Oncogenic EFNA4 Amplification Promotes Lung Adenocarcinoma Lymph Node Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Cell Transfection
2.2. DNA Constructs
2.3. Lentivirus Production
2.4. sgRNA Design
2.5. RNA Extraction and Real-Time PCR
2.6. Immunoblotting
2.7. Cell Proliferation Assay
2.8. Colony Formation Assay
2.9. Migration Assay
2.10. Cell Adhesion Assays
2.11. Confocal Laser Scanning Microscopy
2.12. Xenograft Model
2.13. Lymphatic Metastasis and Survival Analysis of the High Versus Low Gene EFNA4 Expression Tumors
2.14. HPA Analysis
2.15. Statistical Analyses
3. Results
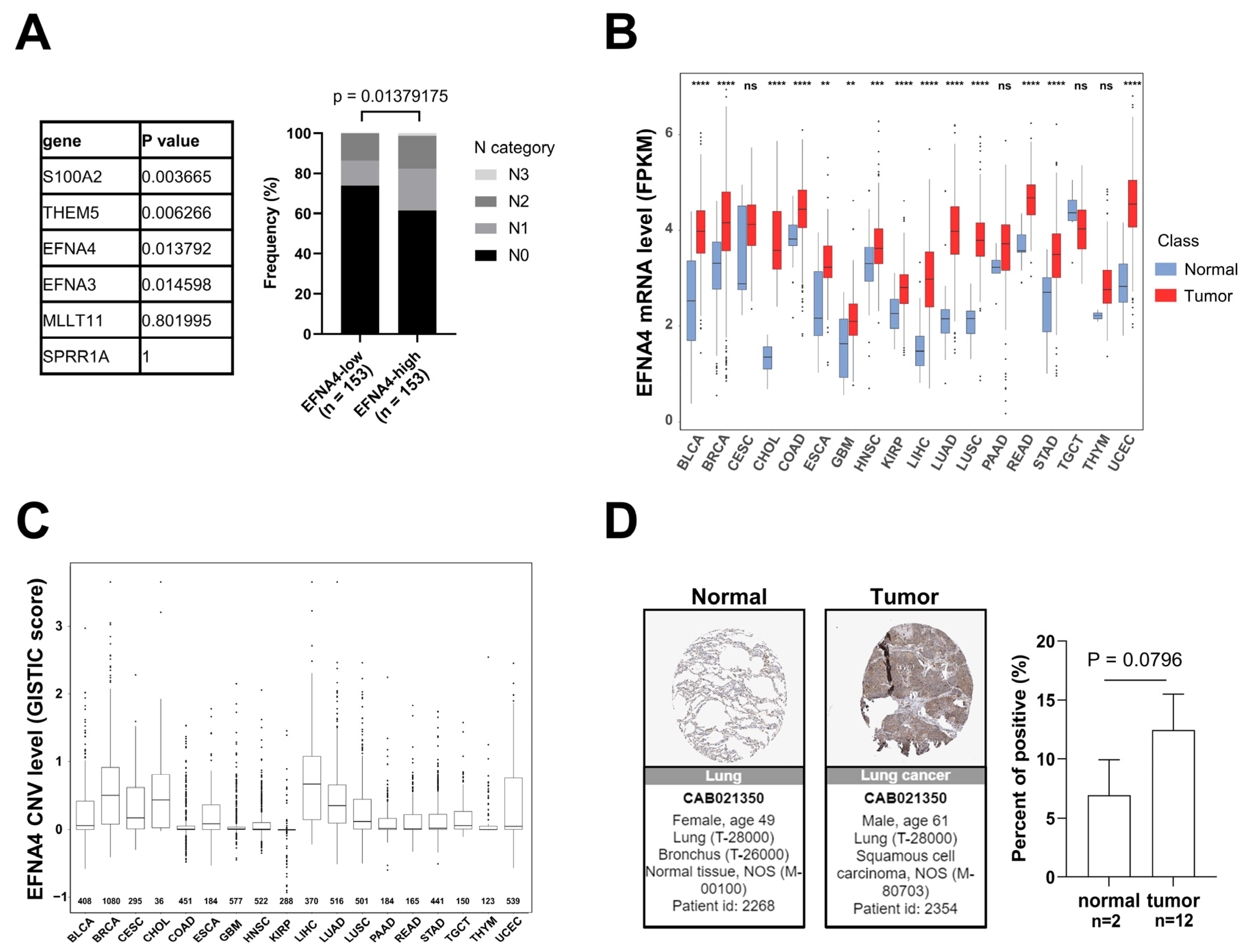
3.1. EFNA4 mRNA Up-Regulation and CNV Amplification in Human Tumors
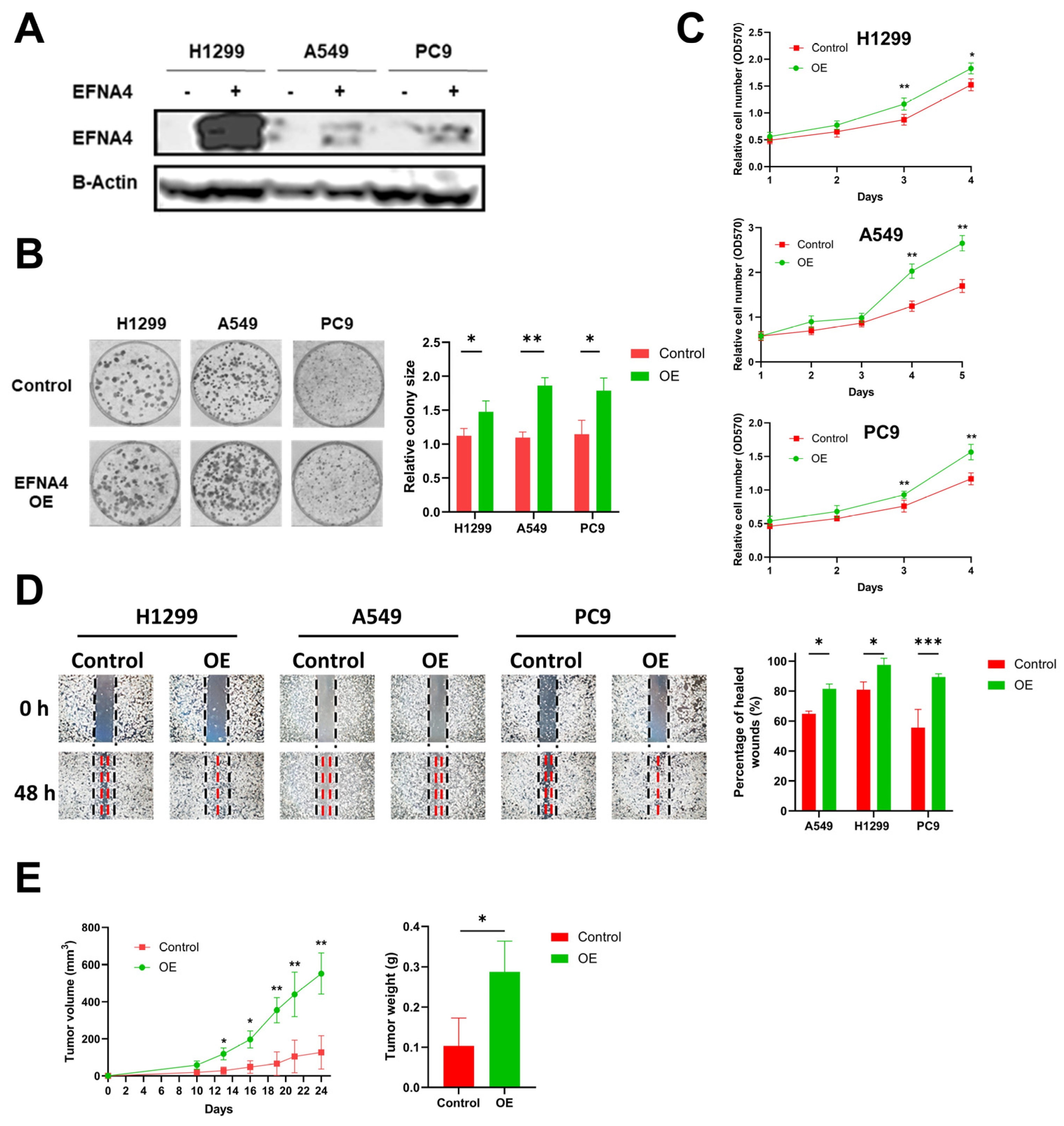
3.2. EFNA4 Overexpression Promotes the Proliferation and Migration of Lung Tumor Cells
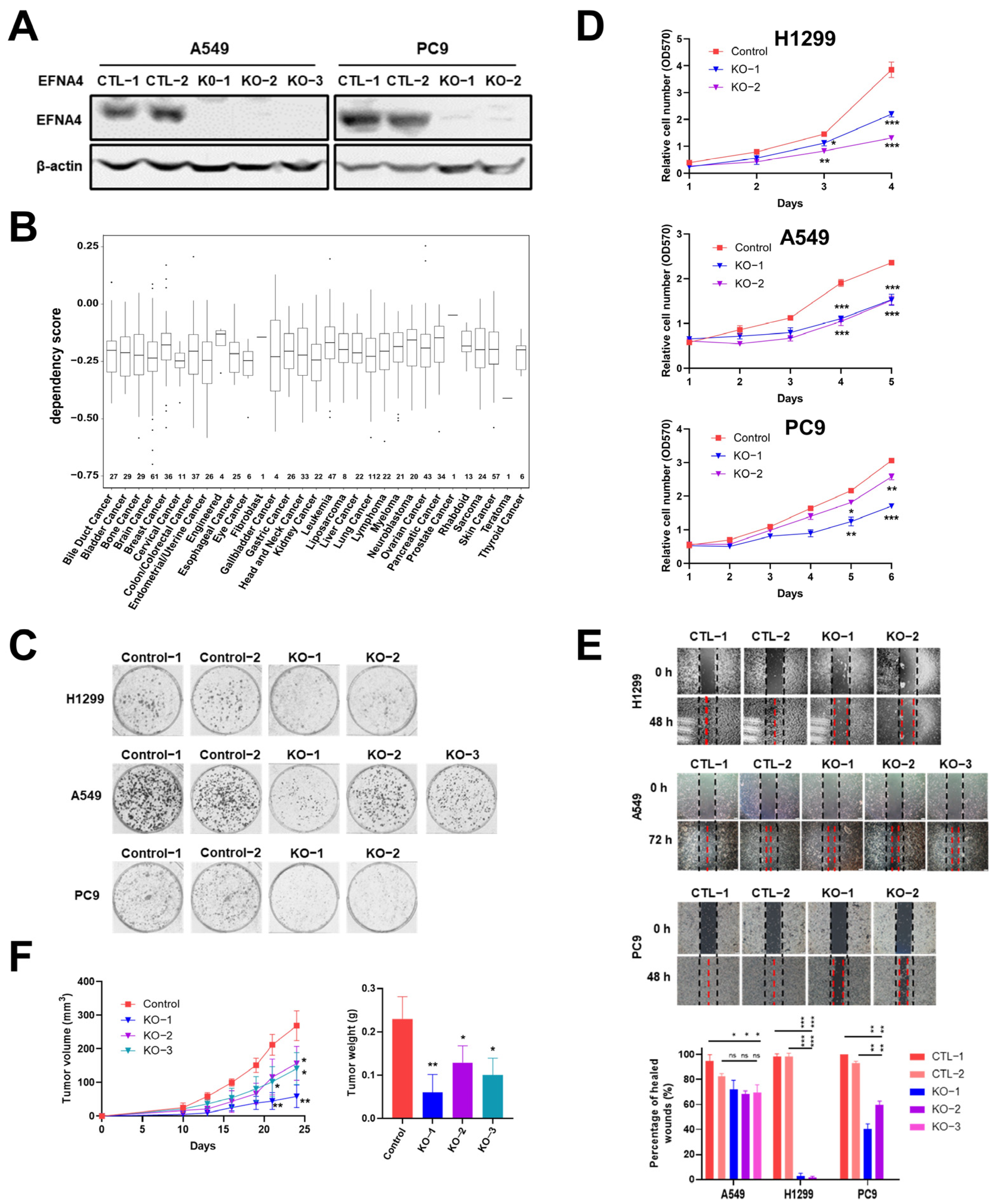
3.3. EFNA4 Depletion Inhibits the Growth and Migration of Lung Tumor Cells
3.4. Ephrin A4 Was Located on the Cell Membrane
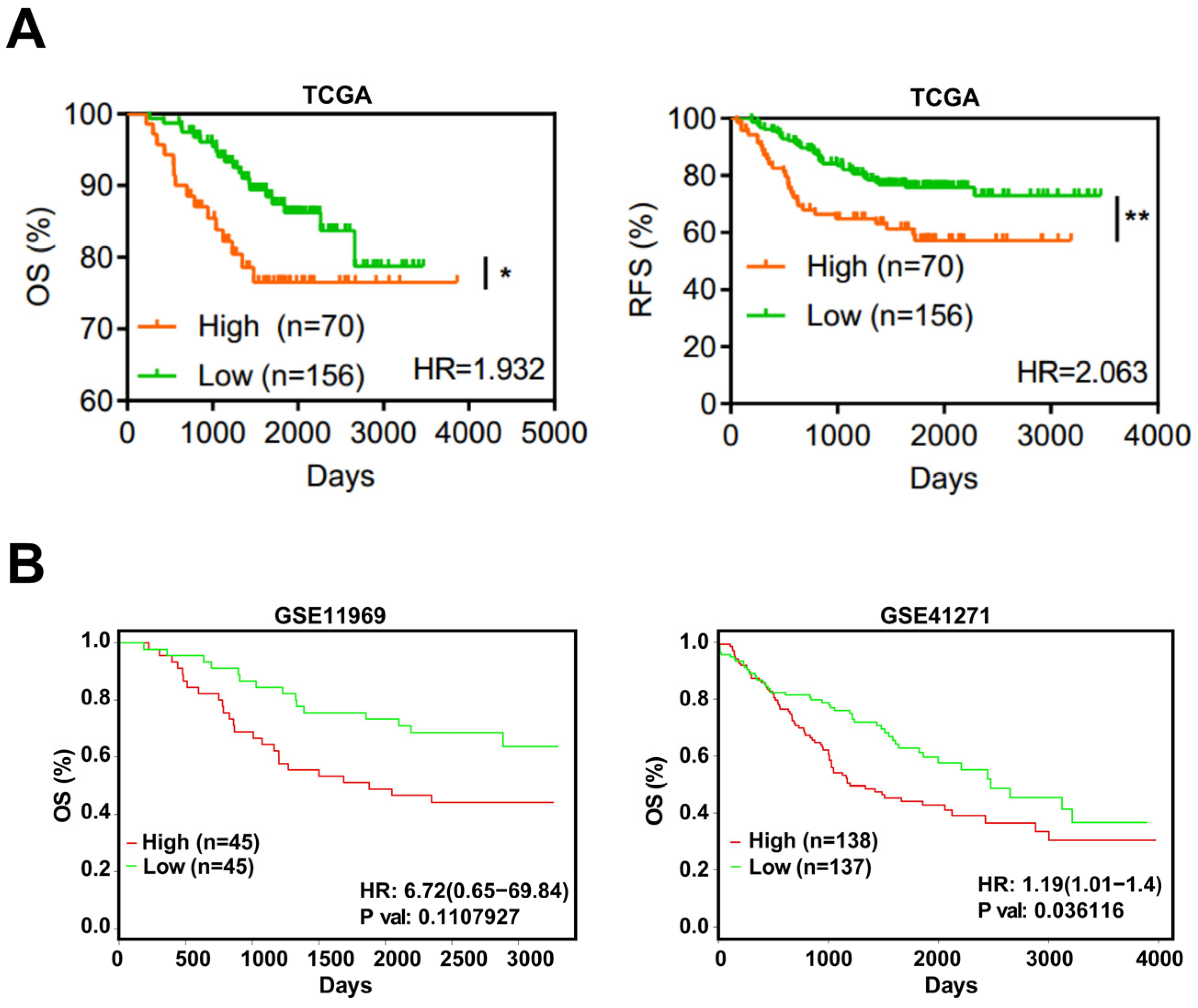
3.5. EFNA4 Could Be a Potential Prognosis Marker in LUAD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhou, X.; Huang, C.; Li, L.; Liu, H.; Liang, N.; Chen, Y.; Ma, D.; Han, Z.; Xu, X.; et al. Lower MiR-340 Expression Predicts Poor Prognosis of Non-Small Cell Lung Cancer and Promotes Cell Proliferation by Targeting CDK4. Gene 2018, 675, 278–284. [Google Scholar] [CrossRef]
