Digital and Interactive Health Interventions Minimize the Physical and Psychological Impact of Breast Cancer, Increasing Women’s Quality of Life: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection—Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Outcomes
2.5. Analysis of the Risk of Bias of the Studies Included and the Quality of the Evidence of the Findings
2.6. Statistical Analysis
2.7. Sensitivity and Subgroup Analyses
3. Results
3.1. Study Selection Process
3.2. Main Characteristics of the Studies Included
3.3. Risk of Bias of the Studies Included
3.4. Outcome Measurements
3.5. Quantitative Synthesis
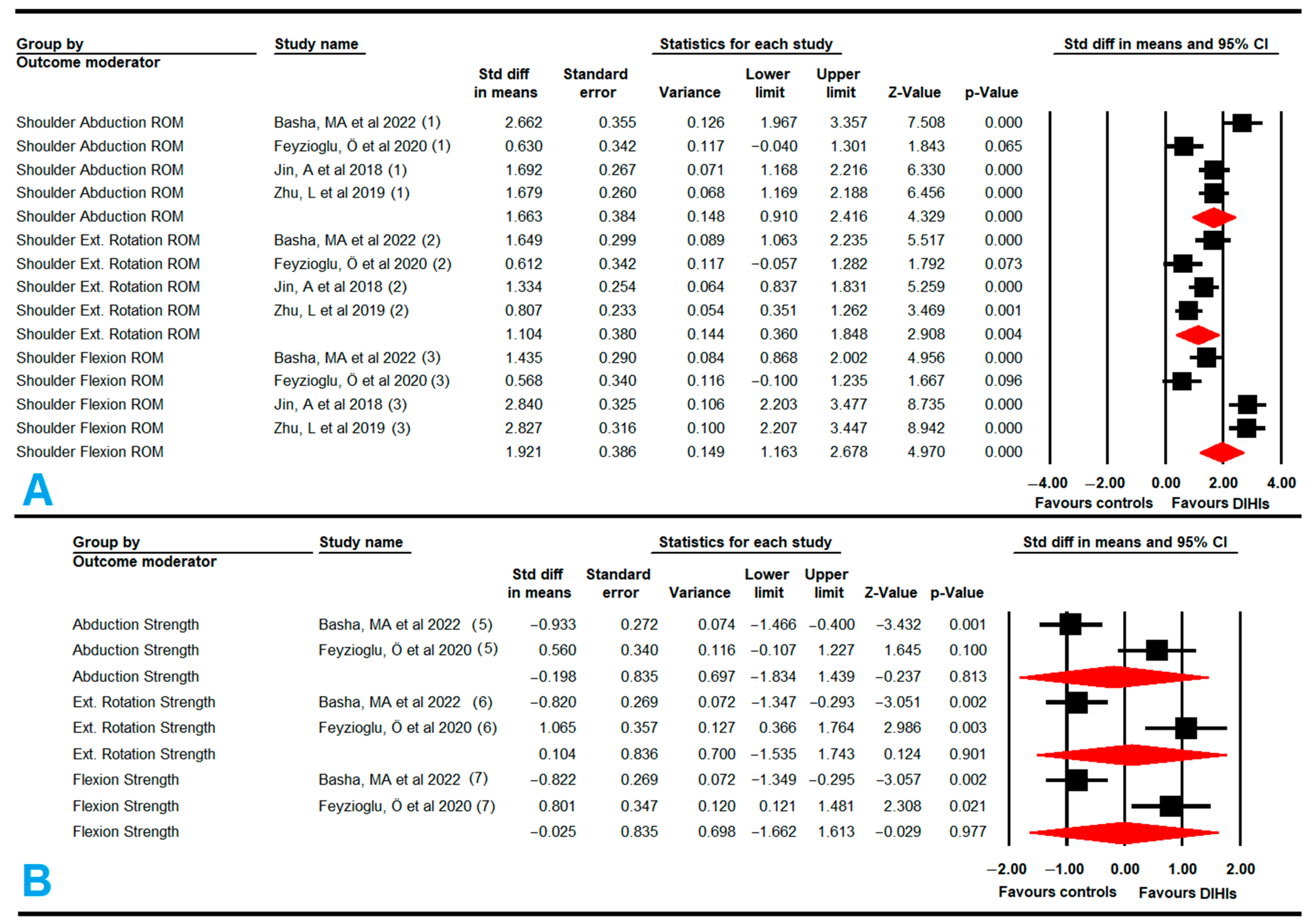
3.5.1. Shoulder Range of Motion (Flexion, Abduction, and External Rotation)
3.5.2. Shoulder Muscle Strength (Flexion, Abduction, and External Rotation)
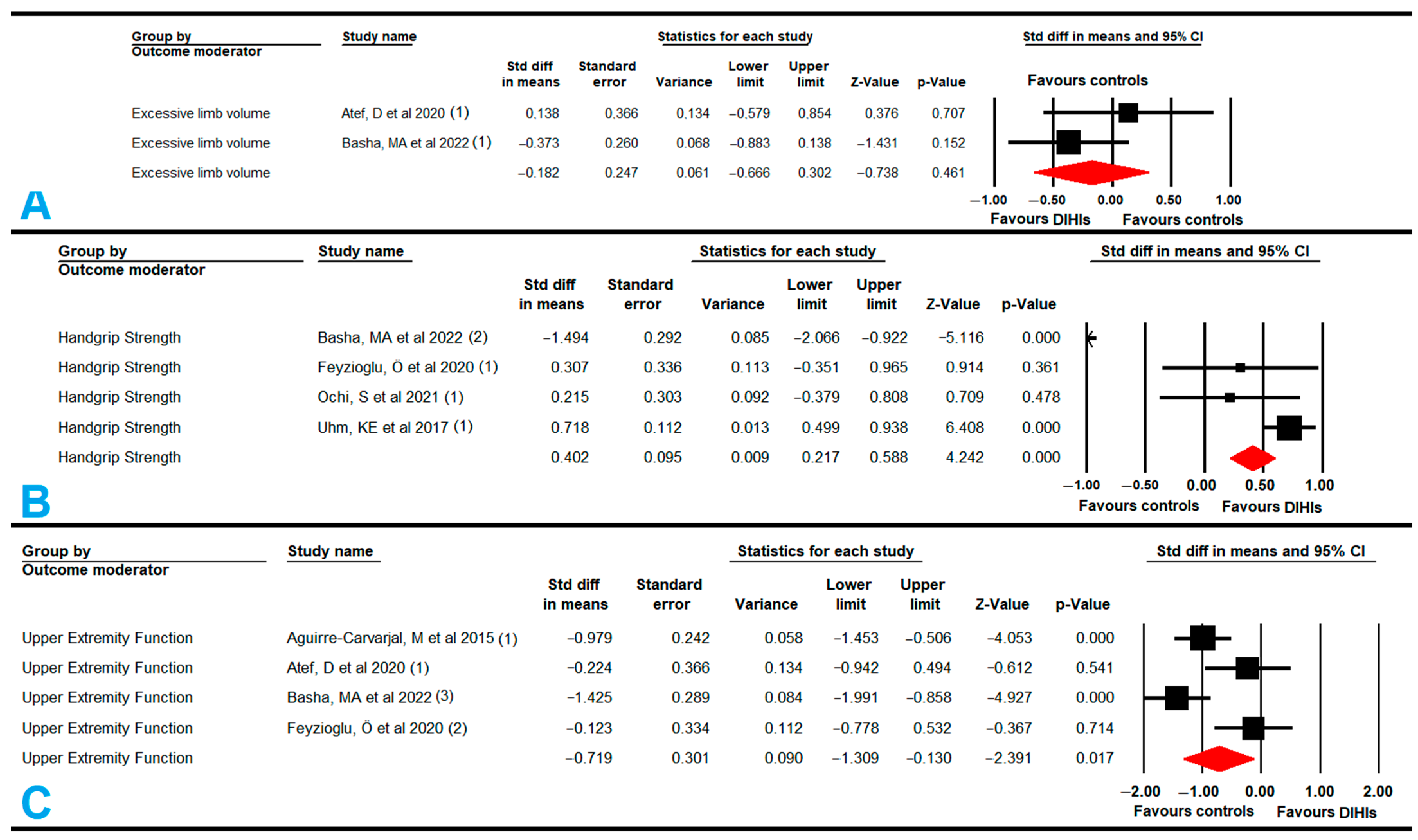
3.5.3. Excess Limb Volume in the Affected Upper Extremity with Lymphedema
3.5.4. Handgrip Strength
3.5.5. Function and Disability of the Affected Upper Extremity with Lymphedema
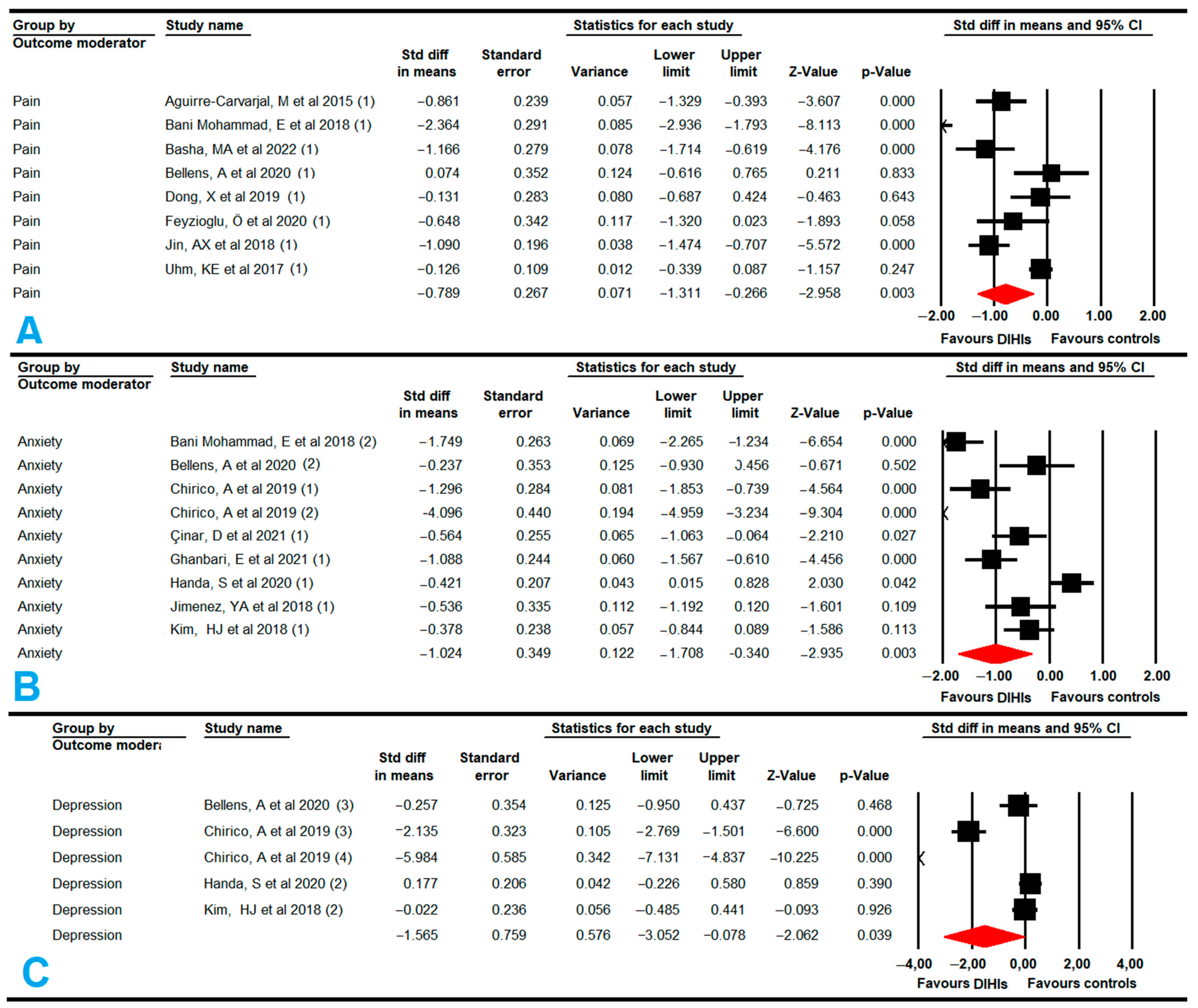
3.5.6. Pain
3.5.7. Anxiety
3.5.8. Depression
3.5.9. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann. Oncol. 2019, 30, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Tsai, W.-C.; Chou, W.-Y.; Hung, Y.-C.; Liu, L.-C.; Huang, K.-F.; Wang, W.-C.; Leung, K.-W.; Hsieh, R.-K.; Kung, P.-T. Quality of life of breast and cervical cancer survivors. BMC Women’s Health 2017, 17, 30. [Google Scholar] [CrossRef]
- Mai, T.T.X.; Choi, J.H.; Lee, M.K.; Chang, Y.J.; Jung, S.-Y.; Cho, H.; Lee, E.S. Prognostic Value of Post-diagnosis Health-Related Quality of Life for Overall Survival in Breast Cancer: Findings from a 10-Year Prospective Cohort in Korea. Cancer Res. Treat. 2019, 51, 1600–1611. [Google Scholar] [CrossRef]
- Firkins, J.; Hansen, L.; Driessnack, M.; Dieckmann, N. Quality of life in “chronic” cancer survivors: A meta-analysis. J. Cancer Surviv. 2020, 14, 504–517. [Google Scholar] [CrossRef]
- Feeney, L.R.; Tormey, S.M.; Harmon, D.C. Breast cancer and chronic pain: A mixed methods review. Irish J. Med. Sci. 2018, 187, 877–885. [Google Scholar] [CrossRef]
- Lorenzo-Gallego, L.; Arranz-Martín, B.; Romay-Barrero, H.; Prieto-Gómez, V.; Lluch, E.; Torres-Lacomba, M. Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series. Int. J. Environ. Res. Public Health 2022, 19, 4055. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Milosavljevic, S.; Dickerson, C.R.; Trask, C.M.; Kim, S.Y. Evidence of rotator cuff disease after breast cancer treatment: Scapular kinematics of post-mastectomy and post-reconstruction breast cancer survivors. Ann. Med. 2022, 54, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.S.; Mestriner, C.; Ribeiro, L.T.N.; Grillo, F.W.; Lemos, T.W.; Carneiro, A.A.; Guirro, R.R.d.J.; Guirro, E.C.O. Relationship between lymphedema after breast cancer treatment and biophysical characteristics of the affected tissue. PLoS ONE 2022, 17, e0264160. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; Abdul Wahhab Ibraheem, S.; Abdullah, M.; Rosli, R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics 2021, 11, 1303. [Google Scholar] [CrossRef]
- De Groef, A.; Devoogdt, N.; Gursen, C.; Moloney, N.; Warpy, V.; Daelemans, J.; Dams, L.; Haenen, V.; Van der Gucht, E.; Heroes, A.-K.; et al. Sensory signs and symptoms in women with self-reported breast cancer–related lymphedema: A case–control study close up. J. Cancer Surviv. 2021, 1–11. [Google Scholar] [CrossRef]
- Meilani, E.; Zanudin, A.; Mohd Nordin, N.A. Psychometric Properties of Quality of Life Questionnaires for Patients with Breast Cancer-Related Lymphedema: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2519. [Google Scholar] [CrossRef]
- Vicini, F.; Shah, C.; Arthur, D. The Increasing Role of Lymphedema Screening, Diagnosis and Management as Part of Evidence-Based Guidelines for Breast Cancer Care. Breast J. 2016, 22, 358–359. [Google Scholar] [CrossRef]
- Naik, H.; Leung, B.; Laskin, J.; McDonald, M.; Srikanthan, A.; Wu, J.; Bates, A.; Ho, C. Emotional distress and psychosocial needs in patients with breast cancer in British Columbia: Younger versus older adults. Breast Cancer Res. Treat. 2020, 179, 471–477. [Google Scholar] [CrossRef]
- Syrjala, K.L.; Jensen, M.P.; Mendoza, M.E.; Yi, J.C.; Fisher, H.M.; Keefe, F.J. Psychological and Behavioral Approaches to Cancer Pain Management. J. Clin. Oncol. 2014, 32, 1703–1711. [Google Scholar] [CrossRef]
- Irelli, A.; Ranieri, J.; Sirufo, M.M.; De Pietro, F.; Casalena, P.; Ginaldi, L.; Cannita, K.; Di Giacomo, D. Allostatic Load as an Insight into the Psychological Burden after Primary Treatment in Women with Breast Cancer: Influence of Physical Side Effects and Pain Perception. J. Clin. Med. 2022, 11, 2144. [Google Scholar] [CrossRef]
