management
6. Therapeutic Cancer Vaccines
6.1. Mechanism of Action
6.2. Renal Adverse Effects
7. Bispecific Monoclonal Antibody (BsAb)
7.1. Mechanism of Action
7.2. Renal Adverse Effects
8. Cytokine Therapy
8.1. Mechanism of Action
8.2. Renal Adverse Effects
9. Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dyba, T.; Randi, G.; Martos, C.; Giusti, F.; Calvalho, R.; Neamtiu, L.; Nicholson, N.; Flego, M.; Dimitrova, N.; Bettio, M. 1501O Long-term estimates of cancer incidence and mortality for the EU and EFTA countries according to different demographic scenarios. Ann. Oncol. 2021, 32, S1102. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Schmielau, J.; Rick, O.; Reuss-Borst, M.; Kalusche-Bontemps, E.-M.; Steimann, M. Rehabilitation of Cancer Survivors with Long-Term Toxicities. Oncol. Res. Treat. 2017, 40, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zindl, C.L.; Chaplin, D.D. Tumor Immune Evasion. Science 2010, 328, 697–698. [Google Scholar] [CrossRef]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of Lymphoidlike Stroma and Immune Escape by Tumors That Express the Chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Choudhury, A.; Lladser, A.; Kiessling, R.; Johansson, C.C. Regulatory T Cells in Cancer. Adv. Cancer Res. 2010, 107, 57–117. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B. The Treatment of Malignat Tumors by Repeated Inoculations of Erysipelas. Am. J. Med Sci. 1893, 105, 487–510. [Google Scholar] [CrossRef]
- Wiemann, B.; Starnes, C.O. Coley’s toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol. Ther. 1994, 64, 529–564. [Google Scholar] [CrossRef]
- Sathyanarayanan, V.; Neelapu, S.S. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol. Oncol. 2015, 9, 2043–2053. [Google Scholar] [CrossRef]
- Karmakar, S.; Dhar, R.; Seethy, A.; Singh, S.; Pethusamy, K.; Srivastava, T.; Talukdar, J.; Rath, G.K. Cancer immunotherapy: Recent advances and challenges. J. Cancer Res. Ther. 2021, 17, 834. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Kwon, E.D.; Hurwitz, A.A.; Foster, B.A.; Madias, C.; Feldhaus, A.L.; Greenberg, N.M.; Burg, M.B.; Allison, J.P. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 8099–8103. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Han, D.; Xu, Z.; Zhuang, Y.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Hematological Malignancies. J. Cancer 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Salter, A.; Pont, M.J.; Riddell, S.R. Chimeric antigen receptor–modified T cells: CD19 and the road beyond. Blood 2018, 131, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.D. Adverse Events of Oncologic Immunotherapy and Their Management. Asia-Pacific J. Oncol. Nurs. 2019, 6, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Teuma, C.; Novel-Catin, E.; Maillet, D.; Souquet, P.; Dalle, S.; Koppe, L.; Fouque, D. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur. J. Cancer 2021, 147, 29–39. [Google Scholar] [CrossRef]
- El Rassy, E.; Kourie, H.R.; Rizkallah, J.; El Karak, F.; Hanna, C.; Chelala, D.N.; Ghosn, M. Immune checkpoint inhibitors renal side effects and management. Immunotherapy 2016, 8, 1417–1425. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Wang, S.; He, Z.; Wang, X.; Li, H.; Liu, X.-S. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. eLife 2019, 8, e49020. [Google Scholar] [CrossRef]
- Zagorulya, M.; Duong, E.; Spranger, S. Impact of anatomic site on antigen-presenting cells in cancer. J. Immunother. Cancer 2020, 8, e001204. [Google Scholar] [CrossRef] [PubMed]
- Diesendruck, Y.; Benhar, I. Novel immune check point inhibiting antibodies in cancer therapy—Opportunities and challenges. Drug Resist. Updat. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Lanzavecchia, A.; Sallusto, F. The instructive role of dendritic cells on T cell responses: Lineages, plasticity and kinetics. Curr. Opin. Immunol. 2001, 13, 291–298. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Horton, B.L.; Fessenden, T.B.; Spranger, S. Tissue Site and the Cancer Immunity Cycle. Trends Cancer 2019, 5, 593–603. [Google Scholar] [CrossRef]
- Cai, X.; Zhan, H.; Ye, Y.; Yang, J.; Zhang, M.; Li, J.; Zhuang, Y. Current Progress and Future Perspectives of Immune Checkpoint in Cancer and Infectious Diseases. Front. Genet. 2021, 12, 785153. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Collins, A.V.; Brodie, D.W.; Gilbert, R.J.C.; Iaboni, A.; Manso-Sancho, R.; Walse, B.; Stuart, D.I.; van der Merwe, P.A.; Davis, S.J. The Interaction Properties of Costimulatory Molecules Revisited. Immunity 2002, 17, 201–210. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-Mediated Inhibition in Regulation of T Cell Responses: Mechanisms and Manipulation in Tumor Immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Esfahani, K.; Meti, N.; Miller, W.H.; Hudson, M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. Can. Med Assoc. J. 2019, 191, E40–E46. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Bodor, J.N.; Borghaei, H. Identifying and managing the adverse effects of immune checkpoint blockade. J. Thorac. Dis. 2018, 10, S480–S489. [Google Scholar] [CrossRef]
- Tang, S.-Q.; Tang, L.-L.; Mao, Y.-P.; Li, W.-F.; Chen, L.; Zhang, Y.; Guo, Y.; Liu, Q.; Sun, Y.; Xu, C.; et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8436 Patients. Cancer Res. Treat. 2021, 53, 339–354. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) V5.0; 2017. Available online: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 25 May 2022).