- Hankey, B.F.; Ries, L.A.; Edwards, B.K. The Surveillance, Epidemiology, and End Results Program: A National Resource. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1117–1121. [Google Scholar]
- Ettinger, D.S.; Akerley, W.; Bepler, G.; Blum, M.G.; Chang, A.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.P.; et al. Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 740–801. [Google Scholar] [CrossRef]
- Patel, T.S.; Shah, M.G.; Gandhi, J.S.; Patel, P. Accuracy of Cytology in Sub Typing Non Small Cell Lung Carcinomas. Diagn. Cytopathol. 2017, 45, 598–603. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Heyneman, L.E.; Herndon, J.E.; Goodman, P.C.; Patz, E.F. Stage Distribution in Patients with a Small (<or =3 cm) Primary Nonsmall Cell Lung Carcinoma. Implication for Lung Carcinoma Screening. Cancer 2001, 92, 3051–3055. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Chang, Y.-C.; Chen, H.-Y.; Chen, C.-H.; Tsui, H.-C.; Yang, P.-C. Tumor Size Matters Differently in Pulmonary Adenocarcinoma and Squamous Cell Carcinoma. Lung Cancer 2010, 67, 296–300. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of Lymph Nodes and Metastatic Lymph Node Ratio Are Associated with Survival in Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Yan, L.; Wang, L.; Jiang, W.; Zhang, Y.; Xi, J.; Chen, L.; Jin, Y.; Qiao, Y.; Shi, Y.; et al. Identification of Reference MiRNAs in Human Tumors by TCGA MiRNA-Seq Data. Biochem. Biophys. Res. Commun. 