- Breidenbach, C.; Heidkamp, P.; Hiltrop, K.; Pfaff, H.; Enders, A.; Ernstmann, N.; Kowalski, C. Prevalence and determinants of anxiety and depression in long-term breast cancer survivors. BMC Psychiatry 2022, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.-M.; Rafiemanesh, H.; Aghamohammadi, T.; Badakhsh, M.; Amirshahi, M.; Sari, M.; Behnamfar, N.; Roudini, K. Prevalence of anxiety among breast cancer patients: A systematic review and meta-analysis. Breast Cancer 2020, 27, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Li, J.-Q.; Shi, J.-F.; Que, J.-Y.; Liu, J.-J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.-Q.; Qiao, Y.-L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef]
- Fancourt, D.; Williamon, A.; Carvalho, L.A.; Steptoe, A.; Dow, R.; Lewis, I. Singing modulates mood, stress, cortisol, cytokine and neuropeptide activity in cancer patients and carers. Ecancermedicalscience 2016, 10, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Del Rocío Ibancos-Losada, M.; Nieto-Escámez, F.A.; Obrero-Gaitán, E.; Catalina Osuna-Pérez, M.; Godinho, C.; Fernandes, J.B. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Cavadino, A.; Chin, S.; Little, Z.; Akroyd, A.; Tennant, G.; Dobson, R.; Broom, R.; Gautier, A. The benefits and acceptability of virtual reality interventions for women with metastatic breast cancer in their homes; a pilot randomised trial. BMC Cancer 2022, 22, 360. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Obrero-Gaitán, E.; Lomas-Vega, R. Nintendo Wii Balance Board therapy for postural control in children with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2021, 61, 1262–1275. [Google Scholar] [CrossRef]
- Błajda, J.; Barnaś, E.; Kucab, A. Application of Personalized Education in the Mobile Medical App for Breast Self-Examination. Int. J. Environ. Res. Public Health 2022, 19, 4482. [Google Scholar] [CrossRef]
- House, G.; Burdea, G.; Grampurohit, N.; Polistico, K.; Roll, D.; Damiani, F.; Hundal, J.; Demesmin, D. A feasibility study to determine the benefits of upper extremity virtual rehabilitation therapy for coping with chronic pain post-cancer surgery. Br. J. Pain 2016, 10, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Suchodolska, G.; Senkus, E. Mobile applications for early breast cancer chemotherapy-related symptoms reporting and management: A scoping review. Cancer Treat. Rev. 2022, 105, 102364. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, U.; Wells, S.J.; Parker, N.; Lyons, E.J.; Swartz, M.D.; Blozinski, A.; Basen-Engquist, K.; Peterson, S.; Swartz, M.C. Use of active video games with or without videoconferencing on health outcomes in adolescent and young adult cancer survivors: A systematic review. J. Cancer Surviv. 2021, 16, 714–727. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, M.; Yu, L.; Yang, S.; Zhang, W. The Efficacy of Virtual Reality-Based Interventions in Breast Cancer-Related Symptom Management: A Systematic Review and Meta-analysis. Cancer Nurs. 2022, 10-1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Zhang, Z.-X.; Zhang, Q. Efficacy of virtual reality-based interventions for patients with breast cancer symptom and rehabilitation management: A systematic review and meta-analysis. BMJ Open 2022, 12, e051808. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Ng, P.H.; Xu, W.; Cheng, Q.; Chen, P.Q.; Cheng, A.S.; Liu, X. The Effectiveness of Virtual Reality–Based Interventions in Rehabilitation Management of Breast Cancer Survivors: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e31395. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Chen, J.; Zhang, Y.; Li, M.; Xiong, C.; Yan, J. Effectiveness of mobile health-based self-management interventions in breast cancer patients: A meta-analysis. Support. Care Cancer 2022, 30, 2853–2876. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Training: London, UK, 2011. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Gotzsche, P.; Juni, P.; Moher, D.; Oxman, A.; Savovic, J.; Schulz, K.; Weeks, L.; Sterne, J. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive meta-analysis software version 3. In Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2020, 12, 19. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Rothman, K.; Greenland, S.; Lash, T. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef]
- Aguirre-Carvajal, M.; Marchant-Pérez, P. Descripción del efecto de los ejercicios de la extremidad superior ipsilateral realizados con realidad virtual en mujeres sometidas a mastectomía. Gac. Mex. Oncol. 2015, 14, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Atef, D.; Elkeblawy, M.M.; El-Sebaie, A.; Abouelnaga, W.A.I. A quasi-randomized clinical trial: Virtual reality versus proprioceptive neuromuscular facilitation for postmastectomy lymphedema. J. Egypt. Natl. Canc. Inst. 2020, 32, 29. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Okuyama, H.; Yamamoto, H.; Nakamura, S.; Kato, Y. Effectiveness of a Smartphone Application as a Support Tool for Patients Undergoing Breast Cancer Chemotherapy: A Randomized Controlled Trial. Clin. Breast Cancer 2020, 20, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, Y.A.; Cumming, S.; Wang, W.; Stuart, K.; Thwaites, D.I.; Lewis, S.J. Patient education using virtual reality increases knowledge and positive experience for breast cancer patients undergoing radiation therapy. Support. Care Cancer 2018, 26, 2879–2888. [Google Scholar] [CrossRef]
