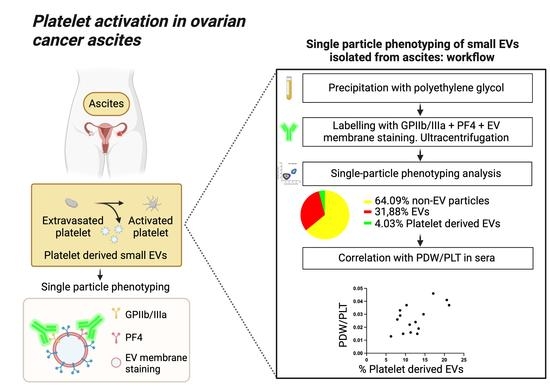
Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Cohort and Samples Collection
2.2. Haemoglobin Detection
2.3. Small-EVs Isolation
2.4. Atomic Force Microscopy (AFM) Imaging and Morphometric Analysis
2.5. Nano-Flow Cytometry
2.6. Protein Expression Analysis by Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Patients’ Selection and Clinical Data
3.2. Haemoglobin Levels in Ascites Are below 0.5% of the Corresponding Sera Level
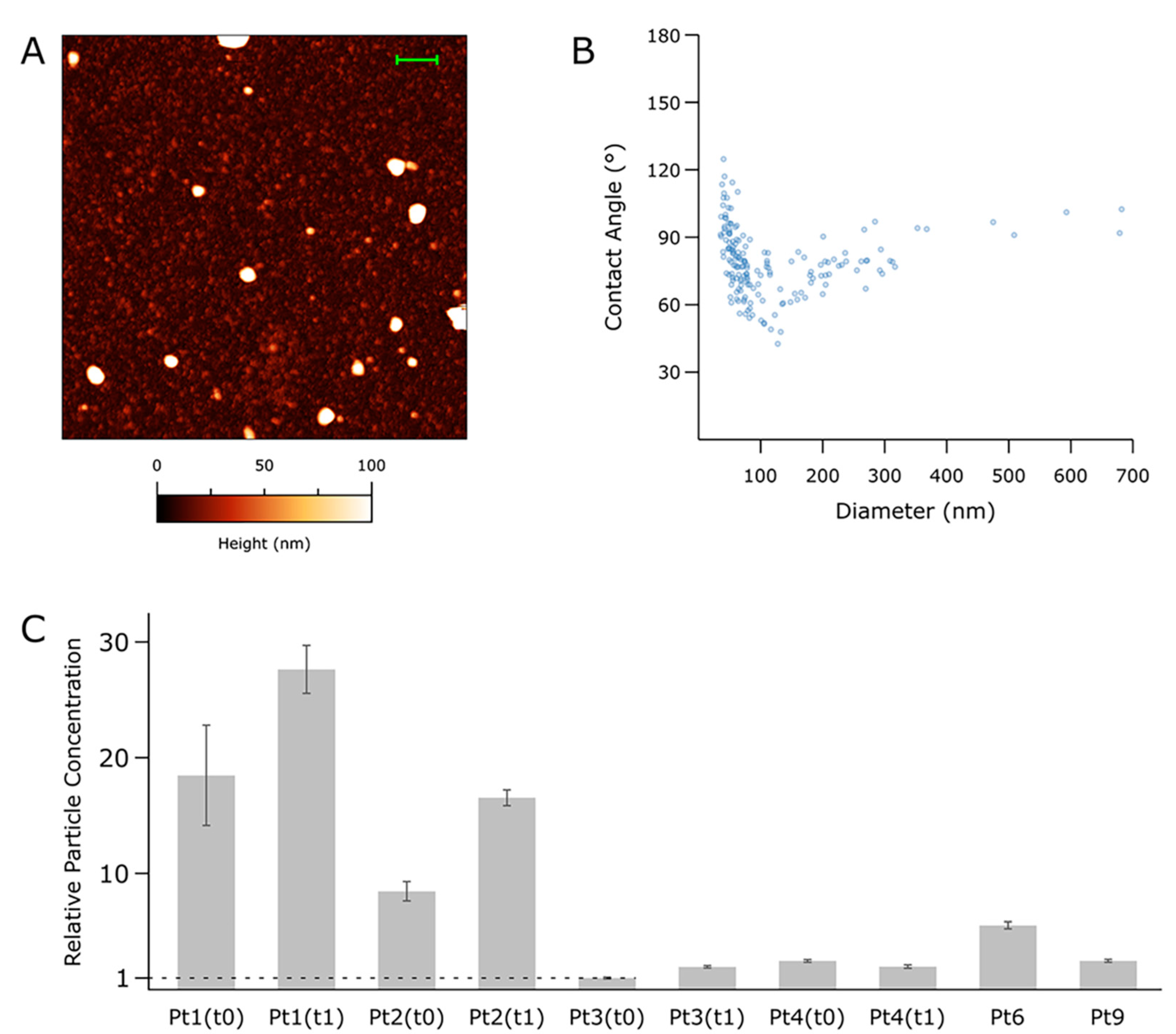
3.3. Nanomechanical Properties of Small-EVs in Ascites Measured by Morphometric Analysis of AFM Images
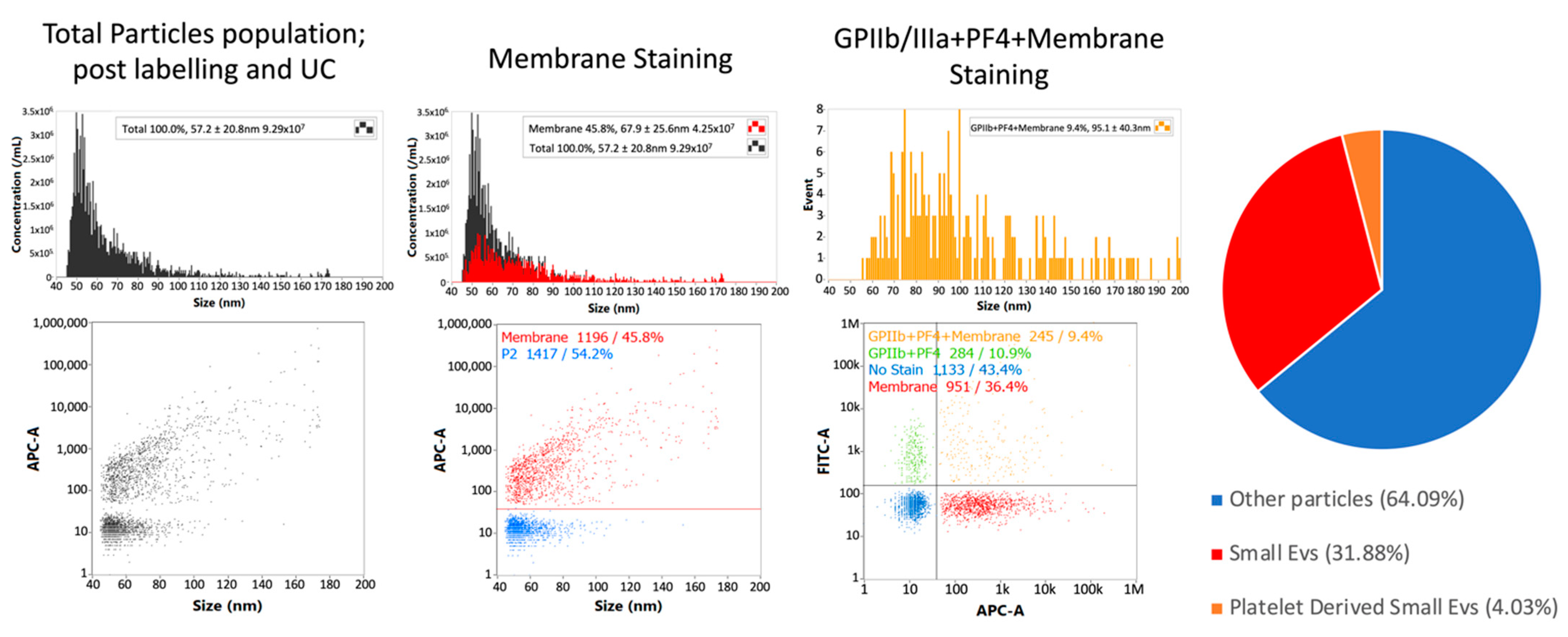
3.4. Single-Particle Phenotyping Analysis of Small-EVs in Ascites Revealed Platelet-Derived GPIIb/IIIa and PF4 in All the Samples
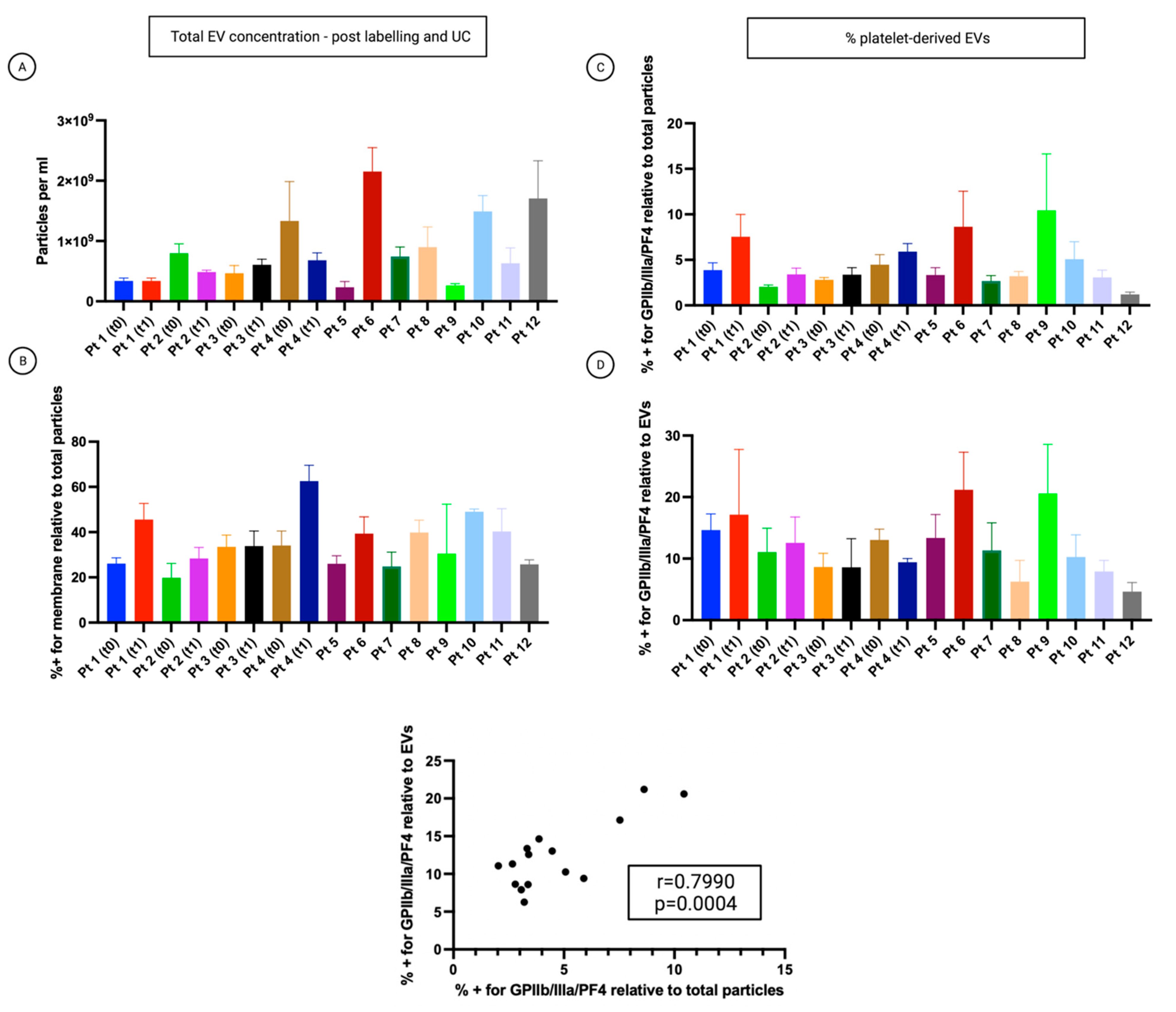
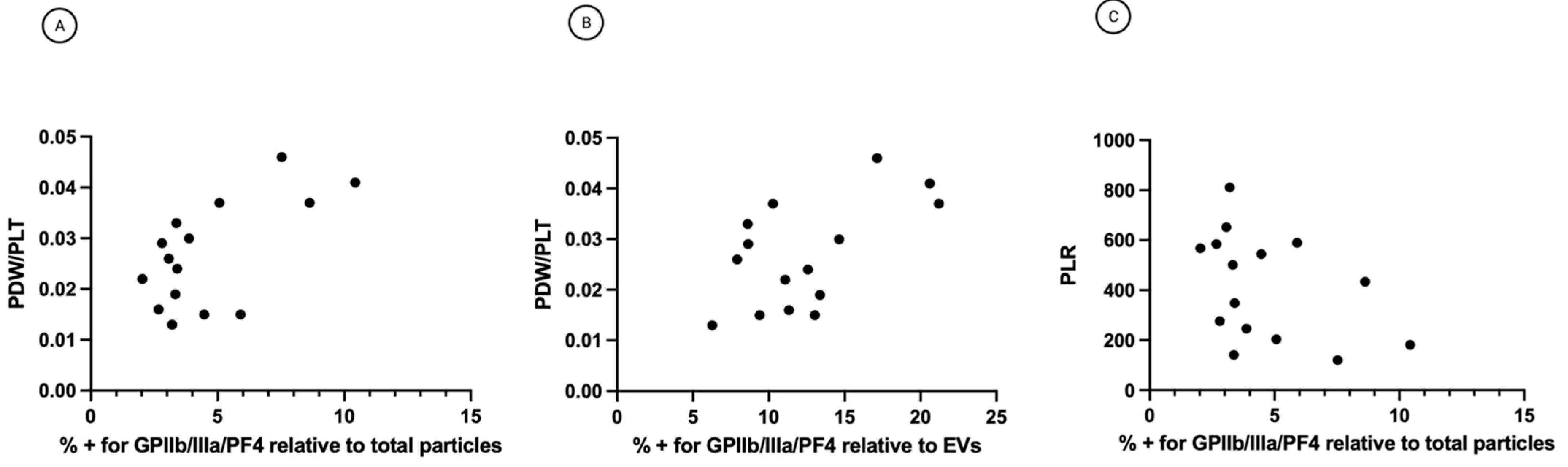
3.5. The Percentage of Platelet Biomarkers Measured in Small-EVs from Ascites Was Positively Correlated with PDW/PLT in Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The Disparate Origins of Ovarian Cancers: Pathogenesis and Prevention Strategies. Nat. Cancer 2017, 17, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Wimberger, P. Cells of Origin of Ovarian Cancer: Ovarian Surface Epithelium or Fallopian Tube? Arch. Gynecol. Obstet. 2017, 296, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Steidel, C.; Ender, F.; Rody, A.; von Bubnoff, N.; Gieseler, F. Biologically Active Tissue Factor-Bearing Larger Ectosome-Like Extracellular Vesicles in Malignant Effusions from Ovarian Cancer Patients: Correlation with Incidence of Thrombosis. Int. J. Mol. Sci. 2021, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical Electron Microscopy in the Study of Human Ovarian Tissues. EuroMediterr. Biomed. J. 2019, 14, 145–151. [Google Scholar] [CrossRef]