2014, 453, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Troup, D.B.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—10 Years On. Nucleic Acids Res. 2011, 39, D1005. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Brückner, K.; Klein, R. Signaling by Eph Receptors and Their Ephrin Ligands. Curr. Opin. Neurobiol. 1998, 8, 375–382. [Google Scholar] [CrossRef]
- Arvanitis, D.; Davy, A. Eph/Ephrin Signaling: Networks. Genes Dev. 2008, 22, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Unified Nomenclature for Eph Family Receptors and Their Ligands, the Ephrins. Eph Nomenclature Committee. Cell 1997, 90, 403–404. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Chen, J. Eph Receptor Tyrosine Kinases in Angiogenesis: From Development to Disease. Angiogenesis 2004, 7, 17–28. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.; Schmidt, S.; Parker, M.; Chen, J. Eph Receptor Tyrosine Kinases in Tumor and Tumor Microenvironment. Curr. Pharm. Des. 2004, 10, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Molecular Mechanisms of Axon Guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Héroult, M.; Schaffner, F.; Augustin, H.G. Eph Receptor and Ephrin Ligand-Mediated Interactions during Angiogenesis and Tumor Progression. Exp. Cell Res. 2006, 312, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Klein, R. Eph/Ephrin Signaling in Morphogenesis, Neural Development and Plasticity. Curr. Opin. Cell Biol. 2004, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Yen, Y.