Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Definitions of Treatment Outcomes and Adverse Events
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Summary of irAEs
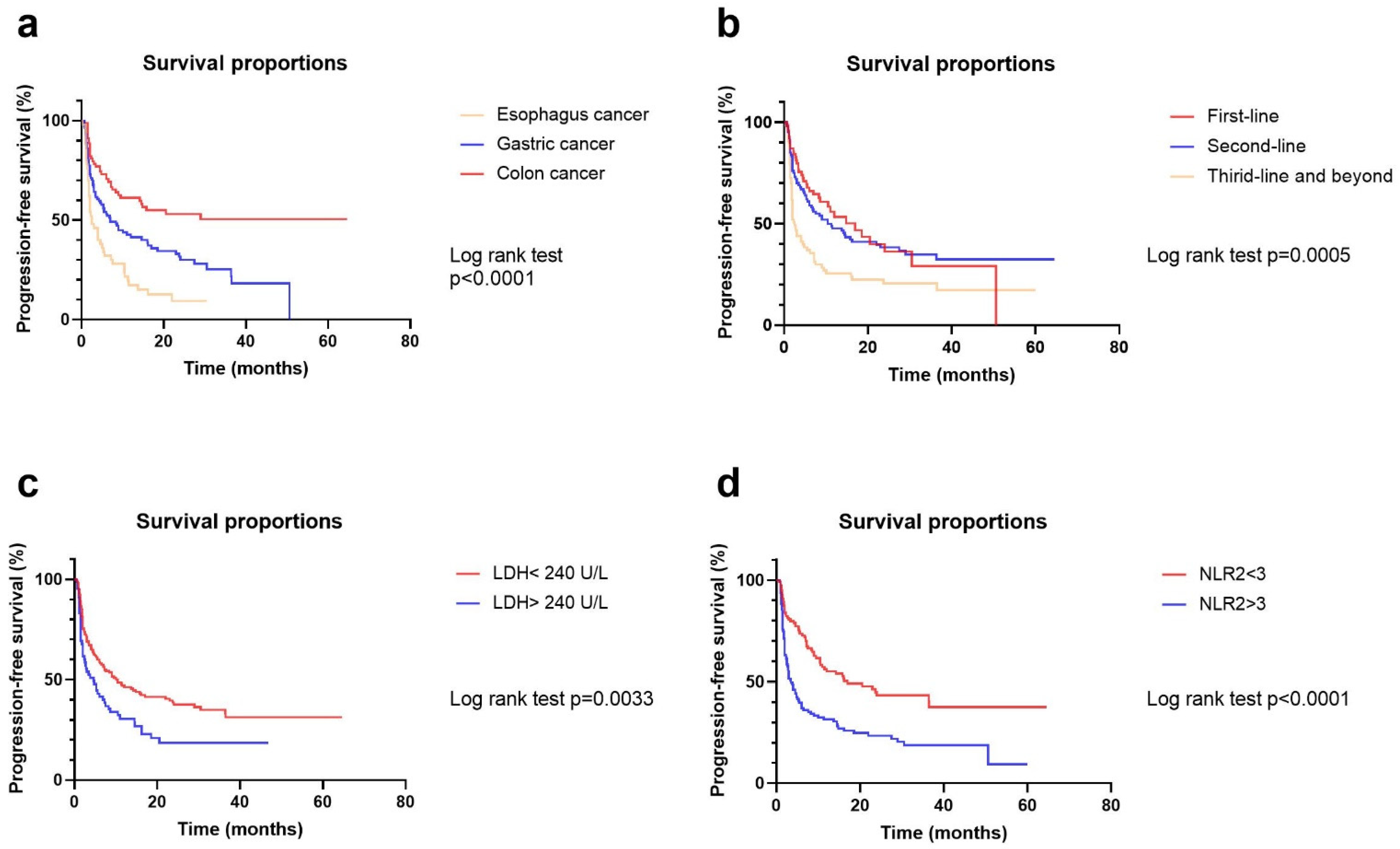
3.3. Univariate and Multivariate Analyses of Risk Factors for ORR and PFS
3.4. Univariate and Multivariate Analyses of Risk Factors for irAEs
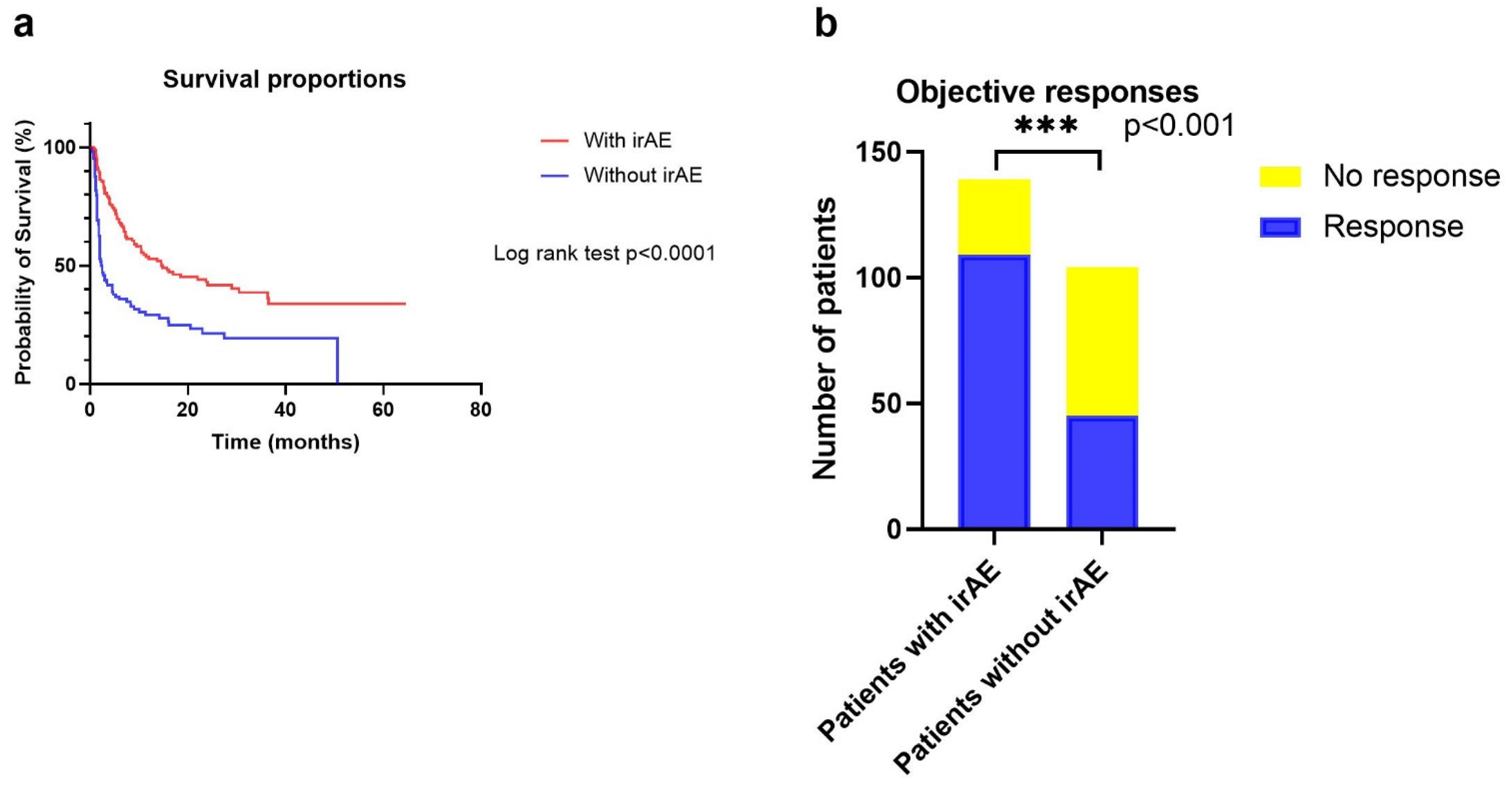
3.5. Correlations between irAEs and Treatment Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Li, Y.; Tang, L.; Qu, X.; Ying, J.; Zhang, J.; Sun, L.; Lin, R.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer. Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Shah, M.A.; Kojima, T.; Hochhauser, D.; Enzinger, P.; Raimbourg, J.; Hollebecque, A.; Lordick, F.; Kim, S.; Tajika, M.; Kim, H.T.; et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol. 2019, 5, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xie, T.; Zhang, X.; Qi, C.; Shen, L.; Peng, Z. Immune checkpoint inhibitors for treatment of advanced gastric or gastroesophageal junction cancer: Current evidence and future perspectives. Chin. J. Cancer Res. 2020, 32, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021, 11, 2168–2185. [Google Scholar] [CrossRef]
- Mcgrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Gambardella, V.; Cervantes, A.; Fleitas, T. Checkpoint inhibitors for gastroesophageal cancers: Dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann. Oncol. 2021, 32, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gerber, D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin. Cancer Biol. 2020, 64, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Skribek, M.; Rounis, K.; Afshar, S.; Grundberg, O.; Friesland, S.; Tsakonas, G.; Ekman, S.; De Petris, L. Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur. J. Cancer 2021, 145, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Chen, Y.; Wei, X.; Wang, Y.; Wang, Z.; Wu, H.; Xu, R.; Yuan, S.; Wang, F. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol. Rep. 2021, 9, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, S.; Tanaka, H.; Nishimura, J.; Sakimura, C.; Tamura, T.; Toyokawa, T.; Muguruma, K.; Yashiro, M.; Hirakawa, K.; Ohira, M. Neutrophils in primary gastric tumors are correlated with neutrophil infiltration in tumor-draining lymph nodes and the systemic inflammatory response. BMC Immunol. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2014, 8, 125–158. [Google Scholar] [CrossRef] [Green Version]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer. 2018, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zou, J.; Liu, C.; Jiao, X.; Gong, J.; Li, J.; Wang, Z.; Lu, M.; Lu, Z.; Shen, L. Baseline derived neutrophil-to-lymphocyte ratio as a prognostic biomarker for non-colorectal gastrointestinal cancer patients treated with immune checkpoint blockade. Clin. Immunol. 2020, 212, 108345. [Google Scholar] [CrossRef]
- Ueda, T.; Chikuie, N.; Takumida, M.; Furuie, H.; Kono, T.; Taruya, T.; Hamamoto, T.; Hattori, M.; Ishino, T.; Takeno, S. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Otolaryngol. 2020, 140, 181–187. [Google Scholar] [CrossRef]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
- Yamada, T.; Hayashi, T.; Inokuchi, Y.; Hayashi, K.; Watanabe, H.; Komori, K.; Kano, K.; Shimoda, Y.; Fujikawa, H.; Shiozawa, M.; et al. Impact of the neutrophil-to-lymphocyte ratio on the survival of patients with gastric cancer treated with nivolumab monotherapy. Target Oncol. 2020, 15, 317–325. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Qu, T.; Wang, Z.; Yan, H.; Si, Y.; Zhang, Y.; Dai, G. Neutrophil-to-lymphocyte ratio (NLR) predicts PD-1 inhibitor survival in patients with metastatic gastric cancer. J. Immunol. Res. 2021, 2021, 2549295. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Di Quattro, R.; Malatino, L.; Fornarini, G.; Addeo, A.; Maruzzo, M.; Urzia, V.; Rundo, F.; Lipari, H.; De Giorgi, U.; et al. Neutrophil-to-lymphocyte ratio and lactate dehydrogenase as biomarkers for urothelial cancer treated with immunotherapy. Clin. Transl. Oncol. 2020, 22, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Signorelli, D.; Metro, G.; Galetta, D.; De Toma, A.; Cantale, O.; Banini, M.; Friedlaender, A.; Pizzutillo, P.; Garassino, M.C.; et al. Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab. Transl. Lung Cancer Res. 2020, 9, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Dharmapuri, S.; özbek, U.; Lin, J.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef]