- Brass, L.F.; Diamond, S.L.; Stalker, T.J. Platelets and Hemostasis: A New Perspective on an Old Subject. Blood Adv. 2016, 1, 5–9. [Google Scholar] [CrossRef]
- Twomey, L.; Wallace, R.G.; Cummins, P.M.; Degryse, B.; Sheridan, S.; Harrison, M.; Moyna, N.; Meade-Murphy, G.; Navasiolava, N.; Custaud, M.-A.; et al. Platelets: From Formation to Function; IntechOpen: London, UK, 2018; ISBN 978-1-78985-078-9. [Google Scholar]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The Immune Nature of Platelets Revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef]
- Kanikarla Marie, P.; Fowlkes, N.W.; Afshar-Kharghan, V.; Martch, S.L.; Sorokin, A.; Shen, J.P.; Morris, V.K.; Dasari, A.; You, N.; Sood, A.K.; et al. The Provocative Roles of Platelets in Liver Disease and Cancer. Front. Oncol. 2021, 11, 643815. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Kopetz, S.; Hawk, E.T.; Millward, S.W.; Sood, A.K.; Gresele, P.; Overman, M.; Honn, K.; Menter, D.G. Bioactive Lipid Metabolism in Platelet “First Responder” and Cancer Biology. Cancer Metastasis Rev. 2018, 37, 439–454. [Google Scholar] [CrossRef]
- Gkolfinopoulos, S.; Jones, R.L.; Constantinidou, A. The Emerging Role of Platelets in the Formation of the Micrometastatic Niche: Current Evidence and Future Perspectives. Front. Oncol. 2020, 10, 374. [Google Scholar] [CrossRef]
- Raghavan, S.; Snyder, C.S.; Wang, A.; McLean, K.; Zamarin, D.; Buckanovich, R.J.; Mehta, G. Carcinoma-Associated Mesenchymal Stem Cells Promote Chemoresistance in Ovarian Cancer Stem Cells via PDGF Signaling. Cancers 2020, 12, E2063. [Google Scholar] [CrossRef]