- Jin, A.; Chen, X.; Zhang, X.; Chen, J. Construction and Application of a VR Platform Based on the Omaha System for Rehabilitation Management of Breast Cancer Patients. Chin. Gen. Pract. 2018, 21, 2987–2993. [Google Scholar]
- Jin, A.; Chen, X.; Zhang, X.; Chen, J. Design and clinical application of rehabilitation VR system for breast cancer patients. Chin. J. Nurs. 2018, 12, 168–172. [Google Scholar]
- Kim, H.J.; Kim, S.M.; Shin, H.; Jang, J.-S.; Kim, Y.I.; Han, D.H. A Mobile Game for Patients with Breast Cancer for Chemotherapy Self-Management and Quality-of-Life Improvement: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e273. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuji, K.; Narisawa, T.; Shimizu, Y.; Kuchiba, A.; Suto, A.; Jimbo, K.; Takayama, S.; Ueno, T.; Sakurai, N.; et al. Cardiorespiratory fitness in breast cancer survivors: A randomised controlled trial of home-based smartphone supported high intensity interval training. BMJ Support. Palliat. Care 2021, 12, 33–37. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.S.; Kim, J.Y.; Bae, S.H. Mobile web-based self-management program for breast cancer patients with chemotherapy-induced amenorrhoea: A quasi-experimental study. Nurs. Open 2022, 9, 655–665. [Google Scholar] [CrossRef]
- Rosen, K.D.; Paniagua, S.M.; Kazanis, W.; Jones, S.; Potter, J.S. Quality of life among women diagnosed with breast Cancer: A randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psychooncology 2018, 27, 2023–2030. [Google Scholar] [CrossRef]
- Uhm, K.E.; Yoo, J.S.; Chung, S.H.; Lee, J.D.; Lee, I.; Kim, J.I.; Lee, S.K.; Nam, S.J.; Park, Y.H.; Lee, J.Y.; et al. Effects of exercise intervention in breast cancer patients: Is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res. Treat. 2017, 161, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, J.; Li, Q. Application of rehabilitation training virtual reality system in postoperative rehabilitation exercise for breast cancer patients. Qilu J. Nurs 2019, 25, 99–102. [Google Scholar]
- Bani Mohammad, E.; Ahmad, M. Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: A randomized control trial. Palliat. Support. Care 2019, 17, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.; Aboelnour, N.H.; Alsharidah, A.S.; Kamel, F.H. Effect of exercise mode on physical function and quality of life in breast cancer–related lymphedema: A randomized trial. Support. Care Cancer 2022, 30, 2101–2110. [Google Scholar] [CrossRef]
- Bellens, A.; Roelant, E.; Sabbe, B.; Peeters, M.; van Dam, P.A. A video-game based cognitive training for breast cancer survivors with cognitive impairment: A prospective randomized pilot trial. Breast 2020, 53, 23–32. [Google Scholar] [CrossRef]
- Chirico, A.; Maiorano, P.; Indovina, P.; Milanese, C.; Giordano, G.G.; Alivernini, F.; Iodice, G.; Gallo, L.; De Pietro, G.; Lucidi, F.; et al. Virtual reality and music therapy as distraction interventions to alleviate anxiety and improve mood states in breast cancer patients during chemotherapy. J. Cell. Physiol. 2020, 235, 5353–5362. [Google Scholar] [CrossRef]
- Çınar, D.; Karadakovan, A.; Erdoğan, A.P. Effect of mobile phone app-based training on the quality of life for women with breast cancer. Eur. J. Oncol. Nurs. 2021, 52, 101960. [Google Scholar] [CrossRef]
- Dong, X.; Yi, X.; Gao, D.; Gao, Z.; Huang, S.; Chao, M.; Chen, W.; Ding, M. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients:a randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 109. [Google Scholar] [CrossRef]
- Feyzioğlu, Ö.; Dinçer, S.; Akan, A.; Algun, Z.C. Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support. Care Cancer 2020, 28, 4295–4303. [Google Scholar] [CrossRef]
- Ghanbari, E.; Yektatalab, S.; Mehrabi, M. Effects of Psychoeducational Interventions Using Mobile Apps and Mobile-Based Online Group Discussions on Anxiety and Self-Esteem in Women with Breast Cancer: Randomized Controlled Trial. JMIR mHealth uHealth 2021, 9, e19262. [Google Scholar] [CrossRef]
- Paek, J.; Choi, Y.J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: A cross-sectional study. BMJ Open 2019, 9, e030938. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Jiménez, C.; Martín-Martín, J.; Pajares, B.; Ribelles, N.; Alba, E.; Cuesta-Vargas, A.I. Factors associated with upper limb function in breast cancer survivors. PM&R 2021, in press. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Speck, R.M.; Rye, S.A.; DiSipio, T.; Hayes, S.C. Prevalence of breast cancer treatment sequelae over 6 years of follow-up. Cancer 2012, 118, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Qu, H.; Wu, Q.; Song, Y. Lymphedema in survivors of breast cancer (Review). Oncol. Lett. 2020, 19, 2085–2096. [Google Scholar] [CrossRef] [Green Version]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef]
- Haraldstad, K.; Sørum, R.; Eide, H.; Natvig, G.K.; Helseth, S. Pain in children and adolescents: Prevalence, impact on daily life, and parents’ perception, a school survey. Scand. J. Caring Sci. 2011, 25, 27–36. [Google Scholar] [CrossRef]