- Ksienski, D.; Wai, E.S.; Alex, D.; Croteau, N.S.; Freeman, A.T.; Chan, A.; Patterson, T.; Clarkson, M.; Fiorino, L.; Poonja, Z.; et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl. Lung Cancer Res. 2021, 10, 355–367. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Peng, Z.; Qi, C.; Gong, J.; Zhang, X.; Li, J.; Shen, L. Association of lymphocyte-to-monocyte ratio with survival in advanced gastric cancer patients treated with immune checkpoint inhibitor. Front. Oncol. 2021, 11, 589022. [Google Scholar] [CrossRef]
- Harutani, Y.; Ozawa, Y.; Murakami, E.; Sato, K.; Oyanagi, J.; Akamatsu, H.; Yoshikawa, T.; Shibaki, R.; Sugimoto, T.; Furuta, K.; et al. Pre-treatment serum protein levels predict survival of non-small cell lung cancer patients without durable clinical benefit by PD-1/L1 inhibitors. Cancer Immunol. Immunother. 2022. [Google Scholar] [CrossRef]
- Brown, J.T.; Liu, Y.; Shabto, J.M.; Martini, D.J.; Ravindranathan, D.; Hitron, E.E.; Russler, G.A.; Caulfield, S.; Yantorni, L.B.; Joshi, S.S.; et al. Baseline modified Glasgow prognostic score associated with survival in metastatic urothelial carcinoma treated with immune checkpoint inhibitors. Oncologist 2021, 26, 397–405. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, X.; Li, S.; Chen, H.; Wei, X.; Liu, C.; Gong, J.; Zhang, X.; Wang, X.; Peng, Z.; et al. Alterations in DNA damage response and repair genes as potential biomarkers for immune checkpoint blockade in gastrointestinal cancer. Cancer Biol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhou, Y.; Fang, J. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liu, Y.; Zhang, Z.; Zhang, X.; Gong, J.; Qi, C.; Li, J.; Shen, L.; Peng, Z. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: A prospective observational study. J. Immunother. 2020, 43, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wu, J.; Luo, H.; Li, Y.; Xiao, J.; Peng, J.; Ye, Z.; Zhou, R.; Yu, Y.; Wang, G.; et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J. Immunother. Cancer 2021, 9, e002467. [Google Scholar] [CrossRef]
- Friedlander, P.; Wood, K.; Wassmann, K.; Christenfeld, A.M.; Bhardwaj, N.; Oh, W.K. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. J. Immunother. Cancer 2018, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Iwama, S.; Sugiyama, D.; Yasuda, Y.; Okuji, T.; Ito, M.; Ito, S.; Sugiyama, M.; Onoue, T.; Takagi, H.; et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002493. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Shahabi, V.; Berman, D.; Chasalow, S.D.; Wang, L.; Tsuchihashi, Z.; Hu, B.; Panting, L.; Jure-Kunkel, M.; Ji, R. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J. Transl. Med. 2013, 11, 75. [Google Scholar] [CrossRef] [Green Version]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Berner, F.; Bomze, D.; Fässler, M.; Diem, S.; Cozzio, A.; Jörger, M.; Früh, M.; Driessen, C.; Lenz, T.L.; et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 2019, 107, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Husain, B.; Kirchberger, M.C.; Erdmann, M.; Schüpferling, S.; Abolhassani, A.; Fröhlich, W.; Berking, C.; Heinzerling, L. Inflammatory markers in autoimmunity induced by checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2021, 147, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 2020, 182, 655–671.e22. [Google Scholar] [CrossRef] [PubMed]
- Matsukane, R.; Watanabe, H.; Minami, H.; Hata, K.; Suetsugu, K.; Tsuji, T.; Masuda, S.; Okamoto, I.; Nakagawa, T.; Ito, T.; et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci. Rep. 2021, 11, 1324. [Google Scholar] [CrossRef]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-lymphocyte ratio predicts development of immune-related adverse events and outcomes from immune checkpoint blockade: A case-control study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef]
- Egami, S.; Kawazoe, H.; Hashimoto, H.; Uozumi, R.; Arami, T.; Sakiyama, N.; Ohe, Y.; Nakada, H.; Aomori, T.; Ikemura, S.; et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: A multicenter retrospective study. J. Cancer 2021, 12, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Ma, F.; Sun, B.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Peripheral blood markers associated with immune-related adverse effects in patients who had advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer. Manag. Res. 2021, 13, 765–771. [Google Scholar] [CrossRef]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Huang, Z.; Xin, L.; Qin, B.; Zhao, X.; Zhang, J.; Shi, W.; Yang, B.; Zhang, G.; Hu, Y. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol. Immunother. 2021, 70, 713–720. [Google Scholar] [CrossRef]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.; Heo, D.S.; Lee, J.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Gomatou, G.; Tzilas, V.; Kotteas, E.; Syrigos, K.; Bouros, D. Immune checkpoint inhibitor-related pneumonitis. Respiration 2020, 99, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4 T-cell immunity in the peripheral blood correlates with response to anti-PD-1 therapy. Cancer. Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef]