-C.; Jang, C.-W.; Wang, S.-H.; Huang, H.-T.; Chen, C.-H.; Hsiao, J.-R.; Chang, J.-Y.; Chen, Y.-W. Ephrin A4-Ephrin Receptor A10 Signaling Promotes Cell Migration and Spheroid Formation by Upregulating NANOG Expression in Oral Squamous Cell Carcinoma Cells. Sci. Rep. 2021, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, C.; Zhang, J.; Song, Z.; Qi, N.; Liu, X.; Zhang, Z.; Li, A.; Chen, F. EFNA4 Promotes Cell Proliferation and Tumor Metastasis in Hepatocellular Carcinoma through a PIK3R2/GSK3β/β-Catenin Positive Feedback Loop. Mol. Ther.-Nucleic Acids 2021, 25, 328–341. [Google Scholar] [CrossRef]
- Xie, R.; Yuan, M.; Jiang, Y. The Pan-Cancer Crosstalk Between the EFNA Family and Tumor Microenvironment for Prognosis and Immunotherapy of Gastric Cancer. Front. Cell Dev. Biol. 2022, 10, 790947. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, X.; Wang, S.; Bai, D.; Zhao, X.; Han, Y.; Hao, P.; Liu, X.-S. Ggct (γ-Glutamyl Cyclotransferase) Plays an Important Role in Erythrocyte Antioxidant Defense and Red Blood Cell Survival. Br. J. Haematol. 2021, 195, 267–275. [Google Scholar] [CrossRef]