- Buche, H.; Michel, A.; Piccoli, C.; Blanc, N. Contemplating or Acting? Which Immersive Modes Should Be Favored in Virtual Reality During Physiotherapy for Breast Cancer Rehabilitation. Front. Psychol. 2021, 12, 631186. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G.; Richards, T.L.; Van Oostrom, T.; Coda, B.A.; Jensen, M.P.; Blough, D.K.; Sharar, S.R. The Analgesic Effects of Opioids and Immersive Virtual Reality Distraction: Evidence from Subjective and Functional Brain Imaging Assessments. Anesth. Analg. 2007, 105, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Bantick, S.J.; Wise, R.G.; Ploghaus, A.; Clare, S.; Smith, S.M.; Tracey, I. Imaging how attention modulates pain in humans using functional MRI. Brain 2002, 125, 310–319. [Google Scholar] [CrossRef]
- Dunn, J.; Yeo, E.; Moghaddampour, P.; Chau, B.; Humbert, S. Virtual and augmented reality in the treatment of phantom limb pain: A literature review. NeuroRehabilitation 2017, 40, 595–601. [Google Scholar] [CrossRef]



| Database | Search Strategy |
|---|---|
| PubMed Medline | (breast neoplasms[mh] or breast neoplasm*[tiab] or breast cancer*[tiab] or breast tumor*[tiab] or mammary cancer*[tiab] or breast cancer lymphedema[mh] or breast cancer lymphedema[tiab] or postmastectomy lymphedema[tiab]) AND (virtual reality[mh] OR virtual reality[tiab] OR virtual reality exposure therapy[mh] OR virtual reality exposure therapy[tiab] OR exergam*[tiab] or videogam*[tiab] or mobile applications[mh] or mobile application*[tiab] or mobile app*[tiab] or smartphone app*[tiab] or mobile game*[tiab] or smartphone game*[tiab]) |
| SCOPUS | TITLE-ABS-KEY (“breast neoplasm” OR “breast cancer” OR “breast tumor” OR “mammary cancer” OR “breast cancer lymphedema” OR “postmastectomy lymphedema”) AND TITLE-ABS-KEY (“virtual reality” OR “virtual reality exposure therapy” OR “mobile applications” OR “mobile app” OR “mobile game” OR “videogame” OR “exergame”) |
| Web of Science | (*breast cancer*) (Topic) AND (*virtual reality* OR *mobile applications* OR *mobile app*) (Topic) |
| PEDro | (virtual reality) AND (breast cancer) (mobile) AND (breast cancer) |
| CINAHL Complete | AB (breast cancer or breast neoplasm or breast carcinoma or breast tumor) AND AB (virtual reality or smartphone applications or mobile apps) |
| SciELO | (breast cancer) AND (virtual reality OR smartphone) |
| Experimental Group | Control Group | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | K | Number of Patients | Sample Characteristics | Intervention Characteristics | Sample Characteristics | Intervention Characteristics | Outcomes | Test | Qualitative Findings |
| Aguirre-Carvajal, M. et al. 2015 [55] (Mexico) Design: Quasi-experimental (NB) Setting: Hospital Carlos Van Bürende Valparaíso (Chile) Funding: No | 2 | 77 post-mastectomy female patients (58.76 ± 1.46 years old) | 41 (57.66 ± 1.65 years old) | Nintendo Wii® videogames for one month, 3 times per week and 32 min per session | 36 (60.03 ± 2.51 years old) | Conventional care | Pain | VAS | Both groups improved, although the Wii group reported a greater reduction in pain |
| UE function | Quick DASH-9 Scale | Both groups improved, although the Wii group reported greater improvement | |||||||
| Atef, D. et al. 2020 [56] (Egypt) Design: Quasi-experimental (NB) Setting: Physical Therapy Department at the National Cairo Institute Funding: No | 2 | 30 female patients between 40 and 65 years old | 15 (54.07 ± 8.28 years old) with post-mastectomy lymphedema | Nintendo Wii® sports videogames for a duration of 30 min, 2 sessions per week for 4 weeks | 15 (53.07 ± 7.24 years old) with post-mastectomy lymphedema | Conventional physical training using PNF for a duration of 30 min, 2 sessions per week for 4 weeks | UE function | Quick DASH-9 Scale | Statistically significant differences in the Nintendo Wii® group (p = 0.001) and in the PNF group (p = 0.003). No significant differences between groups (p = 0.935) |
| Excess limb volume | Milliliters | Each group presented a statistically significant reduction in lymphedema (Nintendo Wii® p = 0.001 and PNF p = 0.004). However, no statistically significant differences were found between the groups (p = 0.9) | |||||||
| Bani Mohammad, E. et al. 2019 [67] (Jordan) Design: RCT (NB) Setting: King Hussein Cancer Center Funding: Yes. Deanship of Scientific Research at the University of Jordan | 2 | 80 female patients (51.99 ± 10.34 years old) in chronic phase | 40 | Immersive VR (“Ocean Rift” or “Happy Place”) after 15 min to give morphine therapy (peak time effect) | 40 | Conventional care | Pain | VAS | Statistically significant differences in each group (p < 0.001 and p < 0.001) and between groups (p < 0.001) |
| Anxiety | STAI | Statistically significant differences in each group (p < 0.001 and p < 0.001) and between groups (p < 0.001), favoring VR | |||||||