| Variables | Number of Patients (N = 234) | Percentage (%) |
|---|---|---|
| Sex | ||
| Female | 71 | 29.2 |
| Male | 172 | 70.8 |
| Age (years) | ||
| <60 | 129 | 53.1 |
| ≥60 | 114 | 46.9 |
| Primary tumor | ||
| Esophagus cancer | 50 | 20.6 |
| Gastric cancer | 115 | 47.3 |
| Colon cancer | 78 | 32.1 |
| ECOG PS | ||
| 0 | 97 | 39.9 |
| 1 | 140 | 57.6 |
| 2 | 6 | 2.5 |
| Treatment types | ||
| Anti-PD-1 * | 143 | 58.8 |
| Anti-PD-L1 ** | 40 | 16.5 |
| Anti-PD-1 + CTLA-4 *** | 60 | 24.7 |
| Line of immunotherapy | ||
| First-line | 70 | 28.8 |
| Second-line | 100 | 41.2 |
| Third-line and beyond | 73 | 30.0 |
| PD-L1 expression | ||
| Positive | 99 | 40.7 |
| Negative | 38 | 15.7 |
| Missing | 106 | 43.6 |
| Immune-Related Adverse Events (Categories) | Total Events (N = 260) | Immune-Related Adverse Events | Total Events (N = 260) | CTCAE Grade 1 (N = 170) | CTCAE Grade 2 (N = 58) | CTCAE Grade 3–4 (N = 32) |
|---|---|---|---|---|---|---|
| Skin | 52 | Pruritus | 2 | 2 | 0 | 0 |
| Rash | 50 | 34 | 12 | 4 | ||
| Rheumatology | 35 | Arthralgia | 8 | 6 | 2 | 0 |
| Myalgia | 2 | 1 | 1 | 0 | ||
| Myositis/Elevated creatine kinase | 20 | 8 | 3 | 9 | ||
| Dry mouth/Dry eye | 1 | 1 | 0 | 0 | ||
| Dental ulcer | 4 | 3 | 1 | 0 | ||
| Pulmonary | 9 | Pneumonitis | 9 | 5 | 4 | 0 |
| Gastrointestinal | 21 | Nausea/Vomiting | 4 | 3 | 1 | 0 |
| Diarrhea/Colitis | 13 | 6 | 5 | 2 | ||
| Elevated amylase | 4 | 2 | 1 | 1 | ||
| Endocrine | 49 | Adrenocortical insufficiency | 4 | 0 | 4 | 0 |
| Thyroiditis | 42 | 39 | 3 | 0 | ||
| Hypophysitis | 3 | 0 | 1 | 2 | ||
| Hepatology | 59 | Transaminitis | 32 | 21 | 5 | 6 |
| Hyperbilirubinemia | 27 | 18 | 6 | 3 | ||
| Cardiology | 6 | Myocarditis | 3 | 0 | 1 | 2 |
| Arrhythmia | 3 | 3 | 0 | 0 | ||
| Nephrology | 7 | Proteinuria | 6 | 2 | 3 | 1 |
| Elevated creatinine | 1 | 0 | 0 | 1 | ||
| Others | 22 | Fever | 6 | 4 | 2 | 0 |
| Leukocytopenia | 11 | 10 | 1 | 0 | ||
| Thrombocytopenia | 2 | 0 | 2 | 0 | ||
| Dizziness/Headache | 2 | 2 | 0 | 0 | ||
| Peripheral neuritis | 1 | 0 | 1 | 0 |