- Xu, C.; Chen, Y.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Wanchoo, R.; Karam, S.; Uppal, N.N.; Barta, V.S.; Deray, G.; Devoe, C.; Launay-Vacher, V.; Jhaveri, K.D.; on behalf of Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am. J. Nephrol. 2017, 45, 160–169. [Google Scholar] [CrossRef]
- Mamlouk, O.; Abudayyeh, A. Cancer immunotherapy and its renal effects. J. Onco-Nephrol. 2019, 3, 151–159. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Eijgelsheim, M.; Sprangers, B. Kidney Biopsy Should Be Performed to Document the Cause of Immune Checkpoint Inhibitor–Associated Acute Kidney Injury: PRO. Kidney360 2020, 1, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Mateus, C.; Boutros, C.; Robert, C.; Rouvier, P.; Amoura, Z.; Mathian, A. Renal effects of immune checkpoint inhibitors. Nephrol. Dial. Transplant. 2016, 32, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Lin, N.; Wang, S.; Wang, Y. Minimal change disease associated with anti-PD1 immunotherapy: A case report. BMC Nephrol. 2018, 19, 156. [Google Scholar] [CrossRef]
- Bickel, A.; Koneth, I.; Enzler-Tschudy, A.; Neuweiler, J.; Flatz, L.; Früh, M. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer 2016, 16, 656. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yamamoto, S.; Hara, S.; Okawara, M.; Teramoto, K.; Ikeda, N.; Kusunoki, Y.; Takeji, M. Nivolumab-induced membranous nephropathy in a patient with stage IV lung adenocarcinoma. CEN Case Rep. 2022, 11, 171–176. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeon, H.; Kim, S.; Lee, W.; Kim, H.J.; Rhee, H.; Song, S.H.; Seong, E.Y. Pembrolizumab-induced focal segmental glomerulosclerosis. Medicine 2021, 100, e27546. [Google Scholar] [CrossRef]
- Hayata, M.; Shimanuki, M.; Ko, T.; Date, R.; Hamaguchi, A.; Tominaga, A.; Miura, R.; Mizumoto, T.; Mukoyama, M. Pembrolizumab-associated thrombotic microangiopathy in a patient with urothelial cancer: A case report and literature review. Ren. Replace. Ther. 2020, 6, 1–6. [Google Scholar] [CrossRef]
- Sury, K.; Perazella, M.A.; Shirali, A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol. 2018, 14, 571–588. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Gao, W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin. Immunol. 2005, 115, 184–191. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Starke, A.; Wüthrich, R.P. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+cytotoxic T cells. Nephrol. Dial. Transplant. 2007, 22, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Borges, T.J.; Yamashita, M.; Riella, L.V. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin. Kidney J. 2016, 9, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Fadel, F.; El Karoui, K.; Knebelmann, B. Anti-CTLA4 Antibody–Induced Lupus Nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Draibe, J.B.; García-Carro, C.; Martinez-Valenzuela, L.; Agraz, I.; Fulladosa, X.; Bolufer, M.; Tango, A.; Torras, J.; Soler, M.J. Acute tubulointerstitial nephritis induced by checkpoint inhibitors versus classical acute tubulointerstitial nephritis: Are they the same disease? Clin. Kidney J. 2020, 14, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Muñoz, A.; Amir, E.; Ng, P.; Avila-Casado, C.; Ragobar, C.; Chan, C.; Kim, J.; Wald, R.; Kitchlu, A. Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J. Immunother. Cancer 2020, 8, e000467. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef]
- Gupta, S.; Short, S.A.P.; E Sise, M.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, S.M.; Abudayyeh, A.; Anand, S.; et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Nast, C.C. Medication-Induced Interstitial Nephritis in the 21st Century. Adv. Chronic Kidney Dis. 2017, 24, 72–79. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Maddukuri, G.; Xie, Y. Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe. Am. J. Kidney Dis. 2020, 75, 497–507. [Google Scholar] [CrossRef]
- Seethapathy, H.; Zhao, S.; Chute, D.F.; Zubiri, L.; Oppong, Y.; Strohbehn, I.; Cortazar, F.B.; Leaf, D.E.; Mooradian, M.J.; Villani, A.-C.; et al. The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin. J. Am. Soc. Nephrol. 2019, 14, 1692–1700. [Google Scholar] [CrossRef]
- Kato, K.; Mizuno, T.; Koseki, T.; Ito, Y.; Hatano, M.; Takahashi, K.; Yamada, S.; Tsuboi, N. Concomitant Proton Pump Inhibitors and Immune Checkpoint Inhibitors Increase Nephritis Frequency. Vivo 2021, 35, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Gueutin, V.; Gharbi, C.; Mateus, C.; Robert, C.; Routier, E.; Thomas, M.; Baumelou, A.; Rouvier, P. Kidney injuries related to ipilimumab. Investig. New Drugs 2014, 32, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Thajudeen, B.; Madhrira, M.; Bracamonte, E.; Cranmer, L.D. Ipilimumab Granulomatous Interstitial Nephritis. Am. J. Ther. 2015, 22, e84–e87. [Google Scholar] [CrossRef]
- Izzedine, H.; Mathian, A.; Champiat, S.; Picard, C.; Mateus, C.; Routier, E.; Varga, A.; Malka, D.; Leary, A.; Michels, J.; et al. Renal toxicities associated with pembrolizumab. Clin. Kidney J. 2018, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ishibuchi, K.; Iwakura, T.; Kaneko, M.; Fukasawa, H.; Furuya, R. Pembrolizumab-associated nephrotic syndrome recovered from transient hemodialysis in a patient with lung cancer. CEN Case Rep. 2020, 9, 215–219. [Google Scholar] [CrossRef]
- Stein, C.; Burtey, S.; Mancini, J.; Pelletier, M.; Sallée, M.; Brunet, P.; Berbis, P.; Grob, J.J.; Honoré, S.; Gaudy, C.; et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: A real-life study in a single-centre cohort. Nephrol. Dial. Transplant. 2020, 36, 1664–1674. [Google Scholar] [CrossRef]