| Basha, M.A. et al. 2022 (Egypt) [68] Design: RCT (SB) Setting: National Cancer Institute and El-Sahel Teaching Hospital (Cairo) Funding: No | 16 | 60 female patients (50.45 ± 2.29 years old) with a mean time since diagnosis of 5 years | 30 (48.83 ± 7 years old) with post-mastectomy lymphedema | Xbox Kinect dance and sports videogames over 8 weeks, 5 days per week and once per day | 30 (52.07 ± 748 years old) with post-mastectomy lymphedema | Physical training combining stretching with resistance exercises using dumbbells for 8 weeks | Pain | VAS | Statistically significant differences favoring Xbox (p = 0.0001) |
| UE function | DASH | Statistically significant differences favoring Xbox (p = 0.0004) | |||||||
| Quality of life | SF-36 | Statistically significant differences favoring Xbox only in general health (p = 0.0005). None in vitality, physical, mental, social, or emotional aspects (p > 0.05) | |||||||
| Handgrip strength | Dynamometer | Statistically significant differences favoring the physical training group (p = 0.0002) | |||||||
| Shoulder ROM | Degree | Greater improvements in flexion (p = 0.0001), abduction (p = 0.0001), and external rotation (p = 0.0001), favoring Xbox | |||||||
| Shoulder strength | Kg | Greater improvements in flexion (p = 0.002), abduction (p = 0.0007), and external rotation (p = 0.004), favoring the physical training group | |||||||
| Excess limb volume | Ml | Each group showed a statistically significant reduction in lymphedema (p < 0.0001 for both). However, no statistically significant differences were found between groups (p = 0.15) | |||||||
| Bellens, A. et al. 2020 (Turkey) [69] Design: RCT Pilot (NB) Setting: Multidisciplinary Breast Clinic of the Antwerp University Hospital Funding: Yes. MyCognition (one author) | 9 | 46 female patients (51.8 ± 0.42 years old) with a mean time since diagnosis of 3.8 years | 23 (51.5 ± 8 years old) | AquaSnap cognitive training videogame for a duration of 3 months of at least 3 times per week in addition to usual care | 23 (52.1 ± 9.1 years old) | Usual care for 3 months | Anxiety/Depression | HADS | Greater reduction in anxiety and depression in the VR group (1.7 and 1.4 points, respectively) |
| Pain | MOS-SF36 | No statistically significant reduction in the control group (p > 0.05) | |||||||
| Quality of life | MOS-SF36 | Greater increase in physical functioning (7 points) and mental health (9.6 points) in the VR group than in the control group | |||||||
| Chirico, A. et al. 2019 (Italy) [70] Design: Quasi-experimental (NB) Setting: Fondazine G. Pascale (Naples, Italy) Funding: Yes. Sbarro Health Research Organization | 4 | 92 female patients (55.69 ± 0.5 years old) in chronic phase | 28 (55.18 ± 5.7 years old) | Walk, climb a mountain, swim in the sea, among others, in relaxed environments created on the Second Life® platform using Immersive VR | 30 (55.7 ± 5.26 years old) | Music therapy for 20 min, 5 min after the start of chemotherapy | Anxiety | SAI | Statistically significant differences between pre- and post-assessments in the VR group (p < 0.001) and the music therapy group (p < 0.001), but not in the control group (p = 0.179). Between groups, statistically significant differences were found between the VR and control groups (p < 0.001) and between the music therapy and control groups (p = 0.049). No statistically significant differences were found between the VR and music therapy groups (p > 0.05) |
| 34 (56.2 ± 6.79 years old) | Usual care | ||||||||
| Depression | SV-POMS | Statistically significant differences between the VR and control groups (p < 0.001) | |||||||
| Çınar, D. et al. 2021 (Turkey) [71] Design: RCT (SB) Setting: State hospital in Turkey Funding: Yes. Mobile app by Balikesir Tuberculosis and Cancer Fight Asociation | 5 | 64 female patients (45.7 ± 9 years old) with a mean time since diagnosis of 2.7 years | 31 (45.9 ± 8.3 years old) | Mobile phone app-based training support (educational and relaxation exercises) in addition to usual care for 12 weeks | 33 (45.5 ± 9.8 years old) | Usual care for 12 weeks | Anxiety | NCCNDTS | Statistically significant differences after therapy in the mobile app group (p = 0.004), but not in the control group (p = 0.082). Statistically significant differences between groups, favoring the mobile app group (p = 0.027) |
| Quality of life | FACT-ES QLS | Statistically significant differences in total (p < 0.001), physical p < 0.0001), and emotional QoL (p = 0.0015) were shown in the mobile app group. Between groups, statistically significant differences, favoring the mobile group, were shown in total, physical, and emotional QoL | |||||||
| Dong, X. et al. 2019 (China) [72] Design: RCT (SB) Setting: The Second Hospital of Shandong University in China Funding: Yes. Specialized Key Subjects of China National S&T Fundamental Work | 7 | 50 female patients (49.81 ± 2.55 years old) in chronic phase | 26 (48 ± 5.54 years old) | Mobile phone app-based exercise and video exercises for 12 weeks, 3 times per week and 30 min per session | 24 (51.63 ± 7.49 years old) | Usual care for 12 weeks | Quality of life | SF-36 | In the exercise app group, statistically significant differences were found in global health (p = 0.024), vitality (p = 0.014), and mental health (p = 0.014). Between groups, statistically significant differences were found favoring the exercise app group in vitality (p = 0.009) and mental health (p = 0.001) |