| Variable | ORR | PFS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | References (HR = 1.000) | Univariate | Multivariate | |||||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Sex | 0.585 | 0.321–1.067 | 0.080 | 0.442 | 0.205–0.954 | 0.037 | Female | 1.407 | 0.984–2.010 | 0.061 | 0.245 | ||
| Age | 1.000 | 0.981–1.019 | 0.995 | 0.794 | <60 year | 0.968 | 0.707–1.325 | 0.839 | 0.645 | 0.454–0.914 | 0.014 | ||
| ECOG PS | 0.701 | 0.433–1.135 | 0.149 | 0 | 1.268 | 0.916–1.756 | 0.152 | ||||||
| Tumor type | 1.771 | 1.216–2.579 | 0.003 | 0.182 | Colorectal | 2.350 | 1.613–3.424 | <0.001 | 2.628 | 1.792–4.014 | <0.001 | ||
| Treatment type | 1.150 | 0.842–1.572 | 0.380 | Monotherapy | 0.809 | 0.551–1.188 | 0.280 | ||||||
| Treatment line | 0.437 | 0.302–0.634 | <0.001 | 0.484 | 0.310–0.754 | 0.001 | ≥3 line | 0.534 | 0.385–0.739 | <0.001 | 0.528 | 0.371–0.751 | <0.001 |
| PD-L1 expression | 1.454 | 1.010–2.094 | 0.044 | 0.050 | Positive | 0.797 | 0.580–1.095 | 0.161 | |||||
| Presence of irAE | 4.393 | 2.519–7.660 | <0.001 | 3.573 | 1.870–6.829 | <0.001 | No | 0.494 | 0.360–0.677 | <0.001 | 0.562 | 0.400–0.791 | 0.001 |
| Highest CTCAE grade of irAE | 1.767 | 1.345–2.320 | <0.001 | 0.991 | Grade 0–1 | 0.684 | 0.490–0.957 | 0.026 | 0.631 | ||||
| Baseline albumin | 1.037 | 0.977–1.101 | 0.232 | <35 g/L | 0.645 | 0.302–1.379 | 0.258 | ||||||
| Baseline LDH | 0.997 | 0.994–1.000 | 0.021 | 0.410 | <240 U/L | 1.642 | 1.171–2.300 | 0.004 | 1.563 | 1.088–2.247 | 0.016 | ||
| Baseline hemoglobin | 1.002 | 0.989–1.014 | 0.777 | <90 g/L | 0.833 | 0.448–1.552 | 0.565 | ||||||
| C1 NLR | 0.972 | 0.900–1.050 | 0.468 | <3 | 1.399 | 1.016–1.926 | 0.040 | 0.060 | |||||
| C1 PLR | 1.000 | 0.998–1.002 | 0.858 | <160 | 1.059 | 0.768–1.460 | 0.725 | ||||||
| C1 LMR | 1.158 | 0.994–1.349 | 0.06 | 0.118 | <3 | 0.603 | 0.440–0.827 | 0.002 | 0.711 | ||||
| C2 NLR | 0.708 | 0.612–0.818 | <0.001 | 0.737 | 0.629–0.864 | <0.001 | <3 | 2.108 | 1.517–2.928 | <0.001 | 1.732 | 1.221–2.457 | 0.002 |
| C2 PLR | 0.997 | 0.994–0.999 | 0.006 | 0.411 | <45 | 1.632 | 1.158–2.301 | 0.005 | 0.744 | ||||
| C2 LMR | 1.618 | 1.322–1.981 | 1.618 | <45 | 0.507 | 0.367–0.701 | <0.001 | 0.162 | |||||
| Variable | Presence of irAE | Highest CTCAE Grade of irAE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Sex | 0.587 | 0.330–1.044 | 0.070 | 0.056 | 0.660 | 0.374–1.165 | 0.152 | 0.151 | ||||