- Haemmerle, M.; Taylor, M.L.; Gutschner, T.; Pradeep, S.; Cho, M.S.; Sheng, J.; Lyons, Y.M.; Nagaraja, A.S.; Dood, R.L.; Wen, Y.; et al. Platelets Reduce Anoikis and Promote Metastasis by Activating YAP1 Signaling. Nat. Commun. 2017, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, A.; Simon-Saez, I.; Perales, S.; Garrido-Navas, C.; Russo, A.; de Miguel-Perez, D.; Puche-Sanz, I.; Alaminos, C.; Ceron, J.; Lorente, J.A.; et al. Exchange of Cellular Components between Platelets and Tumor Cells: Impact on Tumor Cells Behavior. Theranostics 2022, 12, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets Increase the Proliferation of Ovarian Cancer Cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Miyashita, T.; Inokuchi, M.; Hayashi, H.; Oyama, K.; Tajima, H.; Takamura, H.; Ninomiya, I.; Ahmed, A.K.; Harman, J.W.; et al. Platelets Surrounding Primary Tumor Cells Are Related to Chemoresistance. Oncol. Rep. 2016, 36, 787–794. [Google Scholar] [CrossRef]
- Hu, Q.; Hisamatsu, T.; Haemmerle, M.; Cho, M.S.; Pradeep, S.; Rupaimoole, R.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Wong, S.T.C.; Sood, A.K.; et al. Role of Platelet-Derived Tgfβ1 in the Progression of Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5611–5621. [Google Scholar] [CrossRef]
- Cho, M.S.; Li, J.; Gonzalez-Delgado, R.; Lee, H.; Vasquez, M.; He, T.; He, Y.; Liu, K.; Sasano, T.; Nürnberg, B.; et al. The Effect of Platelet G Proteins on Platelet Extravasation and Tumor Growth in the Murine Model of Ovarian Cancer. Blood Adv. 2021, 5, 1947–1951. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Tronolone, J.J.; Chokshi, M.; Lokhande, G.K.; Selahi, A.; Gaharwar, A.K.; Afshar-Kharghan, V.; Sood, A.K.; Bao, G.; et al. Human Tumor Microenvironment Chip Evaluates the Consequences of Platelet Extravasation and Combinatorial Antitumor-Antiplatelet Therapy in Ovarian Cancer. Sci. Adv. 2021, 7, 5283. [Google Scholar] [CrossRef]