| Pain | SF-36 | ||||||||
| Feyzioğlu, Ö. et al. 2020 (Turkey) [73] Design: RCT (SB) Setting: Okmeydanı Training and Research Hospital (Istanbul) Funding: No | 9 | 36 female patients (50.92 ± 0.11 years old) with a chronic duration | 19 (50.84 ± 8.53 years old) | Xbox 360 Kinect dance, sports, and fighting videogames for a duration of 35 min for 8 weeks. CT program added | 17 (51 ± 7.06 years old) | Conventional physical therapy (usual care) for 8 weeks | Pain | VAS | Statistically significant improvement in both groups in pre–post assessment (p = 0.001 in each group). No differences between groups (p = 0.065) |
| UEfunction | DASH Scale | Statistically significant improvement in both groups in pre–post assessment (p = 0.001 in each group). Between groups, statistically significant differences were found favoring the Xbox group (p = 0.025) | |||||||
| Handgrip strength | Dynamometer | Statistically significant improvement in both groups in pre–post assessment (p = 0.001 in each group). No differences between groups (p = 0.302) | |||||||
| Shoulder ROM | Degree | Greater improvements in flexion, abduction, and external rotation in both groups, but no statistically significant differences between them in flexion (p = 0.688), abduction (p = 0.793), or external rotation (p = 0.573) | |||||||
| Shoulder strength | Kg | Greater improvements in flexion, abduction, and external rotation in both groups, but no statistically significant differences between them in flexion (p = 0.203), abduction (p = 0.532), or external rotation (p = 0.666) | |||||||
| Ghanbari, E. et al. 2021 (Iran) [74] Design: RCT (NB) Setting: Shiraz University of Medical Sciences (Shiraz) Funding: Yes. Shiraz University of Medical Sciences | 2 | 77 female patients (46.45 ± 0.63 years old) in chronic phase | 38 (46.9 ± 9.83 years old) in chronic phase | Mobile phone app-based training support (educational exercises) in addition to usual care for 4 weeks | 39 (46 ± 8.8 years old) in chronic phase | Usual care for 4 weeks | Anxiety | STAI | Statistically significant reduction in the mobile app group (p < 0.001) and increase in anxiety in the control group (p = 0.34) |
| Handa, S. et al. 2020 (Japan) [57] Design: RCT (NB) Setting: Showa University Hospital Breast Cancer (Japan) Funding: No | 2 | 95 (49.9 ± 9.7 years old) | 47 (49.9 ± 10.2 years old) in chemotherapy phase | Mobile app support training for 3 weeks (4 courses of chemotherapy) | 48 (49.9 ± 9.2 years old) in chemotherapy phase | Usual care | Anxiety | HADS-A | In both groups, the level of anxiety increased. However, the level of anxiety was lower in the usual care group, with statistically significant differences between groups (p = 0.08) |
| Depression | HADS-D | Usual care group did not show a statistically significantly reduced level of depression (p > 0.05), with no differences between groups (p = 0.35). The mobile app group showed increased depression (p > 0.05) | |||||||
| Jimenez, Y.A. et al. 2018 (Australia) [58] Design: Quasi-experimental (NB) Setting: Crown Princess Mary Cancer Centre, Westmead Hospital, Australia Funding: University of Sydney’s postgraduate research support | 1 | 37 female patients between 35 and 74 years old (major part between 45 and 54 years old) | 19 | VR education using VERT system. A total of 18 sessions of 1 h were carried out | 18 | Usual care education | Anxiety | STAI | VR further reduced the level of anxiety, but no statistically significant differences were found between groups (p = 0.217) |
| Jin, A.X. et al. 2018 [59] (China) Design: RCT (NB) Setting: Zhejiang Provincial People’s Hospital (China) Funding: No | 6 | 120 female patients in chronic phase | 60 | VR exercises using the Omaha system for 3 months | 60 | Conventional physical training for 3 months | QoL | SF-36 | In the VR group, there was an increase in total, physical, mental, emotional, vitality, and social QoL. Statistically significant differences in these dimensions appeared, favoring the VR group (p < 0.05) |
| Jin, A. et al. 2018 (China) [60] Design: RCT (SB) Setting: Zhejiang Provincial People’s Hospital (China) Funding: No | 3 | 72 female patients in chronic phase | 38 | VR-based training for 3 months, twice per day, 15–30 min per session | 38 | Conventional physical training for 3 months | Shoulder ROM | Degree | Patients who performed VR rehabilitation showed greater increases in shoulder flexion, abduction, and external rotation as compared to the conventional physical training group (p < 0.05) |