| Age | 0.983 | 0.965–1.002 | 0.078 | 0.682 | 1.002 | 0.983–1.022 | 0.807 | 0.807 | ||||
| ECOG PS | 0.514 | 0.317–0.835 | 0.007 | 0.571 | 0.334–0.976 | 0.040 | 0.645 | 0.394–1.055 | 0.081 | 0.079 | ||
| Tumor type | 1.486 | 1.036–2.131 | 0.031 | 0.293 | 1.332 | 0.918–1.934 | 0.131 | |||||
| Treatment type | 1.529 | 1.116–2.094 | 0.008 | 0.081 | 1.356 | 0.998–1.843 | 0.051 | 0.050 | ||||
| Treatment line | 0.778 | 0.557–1.087 | 0.142 | 0.968 | 0.687–1.364 | 0.853 | ||||||
| PD–L1 expression | 1.181 | 0.829–1.683 | 0.357 | 1.234 | 0.850–1.792 | 0.268 | ||||||
| Baseline albumin | 1.076 | 1.013–1.142 | 0.017 | 1.067 | 1.000–1.138 | 0.049 | 1.022 | 0.961–1.087 | 0.486 | |||
| Baseline LDH | 1.000 | 0.997–1.003 | 0.939 | 1.001 | 0.998–1.003 | 0.657 | ||||||
| Baseline hemoglobin | 1.001 | 0.989–1.013 | 0.876 | 0.992 | 0.980–1.005 | 0.222 | ||||||
| C1 NLR | 1.014 | 0.938–1.096 | 0.727 | 1.049 | 0.970–1.135 | 0.233 | ||||||
| C1 PLR | 1.001 | 0.999–1.003 | 0.372 | 1.001 | 0.999–1.004 | 0.196 | ||||||
| C1 LMR | 1.137 | 0.985–1.314 | 0.080 | 0.700 | 0.527 | 0.911–1.200 | 0.527 | |||||
| C2 NLR | 0.875 | 0.787–0.972 | 0.013 | 0.894 | 0.801–0.997 | 0.044 | 0.988 | 0.900–1.084 | 0.795 | |||
| C2 PLR | 0.999 | 0.996–1.001 | 0.185 | 1.000 | 0.998–1.002 | 0.867 | ||||||
| C2 LMR | 1.139 | 0.985–1.316 | 0.080 | 0.909 | 0.992 | 0.875–1.124 | 0.895 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xie, T.; Qi, C.; Zhang, X.; Shen, L.; Peng, Z. Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors. Cancers 2022, 14, 3736. https://doi.org/10.3390/cancers14153736
Zhang Z, Xie T, Qi C, Zhang X, Shen L, Peng Z. Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors. Cancers. 2022; 14(15):3736. https://doi.org/10.3390/cancers14153736
Chicago/Turabian StyleZhang, Zhening, Tong Xie, Changsong Qi, Xiaotian Zhang, Lin Shen, and Zhi Peng. 2022. "Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors" Cancers 14, no. 15: 3736. https://doi.org/10.3390/cancers14153736
APA StyleZhang, Z., Xie, T., Qi, C., Zhang, X., Shen, L., & Peng, Z. (2022). Peripheral Blood Biomarkers Predictive of Efficacy Outcome and Immune-Related Adverse Events in Advanced Gastrointestinal Cancers Treated with Checkpoint Inhibitors. Cancers, 14(15), 3736. https://doi.org/10.3390/cancers14153736