- Uchida, N.; Tsuji, S.; Fujita, K.; Koizumi, M.; Moriyoshi, K.; Mio, T. Nivolumab-induced severe acute kidney injury with a long latent phase in a patient with non-small-cell lung cancer: A case report. Clin. Case Rep. 2018, 6, 2185–2188. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Vaios, V.; Leontaridou, E.; Karayannopoulou, G.; Koletsa, T.; Sioulis, A.; Balaskas, E.V.; Zebekakis, P.E. Acute Interstitial Nephritis in a Patient with Non-Small Cell Lung Cancer under Immunotherapy with Nivolumab. Case Rep. Nephrol. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Xipell, M.; Victoria, I.; Hoffmann, V.; Villarreal, J.; Garcia-Herrera, A.; Reig, O.; Rodas, L.; Blasco, M.; Poch, E.; Mellado, B.; et al. Acute tubulointerstitial nephritis associated with atezolizumab, an anti-programmed death-ligand 1 (pd-l1) antibody therapy. OncoImmunology 2018, 7, e1445952. [Google Scholar] [CrossRef]
- Toda, M.G.; Fujii, K.; Kato, A.; Yoshifuji, A.; Komatsu, M.; Amino, Y.; Kitazono, S.; Hashiguchi, A.; Ryuzaki, M. Minimal Change Disease Associated with Durvalumab. Kidney Int. Rep. 2021, 6, 2733–2734. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Rashidi, A.; Herlitz, L.; Tariq, H. Renal tubular acidosis and acute kidney injury secondary to cemiplimab. J. Onco-Nephrol. 2021, 5, 136–139. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Exp. Nephrol. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Lin, J.S.; Mamlouk, O.; Selamet, U.; Tchakarov, A.; Glass, W.F.; Sheth, R.A.; Layman, R.M.; Dadu, R.; Abdel-Wahab, N.; Abdelrahim, M.; et al. Infliximab for the treatment of patients with checkpoint inhibitor associated acute tubular interstitial nephritis. OncoImmunology 2021, 10, 1877415. [Google Scholar] [CrossRef]
- Jessel, S.; Austin, M.; Kluger, H.M. Mycophenolate as Primary Treatment for Immune Checkpoint Inhibitor Induced Acute Kidney Injury in a Patient with Concurrent Immunotherapy-Associated Diabetes: A Case Report. Clin. Oncol. Case Rep. 2021, 4, 156. [Google Scholar]
- Omori, G.; Takada, K.; Murase, K.; Hayasaka, N.; Nakamura, H.; Iyama, S.; Ohnuma, H.; Miyanishi, K.; Fukuta, F.; Tanaka, T.; et al. Successful mycophenolate mofetil treatment of a patient with severe steroid-refractory hepatitis evoked by nivolumab plus ipilimumab treatment for relapsed bladder cancer. Clin. Case Rep. 2020, 9, 654–659. [Google Scholar] [CrossRef]
- Baker, M.L.; Yamamoto, Y.; A Perazella, M.; Dizman, N.; Shirali, A.C.; Hafez, N.; Weinstein, J.; Simonov, M.; Testani, J.M.; Kluger, H.M.; et al. Mortality after acute kidney injury and acute interstitial nephritis in patients prescribed immune checkpoint inhibitor therapy. J. Immunother. Cancer 2022, 10, e004421. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.-P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Kaplan-Lefko, P.J.; Graves, J.D.; Zoog, S.J.; Pan, Y.; Wall, J.; Branstetter, D.G.; Moriguchi, J.; Coxon, A.; Huard, J.N.; Xu, R.; et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol. Ther. 2010, 9, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Coulie, P.G.; Van den Eynde, B.J.; Van Der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Hubert, P.; Amigorena, S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. OncoImmunology 2012, 1, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kono, K.; Mimura, K.; Sugai, H.; Akaike, H.; Fujii, H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int. J. Cancer 2006, 120, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2016, 389, 2415–2429. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Boku, N. HER2-positive gastric cancer. Gastric Cancer 2013, 17, 1–12. [Google Scholar] [CrossRef]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.; Normanno, N.; Rachiglio, A.; Maiello, E.; Latiano, T.; et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: A systematic review. Med. Oncol. 2018, 35, 37. [Google Scholar] [CrossRef]
- Martínez-Trufero, J.; Borbalas, A.L.; Bernad, I.P.; Sanz, M.T.; Izquierdo, E.O.; Cirauqui, B.C.; Rubió-Casadevall, J.; Serrahima, M.P.; Ortega, J.P.; Toledano, I.P.; et al. Sequential chemotherapy regimen of induction with panitumumab and paclitaxel followed by radiotherapy and panitumumab in patients with locally advanced head and neck cancer unfit for platinum derivatives. The phase II, PANTERA/TTCC-2010-06 study. Clin. Transl. Oncol. 2021, 23, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Cymbalista, F.; Sharman, J.; Jacobs, I.; Nava-Parada, P.; Mato, A. The Role of Rituximab in Chronic Lymphocytic Leukemia Treatment and the Potential Utility of Biosimilars. Oncologist 2017, 23, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Jamois, C.; Nielsen, T. Anti-CD20 treatment for B-cell malignancies: Current status and future directions. Expert Opin. Biol. Ther. 2020, 21, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weyden, C.; Dickinson, M.; Whisstock, J.; Prince, H.M. Brentuximab vedotin in T-cell lymphoma. Expert Rev. Hematol. 2018, 12, 5–19. [Google Scholar] [CrossRef]
- Ravandi, F.; O’Brien, S. Alemtuzumab in CLL and Other Lymphoid Neoplasms. Cancer Investig. 2006, 24, 718–725. [Google Scholar] [CrossRef]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. J. Cereb. Blood Flow Metab. 2016, 174, 3727–3748. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Hakroush, S.; Wulf, S.; Gallwas, J.; Tampe, B. Case Report: Collapsing Focal Segmental Glomerulosclerosis After Initiation of Ado-Trastuzumab Emtansine Therapy. Front. Oncol. 2021, 11, 5007. [Google Scholar] [CrossRef]