| Kim, H.J. et al. 2018 (Korea) [61] Design: RCT (NB) Setting: Chung-Ang University Hospital (Korea) Funding: Yes. Nexon 2014 and Korea Creative Content Agency | 3 | 77 female patients (50.95 ± 1.6 years old) with a mean duration of disease of 13.35 years | 34 (49.8 years old) with a mean duration of disease of 13.5 years | Mobile game for a duration of 3 weeks, 3 days per week and more than 30 min per session. | 38 (52.1 years old) with a mean duration of disease of 13.2 years | Conventional therapy for 3 weeks | Anxiety | STAi | Low level of anxiety in the mobile app group (p = 0.11) and no statistically significant differences between groups (p = 0.21) |
| Depression | BDI | Depression increases in both groups (p > 0.5) and no statistically significant differences between groups (p = 0.99) | |||||||
| Quality of life | WHO QoL-BREF Scale | The mobile app group showed higher QoL than the conventional therapy group (p = 0.01). Between groups, greater improvements were found favoring conventional therapy in physical (p = 0.03), mental (p = 0.2), and social QoL (p = 0.67) | |||||||
| Ochi, E. et al. 2021 (Japan) [62] Design: RCT (SB) Setting: National Cancer Center Hospital (Tokyo) Funding: Yes. National Cancer Centre Research and Development Fund | 2 | 44 female patients (48.5 years old) in chronic phase | 21 (48 ± 6 years old) with more than 19 months of evolution | Smartphone app exercise training guidance for 12 weeks and 3 times per week | 23 (49 ± 5 years old) | Usual care for 13 weeks | Handgrip strength | Dynamometer | More improvements in the app group, but with no statistically significant differences between groups (p = 0.53) |
| Quality of life | QoL (EQ-5D) | No statistically significant differences between groups (p = 0.25), although app groups improved more | |||||||
| Park, J.H. et al. 2022 (South Korea) [63] Design: Quasi-experimental (NB) Setting: Breast Cancer Center, Ajou University Medical Center, Suwon (South Korea) Funding: Yes. Basic Science Research Program through the National Research Foundation of Korea | 4 | 51 (42.78 ± 4.7 years old) in chronic phase | 27 (42.78 ± 4.7 years old) | Smartphone app education for 12 weeks | 24 (45 ± 5 years old) | Conventional education for 12 weeks | Quality of life | FACT-G | Statistically significant differences favoring the app group in social support (p = 0.04) and mental QoL (p = 0.003). The app group improved in all QoL dimensions in the pre–post assessment |
| Rosen, K.D. et al. 2018 [64] (United States) Design: RCT (NB) Setting: University of Texas, San Antonio Funding: Yes. ThriveWell Cancer Foundation and Graduate Student Research Award from the University of Texas at San Antonio | 1 | 87 female patients (52.31 ± 1.28 years old), duration of disease between 3 and 5 years | 39 (51.4 ± 10.73 years old) | Mobile app mindfulness (Headspace) mediation training for 8 weeks | 48 (53.22 ± 9.91 years old) | Usual care | Quality of life | FACT-B | After intervention, QoL was higher in the app group than in the control, with statistically significant differences favoring the app group (p < 0.01) |
| Uhm, K.E. et al. 2017 [65] (Korea) Design: Quasi-experimental (NB) Setting: Universities and hospitals in Korea Funding: Yes. National Information Society Agency (Korea) | 6 | 339 female patients (50.3 ± 9.5 years old) with more than 2 years of evolution | 167 (49.3 ± 8 years old) | Mobile app exercise training (mHealth), including aerobic and resistance exercise for 12 weeks | 172 (51.3 ± 10.7 years old) | Conventional exercises for 12 weeks | Handgrip strength | Dynamometer | Statistically significant improvements in the app (p < 0.05) and control (p < 0.05) groups. No statistically significant differences between groups (p > 0.5) |
| Pain | VAS | Statistically significant reduction in app (p < 0.05) and control (p < 0.05) groups. No statistically significant differences between groups (p > 0.22) | |||||||
| QoL | EORTC QLQ-C30 | Global QoL statistically improved in the app (p < 0.05) and control (p < 0.05) groups. Between groups, no statistically significant differences were found in global (p = 0.746), physical (p = 0.337), emotional (p = 0.42), or social (p = 0.608) QoL | |||||||
| Zhu, L. et al. 2019 [66] (China) Design: RCT Setting: Hospital Funding: No | 3 | 80 female patients in chronic phase | 40 | VR exercises for shoulder and hand rehabilitation. Three months, twice per day, 15–30 min each day | 40 | Conventional exercises for 3 months | Shoulder ROM | Degree | The VR rehabilitation group showed greater increases in shoulder flexion, abduction, and external rotation as compared to the conventional physical training group (p < 0.05) |
| STUDY | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | |
|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Concealment Randomization Sequence | Blinding of Participants | Blinding of Assessors | Incomplete Outcome Data | Selective Reporting | Other, Ideally Prespecified | |
| Aguirre-Carvajal, M. et al. 2015 [55] | + | + | + | + | − | ? | ? |
| Atef, D. et al. 2020 [56] | + | + | + | + | − | − | ? |
| Bani Mohammad, E. et al. 2020 [67] | − | − | + | + | − | − | − |
| Basha, M.A. et al. 2022 [68] | − | − | + | − | − | − | − |
| Bellens, A. et al. 2020 [69] | − | − | + | + | ? | − | − |
| Chirico, A. et al. 2019 [70] | + | + | + | + | − | − | ? |