- Malacrida, B.; Nichols, S.; Maniati, E.; Jones, R.; Delanie-Smith, R.; Roozitalab, R.; Tyler, E.J.; Thomas, M.; Boot, G.; Mackerodt, J.; et al. A Human Multi-Cellular Model Shows How Platelets Drive Production of Diseased Extracellular Matrix and Tissue Invasion. iScience 2021, 24, 102676. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Lam, M.; Sorokin, A.V.; Overman, M.J.; Kopetz, S.; Menter, D.G. Platelet Metabolism and Other Targeted Drugs; Potential Impact on Immunotherapy. Front. Oncol. 2018, 8, 107. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and Predictors of Venous Thromboembolism (VTE) among Ambulatory High-Risk Cancer Patients Undergoing Chemotherapy in the United States. Cancer 2013, 119, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and Alpha-Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Baffour, S.; Adjei, J.; Aryeh, C.; Kyeremeh, R.; Kyei, F.; Seidu, M.A. Understanding the Biosynthesis of Platelets-Derived Extracellular Vesicles. Immun. Inflamm. Dis. 2015, 3, 133–140. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C.; Zhang, C.-Q. Platelet-Derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef]
- Fagotti, A.; Vizzielli, G.; De Iaco, P.; Surico, D.; Buda, A.; Mandato, V.D.; Petruzzelli, F.; Ghezzi, F.; Garzarelli, S.; Mereu, L.; et al. A Multicentric Trial (Olympia-MITO 13) on the Accuracy of Laparoscopy to Assess Peritoneal Spread in Ovarian Cancer. Am. J. Obstet. Gynecol. 2013, 209, e1–e462. [Google Scholar] [CrossRef] [PubMed]
- Bortot, B.; Apollonio, M.; Rampazzo, E.; Valle, F.; Brucale, M.; Ridolfi, A.; Ura, B.; Addobbati, R.; Di Lorenzo, G.; Romano, F.; et al. Small Extracellular Vesicles from Malignant Ascites of Patients with Advanced Ovarian Cancer Provide Insights into the Dynamics of the Extracellular Matrix. Mol. Oncol. 2021, 15, 3596–3614. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-force Microscope Tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and Efficiency Assessment of Six Extracellular Vesicle Isolation Methods by Nano-Flow Cytometry. J. Extracell. Vesicles 2019, 9, 1697028. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, L.; Long, Y.; Gao, M.; Huang, T.; Hang, W.; Yan, X. Size Differentiation and Absolute Quantification of Gold Nanoparticles via Single Particle Detection with a Laboratory-Built High-Sensitivity Flow Cytometer. J. Am. Chem. Soc. 2010, 132, 12176–12178. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Yan, Q.; Li, S.; Li, B.; Feng, Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio Are Predictive of Chemotherapeutic Response and Prognosis in Epithelial Ovarian Cancer Patients Treated with Platinum-Based Chemotherapy. Cancer Biomark. 2016, 17, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, H.; Guan, Z.; Lv, M.; Qian, T.; Wu, Y. Effect of Rho GTPase Activating Protein 9 Combined with Preoperative Ratio of Platelet Distribution Width to Platelet Count on Prognosis of Patients with Serous Ovarian Cancer. Transl. Cancer Res. 2021, 10, 4440–4453. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, R.; Musicò, A.; Brucale, M.; Ridolfi, A.; Galbiati, S.; Vago, R.; Bergamaschi, G.; Ferretti, A.M.; Chiari, M.; Valle, F.; et al. Extracellular Vesicles Analysis in the COVID-19 Era: Insights on Serum Inactivation Protocols towards Downstream Isolation and Analysis. Cells 2021, 10, 544. [Google Scholar] [CrossRef]
- Fullard, J.F. The Role of the Platelet Glycoprotein IIb/IIIa in Thrombosis and Haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef]
- Davis, A.N.; Afshar-Kharghan, V.; Sood, A.K. Platelet Effects on Ovarian Cancer. Semin. Oncol. 2014, 41, 378–384. [Google Scholar] [CrossRef]