- Russo, G.; Cioffi, G.; Di Lenarda, A.; Tuccia, F.; Bovelli, D.; Di Tano, G.; Alunni, G.; Gori, S.; Faggiano, P.; Tarantini, L. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern. Emerg. Med. 2012, 7, 439–446. [Google Scholar] [CrossRef]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: An evidence-based review of its safety, efficacy, and place in therapy. Core EÉvid. 2019, 14, 51–70. [Google Scholar] [CrossRef]
- Zhu, C.; Ling, W.; Zhang, J.; Gao, H.; Shen, K.; Ma, X. Safety and efficacy evaluation of pertuzumab in patients with solid tumors. Medicine 2017, 96, e6870. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mirza, M.M.; Ganti, A.K.; Tendulkar, K. Renal Toxicities of Targeted Therapies. Target. Oncol. 2015, 10, 487–499. [Google Scholar] [CrossRef]
- Cosmai, L.; Gallieni, M.; Porta, C. Renal toxicity of anticancer agents targeting HER2 and EGFR. J. Nephrol. 2015, 28, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Barni, S. Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: Systematic review and pooled analysis of randomized studies. Expert Opin. Drug Saf. 2011, 11, S9–S19. [Google Scholar] [CrossRef]
- Groenestege, W.M.T.; Thébault, S.; van der Wijst, J.; Berg, D.V.D.; Janssen, R.; Tejpar, S.; Heuvel, L.P.V.D.; Van Cutsem, E.; Hoenderop, J.G.; Knoers, N.V.; et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Investig. 2007, 117, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, L.; Liao, C.; Tan, A.; Gao, F. Meta-analysis of incidence and risk of hypokalemia with cetuximab-based therapy for advanced cancer. Cancer Chemother. Pharmacol. 2009, 66, 37–42. [Google Scholar] [CrossRef]
- Giusti, R.M.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA Review of a Panitumumab (Vectibix™) Clinical Trial for First-Line Treatment of Metastatic Colorectal Cancer. Oncologist 2009, 14, 284–290. [Google Scholar] [CrossRef]
- Boku, N.; Sugihara, K.; Kitagawa, Y.; Hatake, K.; Gemma, A.; Yamazaki, N.; Muro, K.; Hamaguchi, T.; Yoshino, T.; Yana, I.; et al. Panitumumab in Japanese patients with unresectable colorectal cancer: A post-marketing surveillance study of 3085 patients. Jpn. J. Clin. Oncol. 2014, 44, 214–223. [Google Scholar] [CrossRef]
- Kamo, H.; Shinozaki, E.; Sugase, T.; Mizunuma, N.; Taniguchi, S.; Gotoh, T.; Chin, K.; Tanaka, T.; Koga, K.; Yamaguchi, K. Leukocytoclastic vasculitis with purpura and renal failure induced by the anti-epidermal growth factor receptor antibody panitumumab: A case report. J. Med. Case Rep. 2019, 13, 13. [Google Scholar] [CrossRef]
- Manthri, S.; Bandaru, S.; Chang, A.; Hudali, T. Cetuximab-Associated Crescentic Diffuse Proliferative Glomerulonephritis. Case Rep. Nephrol. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Sasaki, K.; Anderson, E.; Shankland, S.J.; Nicosia, R.F. Diffuse Proliferative Glomerulonephritis Associated With Cetuximab, an Epidermal Growth Factor Receptor Inhibitor. Am. J. Kidney Dis. 2013, 61, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Takahashi, M.; Murata, M.; Kikuchi, Y.; Seta, K.; Yahata, K. Thrombotic microangiopathy associated with cetuximab, an epidermal growth factor receptor inhibitor. Clin. Nephrol. 2017, 87, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.L.; Sehn, L.H. A tale of two antibodies: Obinutuzumabversusrituximab. Br. J. Haematol. 2018, 182, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Korycka-Wołowiec, A.; Wołowiec, D.; Robak, T. Ofatumumab for treating chronic lymphocytic leukemia: A safety profile. Expert Opin. Drug Saf. 2015, 14, 1945–1959. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Gu, Y.; Xia, J.; Kong, X.; Qian, Q.; Hong, Y. Safety and efficacy of Ofatumumab in chronic lymphocytic leukemia: A systematic review and meta-analysis. Hematology 2017, 22, 578–584. [Google Scholar] [CrossRef]
- Howard, S.C.; Trifilio, S.; Gregory, T.K.; Baxter, N.; McBride, A. Tumor lysis syndrome in the era of novel and targeted agents in patients with hematologic malignancies: A systematic review. Ann. Hematol. 2016, 95, 563–573. [Google Scholar] [CrossRef]
- Kasi, P.M.; A Tawbi, H.; Oddis, C.V.; Kulkarni, H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. Crit. Care 2012, 16, 231. [Google Scholar] [CrossRef]
- Caldito, N.G.; Shirani, A.; Salter, A.; Stuve, O. Adverse event profile differences between rituximab and ocrelizumab: Findings from the FDA Adverse Event Reporting Database. Mult. Scler. J. 2020, 27, 1066–1076. [Google Scholar] [CrossRef]
- Scott, L.J. Brentuximab Vedotin: A Review in CD30-Positive Hodgkin Lymphoma. Drugs 2017, 77, 435–445. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; A Johnson, N.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef]
- Robak, T. Alemtuzumab for B-cell chronic lymphocytic leukemia. Expert Rev. Anticancer Ther. 2008, 8, 1033–1051. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Skotnicki, A.B.; Robak, T.; Jaksic, B.; Dmoszynska, A.; Wu, J.; Sirard, C.; Mayer, J. Alemtuzumab Compared With Chlorambucil As First-Line Therapy for Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2007, 25, 5616–5623. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.; A Winston, J.; Wynn, D.; Habek, M.; Hartung, H.-P.; Havrdová, E.K.; Markowitz, G.S.; Margolin, D.H.; E Rodriguez, C.; Baker, D.P.; et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult. Scler. J. 2019, 25, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Thyss, A.; Gari-Toussaint, M. Prostatic and renal aspergillosis due to Aspergillus fumigatus in a patient receiving alemtuzumab for chronic lymphocytic leukaemia. J. Mycol. Médicale 2013, 23, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Perica, K.; Varela, J.C.; Oelke, M.; Schneck, J.P. Adoptive T Cell Immunotherapy for Cancer. Rambam Maimonides Med J. 2015, 6, e0004. [Google Scholar] [CrossRef]