| Çinar, D. et al. 2021 [71] | − | ? | + | − | − | − | − |
| Dong, X. et al. 2019 [72] | − | − | + | − | − | − | − |
| Feyzioğlu et al. 2020 [73] | − | − | + | + | − | ? | − |
| Ghanbari, E. et al. 2021 [74] | − | − | + | + | − | − | − |
| Handa, S. et al. 2020 [57] | − | ? | + | + | − | − | − |
| Jimenez, Y.A. et al. 2018 [58] | + | + | + | + | − | − | ? |
| Jin, A.X. et al. 2018 [59] | − | − | + | + | − | − | − |
| Jin, A. et al. 2018 [60] | − | − | + | + | − | − | − |
| Kim, H.J. et al. 2018 [61] | − | − | + | + | − | − | − |
| Ochi, E. et al. 2021 [62] | − | − | + | − | − | − | − |
| Park, J.H. et al. 2022 [63] | + | + | + | + | − | − | ? |
| Rosen, K.D. et al. 2018 [64] | − | − | + | + | − | − | − |
| Uhm, K.E. et al. 2017 [65] | + | + | + | + | − | − | ? |
| Zhu, L. et al. 2019 [66] | − | − | + | + | − | − | − |
| Findings Summary | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Heter | Publication Bias | ||||||||||||
| K | N | Ns | SMD | 95% CI | p | Q (df) | I2 (p) | Risk | Funnel Plot (Egger p) | Trim and Fill | Risk | |||
| Adj SMD | % var | |||||||||||||
| UPPER EXTREMITY | Flexion ROM | 4 | 248 | 62 | 1.92 | 1.16 to 2.68 | <0.001 | 3.18 (3) | 5.72% (0.36) | No | Symmetric (0.92) | 1.92 | 0% | No |
| Abduction ROM | 4 | 248 | 62 | 1.66 | 0.91 to 2.42 | <0.001 | 3.78 (3) | 20.71% (0.29) | Low | Asymmetric (0.48) | 1.35 | 19% | High | |
| External Rotation ROM | 4 | 248 | 62 | 1.1 | 0.36 to 1.85 | 0.004 | 3.17 (3) | 5.64% (0.35) | No | Symmetric (0.97) | 1.1 | 0% | No | |
| Flexion Strength | 2 | 86 | 43 | −0.03 | −1.66 to 1.62 | 0.97 | 1 (1) | 0% (0.32) | No | NP | NP | NP | Possible | |
| Abduction Strength | 2 | 86 | 43 | −0.2 | −1.83 to 1.44 | 0.81 | 1 (1) | 0% (0.32) | No | NP | NP | NP | Possible | |
| External Rotation Strength | 2 | 86 | 43 | 0.1 | −1.54 to 1.74 | 0.9 | 1 (1) | 0% (0.32) | No | NP | NP | NP | Possible | |
| Excessive Limb Volume | 2 | 90 | 45 | −0.18 | −0.66 to 0.3 | 0.46 | 1 (1) | 0% (0.32) | No | NP | NP | NP | Possible | |
| Handgrip Strength | 4 | 477 | 119 | 0.4 | 0.21 to 0.59 | <0.001 | 50.6(3) | 67% (0.0001) | Large | Asymmetric (0.15) | 0.67 | 65% | High | |
| Function | 4 | 203 | 51 | −0.72 | −1.31 to −0.13 | 0.017 | 3.17 (3) | 5.5% (0.35) | No | Symmetric (0.97) | −0.72 | 0% | No | |
| PAIN | 8 | 758 | 95 | −0.8 | −1.31 to −0.26 | 0.003 | 7.53 (7) | 7.14% (0.38) | No | Asymmetric (0.08) | −0.9 | 13% | High | |
| ANXIETY | 9 | 660 | 73 | −1.02 | −1.71 to −0.34 | 0.003 | 11.8 (8) | 32% (0.16) | Medium | Asymmetric (0.02) | −1.35 | 32% | High | |
| DEPRESSION | 5 | 402 | 80 | −1.57 | −3.1 to −0.08 | 0.039 | 8.96(4) | 46% (0.06) | Medium | Asymmetric (0.01) | −2.05 | 29% | High | |
| QUALITY OF LIFE | Overall Health Perception | 10 | 888 | 89 | 0.6 | 0.31 to 0.89 | <0.001 | 5.8 (9) | 0% (0.76) | No | Asymmetric (0.02) | 0.35 | 42% | High |
| Physical Role | 8 | 759 | 95 | 0.41 | 0.08 to 0.74 | 0.012 | 6.4 (7) | 0% (0.5) | No | Asymmetric (0.3) | 0.5 | 19% | High | |
| Mental Role | 7 | 695 | 99 | 0.37 | 0.03 to 0.72 | 0.035 | 5.76 (6) | 0% (0.45) | No | Symmetric (0.73) | 0.37 | 0% | No | |
| Emotional Role | 5 | 292 | 58 | 0.45 | 0.04 to 0.87 | 0.033 | 3.24 (4) | 0% (0.52) | No | Asymmetric (0.01) | 0.53 | 18% | High | |
| Social Functioning | 8 | 759 | 95 | 0.28 | −0.04 to 0.6 | 0.09 | 6.62 (7) | 0% (0.47) | No | Asymmetric (0.34) | 0.16 | 47% | High | |
| Vitality | 4 | 228 | 57 | 0.62 | 0.15 to 1.1 | 0.009 | 3.06 (3) | 1.8% (0.38) | No | Asymmetric (0.03) | 0.82 | 32% | High | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrero-Gaitán, E.; Cortés-Pérez, I.; Calet-Fernández, T.; García-López, H.; López Ruiz, M.d.C.; Osuna-Pérez, M.C. Digital and Interactive Health Interventions Minimize the Physical and Psychological Impact of Breast Cancer, Increasing Women’s Quality of Life: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4133. https://doi.org/10.3390/cancers14174133
Obrero-Gaitán E, Cortés-Pérez I, Calet-Fernández T, García-López H, López Ruiz MdC, Osuna-Pérez MC. Digital and Interactive Health Interventions Minimize the Physical and Psychological Impact of Breast Cancer, Increasing Women’s Quality of Life: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(17):4133. https://doi.org/10.3390/cancers14174133
Chicago/Turabian StyleObrero-Gaitán, Esteban, Irene Cortés-Pérez, Tania Calet-Fernández, Héctor García-López, María del Carmen López Ruiz, and María Catalina Osuna-Pérez. 2022. "Digital and Interactive Health Interventions Minimize the Physical and Psychological Impact of Breast Cancer, Increasing Women’s Quality of Life: A Systematic Review and Meta-Analysis" Cancers 14, no. 17: 4133. https://doi.org/10.3390/cancers14174133
APA StyleObrero-Gaitán, E., Cortés-Pérez, I., Calet-Fernández, T., García-López, H., López Ruiz, M. d. C., & Osuna-Pérez, M. C. (2022). Digital and Interactive Health Interventions Minimize the Physical and Psychological Impact of Breast Cancer, Increasing Women’s Quality of Life: A Systematic Review and Meta-Analysis. Cancers, 14(17), 4133. https://doi.org/10.3390/cancers14174133