- Orellana, R.; Kato, S.; Erices, R.; Bravo, M.L.; Gonzalez, P.; Oliva, B.; Cubillos, S.; Valdivia, A.; Ibañez, C.; Brañes, J.; et al. Platelets Enhance Tissue Factor Protein and Metastasis Initiating Cell Markers, and Act as Chemoattractants Increasing the Migration of Ovarian Cancer Cells. BMC Cancer 2015, 15, 290. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles. Arter. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef]
- Valkonen, S.; Mallas, B.; Impola, U.; Valkeajärvi, A.; Eronen, J.; Javela, K.; Siljander, P.R.-M.; Laitinen, S. Assessment of Time-Dependent Platelet Activation Using Extracellular Vesicles, CD62P Exposure, and Soluble Glycoprotein V Content of Platelet Concentrates with Two Different Platelet Additive Solutions. Transfus. Med. Hemotherapy 2019, 46, 267–275. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, B.; Song, Y.S. Ascites Modulates Cancer Cell Behavior, Contributing to Tumor Heterogeneity in Ovarian Cancer. Cancer Sci. 2016, 107, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.-L.T.; Schmittgen, T.D.; et al. Detection of MicroRNA Expression in Human Peripheral Blood Microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.M.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Turiák, L.; Wright, M.; Herczeg, P.; Lédeczi, Z.; Kittel, A.; Polgár, A.; Tóth, K.; Dérfalvi, B.; et al. Improved Flow Cytometric Assessment Reveals Distinct Microvesicle (Cell-Derived Microparticle) Signatures in Joint Diseases. PLoS ONE 2012, 7, e49726. [Google Scholar] [CrossRef]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E.; Moses, M.A.; et al. Platelet-Derived Extracellular Vesicles Infiltrate and Modify the Bone Marrow during Inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Winnicka, A. Platelets Extracellular Vesicles as Regulators of Cancer Progression—An Updated Perspective. Int. J. Mol. Sci. 2020, 21, 5195. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Pt | CA125 UI/mL (t0) | CA125 UI/mL (t1) | Ascites mL (t0) | Ascites mL (t1) | PDW/PLT (t0) | PDW/PLT (t1) | PLR (t0) | PLR (t1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1022.4 * | 443.2 * | 1800 | 300 | 0.03 | 0.046 | 246.41 | 120 |