- Mohanty, R.; Chowdhury, C.R.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Liu, Y.; Han, W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. JNCI J. Natl. Cancer Inst. 2016, 108, djv439. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef]
- Wäsch, R.; Munder, M.; Marks, R. Teaming up for CAR-T cell therapy. Haematologica 2019, 104, 2335–2336. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ping, J.; Huang, Z.; Zhang, X.; Zhou, J.; Wang, G.; Liu, S.; Ma, J. CAR-T Cell Therapy in Cancer: Tribulations and Road Ahead. J. Immunol. Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.Y.; Williams, G.R.; Paluri, R.K. CAR T Cell Therapy in Pancreaticobiliary Cancers: A Focused Review of Clinical Data. J. Gastrointest. Cancer 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; Von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Lee, D.W.; A Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.C.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Zhang, P.; Zhang, Y.; Chen, Y.; Fan, X.; Cao, X.; Liu, J.; Yang, Y.; Wang, B.; et al. Management of cytokine release syndrome related to CAR-T cell therapy. Front. Med. 2019, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-J.; Tang, Y.-M. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett. 2014, 343, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Turtle, C.J. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr. Res. Transl. Med. 2018, 66, 50–52. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, J. Cytokine release syndrome: Grading, modeling, and new therapy. J. Hematol. Oncol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Frey, N.V.; Porter, D.L. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology 2016, 2016, 567–572. [Google Scholar] [CrossRef]
- Jhaveri, K.D.; Rosner, M.H. Chimeric Antigen Receptor T Cell Therapy and the Kidney. Clin. J. Am. Soc. Nephrol. 2018, 13, 796–798. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, M.; Cui, L.; Jiang, J. Chimeric antigen receptor T cell therapy and nephrotoxicity: From diagnosis to treatment strategies. Int. Immunopharmacol. 2020, 89, 107072. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.; Grupp, S.A. Managing Cytokine Release Syndrome Associated with Novel T Cell-Engaging Therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Siddall, E.; Khatri, M.; Radhakrishnan, J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017, 92, 37–46. [Google Scholar] [CrossRef]
- Shalabi, H.; Sachdev, V.; Kulshreshtha, A.; Cohen, J.W.; Yates, B.; Rosing, D.R.; Sidenko, S.; Delbrook, C.; Mackall, C.; Wiley, B.; et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J. Immunother. Cancer 2020, 8, e001159. [Google Scholar] [CrossRef]
- Kellum, J.A. Persistent Acute Kidney Injury*. Crit. Care Med. 2015, 43, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. Inflammatory cytokines in acute renal failure. Kidney Int. 2004, 66, S56–S61. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Yu, X.; Lan, H.-Y. Smad3 Signatures in Renal Inflammation and Fibrosis. Int. J. Biol. Sci. 2022, 18, 2795–2806. [Google Scholar] [CrossRef]
- Cairo, M.S.; Bishop, M. Tumour lysis syndrome: New therapeutic strategies and classification. Br. J. Haematol. 2004, 127, 3–11. [Google Scholar] [CrossRef]
- Abu-Alfa, A.K.; Younes, A. Tumor Lysis Syndrome and Acute Kidney Injury: Evaluation, Prevention, and Management. Am. J. Kidney Dis. 2010, 55, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.R.; Keenan, C.; Alfaro, G.M.; Cheng, C.; Zhou, Y.; Sharma, A.; Hurley, C.; Nichols, K.E.; Gottschalk, S.; Triplett, B.M.; et al. Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br. J. Haematol. 2021, 194, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, D.; Hogan, J.; D’Agati, V.D. Hemophagocytic Syndrome With Histiocytic Glomerulopathy and Intraglomerular Hemophagocytosis. Am. J. Kidney Dis. 2016, 67, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Malaga-Dieguez, L.; Ming, W.; Trachtman, H. Direct Reversible Kidney Injury in Familial Hemophagocytic Lymphohistiocytosis Type 3. J. Am. Soc. Nephrol. 2015, 26, 1777–1780. [Google Scholar] [CrossRef]
- Gupta, S.; Seethapathy, H.; Strohbehn, I.A.; Frigault, M.J.; O’Donnell, E.K.; Jacobson, C.A.; Motwani, S.S.; Parikh, S.M.; Curhan, G.C.; Reynolds, K.L.; et al. Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma. Am. J. Kidney Dis. 2020, 76, 63–71. [Google Scholar] [CrossRef]
- Gutgarts, V.; Jain, T.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Pennisi, M.; Jaimes, E.A.; Perales, M.-A.; Sathick, J. Acute Kidney Injury after CAR-T Cell Therapy: Low Incidence and Rapid Recovery. Biol. Blood Marrow Transplant. 2020, 26, 1071–1076. [Google Scholar] [CrossRef]
- Lee, M.D.; Strohbehn, I.A.; Seethapathy, H.S.; Rusibamayila, N.; Casey, K.S.; Gupta, S.; Leaf, D.E.; Frigault, M.J.; Sise, M.E. Acute Kidney Injury After the CAR-T Therapy Tisagenlecleucel. Am. J. Kidney Dis. 2021, 77, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, S.R.; Cheungpasitporn, W.; Thongprayoon, C.; Petnak, T.; Lin, Y.; Kovvuru, K.; Manohar, S.; Kashani, K.; Herrmann, S.M. Systematic Review of Risk factors and Incidence of Acute Kidney Injury among Patients Treated with CAR-T Cell Therapies. Kidney Int. Rep. 2021, 6, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Druml, W.; Forni, L.G.; Groeneveld, A.B.J.; Honore, P.M.; Hoste, E.; Ostermann, M.; Straaten, H.M.O.-V.; Schetz, M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017. Intensiv. Care Med. 2017, 43, 730–749. [Google Scholar] [CrossRef]
- Burstein, D.S.; Maude, S.; Grupp, S.; Griffis, H.; Rossano, J.; Lin, K.; Burstein, D.S.; Maude, S.; Grupp, S.; Griffis, H.; et al. Cardiac Profile of Chimeric Antigen Receptor T Cell Therapy in Children: A Single-Institution Experience. Biol. Blood Marrow Transplant. 2018, 24, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef]