| 2 | 1851.0 * | 156.9 * | 5300 | <100 | 0.022 | 0.024 | 568.18 | 348.6 |
| 3 | 2426.0 * | 157.0 * | 8000 | <100 | 0.029 | 0.033 | 276.06 | 141.5 |
| 4 | 318.0 * | 166.5 * | 2200 | 5000 | 0.015 | 0.015 | 544.8 | 589.9 |
| 5 | 332.6 * | 11.7 | 2000 | 0.019 | 500.98 | |||
| 6 | 3099.1 * | 12.3 | 4000 | 0.037 | 433.75 | |||
| 7 | 9797.0 * | 43.6 | 7000 | 0.016 | 584.68 | |||
| 8 | 290.3 * | 5.5 | 3500 | 0.013 | 811.54 | |||
| 9 | 995.6 * | 27.5 | <100 | 0.041 | 181.18 | |||
| 10 | 20,597.0 * | 157.6 | 200 | 0.037 | 203.35 | |||
| 11 | 2875.2 * | 132.8 | 3700 | 0.026 | 652.54 | |||
| 12 | 5268.3 * | 38.6 | 4900 | 0.016 | 662.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortot, B.; Mangogna, A.; Peacock, B.; Lees, R.; Valle, F.; Brucale, M.; Tassinari, S.; Romano, F.; Ricci, G.; Biffi, S. Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers 2022, 14, 4100. https://doi.org/10.3390/cancers14174100
Bortot B, Mangogna A, Peacock B, Lees R, Valle F, Brucale M, Tassinari S, Romano F, Ricci G, Biffi S. Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers. 2022; 14(17):4100. https://doi.org/10.3390/cancers14174100
Chicago/Turabian StyleBortot, Barbara, Alessandro Mangogna, Ben Peacock, Rebecca Lees, Francesco Valle, Marco Brucale, Sara Tassinari, Federico Romano, Giuseppe Ricci, and Stefania Biffi. 2022. "Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis" Cancers 14, no. 17: 4100. https://doi.org/10.3390/cancers14174100
APA StyleBortot, B., Mangogna, A., Peacock, B., Lees, R., Valle, F., Brucale, M., Tassinari, S., Romano, F., Ricci, G., & Biffi, S. (2022). Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers, 14(17), 4100. https://doi.org/10.3390/cancers14174100