- Kotch, C.; Barrett, D.; Teachey, D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev. Clin. Immunol. 2019, 15, 813–822. [Google Scholar] [CrossRef]
- Bergsten, E.; Horne, A.; Aricó, M.; Astigarraga, I.; Egeler, R.M.; Filipovich, A.H.; Ishii, E.; Janka, G.; Ladisch, S.; Lehmberg, K.; et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: Long-term results of the cooperative HLH-2004 study. Blood 2017, 130, 2728–2738. [Google Scholar] [CrossRef]
- Belay, Y.; Yirdaw, K.; Enawgaw, B. Tumor Lysis Syndrome in Patients with Hematological Malignancies. J. Oncol. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Athanasiou, A.; Bowden, S.; Paraskevaidi, M.; Fotopoulou, C.; Martin-Hirsch, P.; Paraskevaidis, E.; Kyrgiou, M. HPV vaccination and cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 109–124. [Google Scholar] [CrossRef]
- Chang, M.H. Hepatitis B virus and cancer prevention. Recent results in cancer research. Fortschritte der Krebsforschung. Prog. Rech. Cancer 2011, 188, 75–84. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, P.J.; Bilusic, M. Cancer Vaccines. Hematol. Clin. North Am. 2019, 33, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side Effects of Bacillus Calmette-Guérin (BCG) in the Treatment of Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Bladder: Results of the EORTC Genito-Urinary Cancers Group Randomised Phase 3 Study Comparing One-third Dose with Full Dose and 1 Year with 3 Years of Maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Peyriere, H.; Klouche, K.; Béraud, J.-J.; Blayac, J.-P.; Hillaire-Buys, D. Fatal Systemic Reaction after Multiple Doses of Intravesical Bacillus Calmette-Guérin for Polyposis. Ann. Pharmacother. 2000, 34, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, W.; Decaestecker, K. Managing the adverse events of intravesical bacillus Calmette–Guérin therapy. Res. Rep. Urol. 2015, 7, 157–163. [Google Scholar] [CrossRef]
- Mohammed, A.; Arastu, Z. Emerging concepts and spectrum of renal injury following Intravesical BCG for non-muscle invasive bladder cancer. BMC Urol. 2017, 17, 114. [Google Scholar] [CrossRef]
- Modesto, A.; Marty, L.; Suc, J.-M.; Kleinknecht, D.; De Frémont, J.-F.; Marsepoil, T.; Veyssier, P. Renal Complications of Intravesical Bacillus Calmette-Guérin Therapy. Am. J. Nephrol. 1991, 11, 501–504. [Google Scholar] [CrossRef]
- Fry, A.; Saleemi, A.; Griffiths, M.; Farrington, K. Acute renal failure following intravesical bacille Calmette-Guerin chemotherapy for superficial carcinoma of the bladder. Nephrol. Dial. Transplant. 2005, 20, 849–850. [Google Scholar] [CrossRef]
- Tsukada, H.; Miyakawa, H. Henoch Schönlein Purpura Nephritis Associated with Intravesical Bacillus Calmette-Guerin (BCG) Therapy. Intern. Med. 2017, 56, 541–544. [Google Scholar] [CrossRef]
- Singh, N.P.; Prakash, A.; Kubba, S.; Ganguli, A.; Agarwal, S.K.; Dinda, A.K.; Aggarwal, P.N. Nephrotic Syndrome as a Complication of Intravesical BCG Treatment of Transitional Cell Carcinoma of Urinary Bladder. Ren. Fail. 2007, 29, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Srinivasa, Y.; Paul, F. Asymptomatic renal BCG granulomatosis: An unusual complication of intravesical BCG therapy for carcinoma urinary bladder. Indian J. Urol. 2015, 31, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaoud, T.; Brimo, F.; Aprikian, A.G.; Andonian, S. BCG-Related Renal Granulomas Managed Conservatively. Can. Urol. Assoc. J. 2015, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Dores, G.M.; Bryant-Genevier, M.; Perez-Vilar, S. Adverse Events Associated With the Use of Sipuleucel-T Reported to the US Food and Drug Administration’s Adverse Event Reporting System, 2010–2017. JAMA Netw. Open 2019, 2, e199249. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2015, 76, 147–154. [Google Scholar] [CrossRef]
- Harrington, K.J.; Andtbacka, R.H.; Collichio, F.; Downey, G.; Chen, L.; Szabo, Z.; Kaufman, H.L. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: Subanalysis of the Phase III OPTiM trial. OncoTargets Ther. 2016, 9, 7081–7093. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 1555. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Huang, S.; van Duijnhoven, S.M.J.; Sijts, A.J.A.M.; van Elsas, A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J. Cancer Res. Clin. Oncol. 2020, 146, 3111–3122. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Suurs, F.V.; Hooge, M.N.L.-D.; de Vries, E.G.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Hofmeister, R.; Kufer, P.; Schlereth, B.; Baeuerle, P.A. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 2005, 10, 1237–1244. [Google Scholar] [CrossRef]
- Wang, S.; Chen, K.; Lei, Q.; Ma, P.; Yuan, A.Q.; Zhao, Y.; Jiang, Y.; Fang, H.; Xing, S.; Fang, Y.; et al. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol. Med. 2021, 13, e14291. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Ko, C.-W.; Deisseroth, A.; Yancey, C.L.; Candau-Chacon, R.; Chiu, H.-J.; Gehrke, B.J.; Gomez-Broughton, C.; Kane, R.C.; Kirshner, S.; et al. FDA Approval: Blinatumomab. Clin. Cancer Res. 2015, 21, 4035–4039. [Google Scholar] [CrossRef]
- Syed, Y.Y. Amivantamab: First Approval. Drugs 2021, 81, 1349–1353. [Google Scholar] [CrossRef]
- European Commission Approves Roche’s First-in-Class Bispecific Antibody Lunsumio for People with Relapsed or Refractory Follicular Lymphoma; Roche Media&Investor Relase. Available online: https://assets.cwp.roche.com/imported/01_08062022_MR_Lunsumio_En.pdf (accessed on 21 June 2022).
- EMA. Lunsumio: Pending EC Decision–European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/lunsumio (accessed on 21 June 2022).
- Pulte, E.D.; Vallejo, J.; Przepiorka, D.; Nie, L.; Farrell, A.T.; Goldberg, K.B.; McKee, A.E.; Pazdur, R. FDA Supplemental Approval: Blinatumomab for Treatment of Relapsed and Refractory Precursor B-Cell Acute Lymphoblastic Leukemia. Oncologist 2018, 23, 1366–1371. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; A Larson, R.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of postreinduction therapy consolidation with blinatumomab vs. chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.-S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients With Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Xia, Y.; Protzer, U. Control of Hepatitis B Virus by Cytokines. Viruses 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Au, J.S.; Pockros, P.J. Novel Therapeutic Approaches for Hepatitis C. Clin. Pharmacol. Ther. 2013, 95, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Karadag, O.; Bolek, E.C. Management of Behcet’s syndrome. Rheumatology 2020, 59, iii108–iii117. [Google Scholar] [CrossRef]
- Atallah-Yunes, S.A.; Robertson, M.J. Cytokine Based Immunotherapy for Cancer and Lymphoma: Biology, Challenges and Future Perspectives. Front. Immunol. 2022, 13, 872010. [Google Scholar] [CrossRef]
- Solal-Celigny, P.; Lepage, E.; Brousse, N.; Reyes, F.; Haioun, C.; Leporrier, M.; Peuchmaur, M.; Bosly, A.; Parlier, Y.; Brice, P.; et al. Recombinant Interferon Alfa-2b Combined with a Regimen Containing Doxorubicin in Patients with Advanced Follicular Lymphoma. N. Engl. J. Med. 1993, 329, 1608–1614. [Google Scholar] [CrossRef]
- Golomb, H.M.; Jacobs, A.; Fefer, A.; Ozer, H.; Thompson, J.; Portlock, C.; Ratain, M.; Golde, D.; Vardiman, J.; Burke, J.S. Alpha-2 interferon therapy of hairy-cell leukemia: A multicenter study of 64 patients. J. Clin. Oncol. 1986, 4, 900–905. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Strawderman, M.H.; Ernstoff, M.S.; Smith, T.J.; Borden, E.C.; Blum, R.H. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996, 14, 7–17. [Google Scholar] [CrossRef]
- Groopman, J.E.; Gottlieb, M.S.; Goodman, J.; Mitsuyasu, R.T.; Conant, M.A.; Prince, H.; Fahey, J.L.; Derezin, M.; Weinstein, W.M.; Casavante, C.; et al. Recombinant Alpha-2 Interferon Therapy for Kaposi’s Sarcoma Associated with the Acquired Immunodeficiency Syndrome. Ann. Intern. Med. 1984, 100, 671–676. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Sleijfer, S.; Bannink, M.; Van Gool, A.R.; Kruit, W.H.J.; Stoter, G. Side Effects of Interferon-α Therapy. Pharm. Weekbl. Sci. Ed. 2005, 27, 423–431. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Bender, C.; Agarwala, S.; Tarhini, A.; Shipe-Spotloe, J.; Smelko, B.; Donnelly, S.; Stover, L.; Goh, B.-C.; Lee, S.-C.; et al. Mechanisms and Management of Toxicities Associated With High-Dose Interferon Alfa-2b Therapy. J. Clin. Oncol. 2002, 20, 3703–3718. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.R.; Talpaz, M.; Rios, A.; Kurzrock, R.; Gutterman, J.U. Clinical toxicity of interferons in cancer patients: A review. J. Clin. Oncol. 1986, 4, 234–243. [Google Scholar] [CrossRef]
- Phillips, T.M. Interferon-α induces renal dysfunction and injury. Curr. Opin. Nephrol. Hypertens. 1996, 5, 380–383. [Google Scholar] [CrossRef]
- Selby, P.; Kohn, J.; Raymond, J.; Judson, I.; McElwain, T. Nephrotic syndrome during treatment with interferon. BMJ 1985, 290, 1180. [Google Scholar] [CrossRef]
- Horowitz, R.; Glicklich, D.; Sablay, L.B.; Wiernik, P.H.; Wadler, S. Interferon-induced acute renal failure: A case report and literature review. Med. Oncol. 1995, 12, 55–57. [Google Scholar] [CrossRef]
- Averbuch, S.D.; Austin, H.A.; Sherwin, S.A.; Antonovych, T.; Bunn, P.A.; Longo, D.L. Acute Interstitial Nephritis with the Nephrotic Syndrome Following Recombinant Leukocyte A Interferon Therapy for Mycosis Fungoides. N. Engl. J. Med. 1984, 310, 32–35. [Google Scholar] [CrossRef]
- Galesic, K.; Bozic, B.; Racic, I.; Scukanec-Spoljar, M. Thrombotic microangiopathy associated with alpha-interferon therapy for chronic myeloid leukaemia (Case Report). Nephrology 2006, 11, 49–52. [Google Scholar] [CrossRef]
- Antony, G.K. Interleukin 2 in Cancer Therapy. Curr. Med. Chem. 2013, 17, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.P.; Puri, R.K. Interleukin-2 toxicity. J. Clin. Oncol. 1991, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.; Faulds, D. Interleukin-2. A Review of Its Pharmacological Properties and Therapeutic Use in Patients with Cancer. Drugs 1993, 46, 446–514. [Google Scholar] [CrossRef] [PubMed]
- Shalmi, C.L.; Dutcher, J.P.; A Feinfeld, D.; Chun, K.J.; Saleemi, K.R.; Freeman, L.M.; I Lynn, R.; Wiernik, P.H. Acute renal dysfunction during interleukin-2 treatment: Suggestion of an intrinsic renal lesion. J. Clin. Oncol. 1990, 8, 1839–1846. [Google Scholar] [CrossRef]
- Feinfeld, D.A.; D’Agati, V.; Dutcher, J.P.; Werfel, S.B.; Lynn, R.I.; Wiernik, P.H. Interstitial Nephritis in a Patient Receiving Adoptive Immunotherapy with Recombinant Interleukin-2 and Lymphokine-Activated Killer Cells. Am. J. Nephrol. 1991, 11, 489–492. [Google Scholar] [CrossRef]
| Target | Drug |
|---|---|
| TLA-4 | Ipilimumab (Yervoy®) Tremelimumab * |
| PD-1 | Nivolumab (Opdivo®) Pembrolizumab (Keytruda®) Cemiplimab (Libtayo®) Dostarlimab (Jemperli®) |
| PD-L1 | Atezolizumab (Tecentriq®) |
| Avelumab (Bavencio®) Durvalumab (Imfinzi®) |
| Risk Factor | AEs | OR | 95%CI | p-Value |
|---|---|---|---|---|
| The presence of other irAEs | AKI [66] | 3.2 | 1.6–6.0 | <0.001 |
| AKI [68] | 2.07 | 1.53–2.78 | No data | |
| Hypertension | AKI [66] | 4.3 | 1.8–6.1 | <0.001 |
| Cerebrovascular disease | AKI [66] | 9.2 | 2.1–40 | <0.001 |
| ACEI/ARB | AKI [66] | 2.9 | 1.5–5.7 | <0.01 |
| Diuretics | AKI [66] | 4.3 | 1.9–9.8 | <0.001 |
| Corticosteroids | AKI [66] | 1.9 | 1.1–3.6 | <0.05 |
| eGFR < 30 mL/min/1.73 m2 | AKI [67] | 1.99 | 1.43–2.76 | <0.001 |
| PPIs | AKI [67] | 2.38 | 1.57–3.62 | <0.001 |
| AKI [68] | 2.40 | 1.79–3.23 | No data | |
| Anti-CTLA-4 with anti-PD1/anti-PD-L1 combination | AKI [67] | 2.71 | 1.62–4.53 | <0.001 |
| Drug | Target | AEs |
|---|---|---|
| Ipilimumab (Yervoy®) | CTLA-4 | Nephrotic syndrome [64] |
| Interstitial inflammation [73] | ||
| Granulomatous interstitial nephritis [74] | ||
| Acute interstitial nephritis [52] | ||
| Thrombotic microangiopathy [52] | ||
| Tubulointerstitial nephritis [25] | ||
| Pembrolizumab (Keytruda®) | PD-1 | Acute tubular injury [75] |
| Minimal change disease [75] | ||
| Nivolumab (Opdivo®) | PD-1 | Acute tubulointerstitial nephritis [79] |
| IgA nephropathy [79] | ||
| Diffusive tubular injury [79] | ||
| Complex-mediated glomerulonephritis [79] | ||
| Atezolizumab (Tecentriq®) | PD-L1 | Acute interstitial nephritis [80] |
| Durvalumab (Imfinzi®) | PD-L1 | Nephrotic syndrome [81] |
| Minimal change disease [81] | ||
| Cemiplimab | PD-L1 | Acute interstitial nephritis [83] |
| Drug | Target | AEs |
|---|---|---|
| Ado-trastuzumab Emtansine (Kadcyla®) | HER-2 | Nephrotic syndrome [109] |
| Focal segmental glomerulosclerosis [109] | ||
| Acute tubular injury [109] | ||
| Panitumumab (Vectibix®) | EGFR | Hypomagnesemia [113,114] |
| Hypokalemia [118] | ||
| Renal and urinary disorders [119] | ||
| Nephrotic syndrome [120] | ||
| Acute kidney injury [120] | ||
| Leukocytoclastic vasculitis [120] | ||
| Cetuximab (Erbitux®) | EGFR | Hypomagnesemia [113,114] |
| Hypokalemia [117] | ||
| Nephrotic syndrome [120] | ||
| Acute kidney injury [120] | ||
| Crescentic diffuse proliferativeglomerulonephritis [121] | ||
| Diffuse proliferative glomerulonephritis [122] | ||
| Thrombotic microangiopathy [123] | ||
| Rituximab (Mabthera®) | CD20 | Acute kidney injury [127,128] Urinary tract infections [129] |
| Obinutuzumab (Gazyvaro®) | ||
| Ofatumumab (Kesimpta®) | ||
| Brentuximab vedotin (Adcetris®) | CD30 | Acute tubulointerstitial nephritis [131] |
| Alemtuzumab (Lemtrada®) | CD52 | Anti-glomerular basement membrane disease [134] |
| Membranous glomerulopathy [134] | ||
| Kidney aspergillosis [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borówka, M.; Łącki-Zynzeling, S.; Nicze, M.; Kozak, S.; Chudek, J. Adverse Renal Effects of Anticancer Immunotherapy: A Review. Cancers 2022, 14, 4086. https://doi.org/10.3390/cancers14174086
Borówka M, Łącki-Zynzeling S, Nicze M, Kozak S, Chudek J. Adverse Renal Effects of Anticancer Immunotherapy: A Review. Cancers. 2022; 14(17):4086. https://doi.org/10.3390/cancers14174086
Chicago/Turabian StyleBorówka, Maciej, Stanisław Łącki-Zynzeling, Michał Nicze, Sylwia Kozak, and Jerzy Chudek. 2022. "Adverse Renal Effects of Anticancer Immunotherapy: A Review" Cancers 14, no. 17: 4086. https://doi.org/10.3390/cancers14174086
APA StyleBorówka, M., Łącki-Zynzeling, S., Nicze, M., Kozak, S., & Chudek, J. (2022). Adverse Renal Effects of Anticancer Immunotherapy: A Review. Cancers, 14(17), 4086. https://doi.org/10.3390/cancers14174086