- Ahmadian, S.; Barar, J.; Saei, A.A.; Fakhree, M.A.A.; Omidi, Y. Cellular Toxicity of Nanogenomedicine in MCF-7 Cell Line: MTT Assay. JoVE 2009, 26, e1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiong, Y.; Zhao, L.; Gu, K.; Li, Y.; Zhao, F.; Li, J.; Wang, M.; Wang, H.; Tao, Z.; et al. UCSCXenaShiny: An R/CRAN Package for Interactive Analysis of UCSC Xena Data. Bioinformatics 2021, 38, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A Comprehensive and Easy-to-Use Web Service for Boosting Publication-Ready Biomedical Data Visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Song, M.; Tao, Z.; Wu, T.; He, Z.; Zhao, X.; Wu, K.; Liu, X.-S. Copy Number Signature Analysis Tool and Its Application in Prostate Cancer Reveals Distinct Mutational Processes and Clinical Outcomes. PLoS Genet. 2021, 17, e1009557. [Google Scholar] [CrossRef] [PubMed]
- Mermel, C.H.; Schumacher, S.E.; Hill, B.; Meyerson, M.L.; Beroukhim, R.; Getz, G. GISTIC2.0 Facilitates Sensitive and Confident Localization of the Targets of Focal Somatic Copy-Number Alteration in Human Cancers. Genome Biol. 2011, 12, R41. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Bankovich, A.; Park, A.; Aguilar, J.; Anderson, W.; Santaguida, M.; Aujay, M.; Fong, S.; Khandke, K.; Pulito, V.; et al. Anti-EFNA4 Calicheamicin Conjugates Effectively Target Triple-Negative Breast and Ovarian Tumor-Initiating Cells to Result in Sustained Tumor Regressions. Clin. Cancer Res. 2015, 21, 4165–4173. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Krop, I.; Burris, H.A.; Hamilton, E.; Braiteh, F.; Weise, A.M.; Abu-Khalaf, M.; Werner, T.L.; Pirie-Shepherd, S.; Zopf, C.J.; et al. First-in-Human, Phase I Study of PF-06647263, an Anti-EFNA4 Calicheamicin Antibody-Drug Conjugate, in Patients with Advanced Solid Tumors. Int. J. Cancer 2019, 145, 1798–1808. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Chen, Y.; Sun, X.; He, Z.; Wu, T.; Wu, C.; Chen, J.; Wang, J.; Diao, K.; Liu, X.-S. Oncogenic EFNA4 Amplification Promotes Lung Adenocarcinoma Lymph Node Metastasis. Cancers 2022, 14, 4226. https://doi.org/10.3390/cancers14174226
Zhao X, Chen Y, Sun X, He Z, Wu T, Wu C, Chen J, Wang J, Diao K, Liu X-S. Oncogenic EFNA4 Amplification Promotes Lung Adenocarcinoma Lymph Node Metastasis. Cancers. 2022; 14(17):4226. https://doi.org/10.3390/cancers14174226
Chicago/Turabian StyleZhao, Xiangyu, Yuxing Chen, Xiaoqin Sun, Zaoke He, Tao Wu, Chenxu Wu, Jing Chen, Jinyu Wang, Kaixuan Diao, and Xue-Song Liu. 2022. "Oncogenic EFNA4 Amplification Promotes Lung Adenocarcinoma Lymph Node Metastasis" Cancers 14, no. 17: 4226. https://doi.org/10.3390/cancers14174226
APA StyleZhao, X., Chen, Y., Sun, X., He, Z., Wu, T., Wu, C., Chen, J., Wang, J., Diao, K., & Liu, X.-S. (2022). Oncogenic EFNA4 Amplification Promotes Lung Adenocarcinoma Lymph Node Metastasis. Cancers, 14(17), 4226. https://doi.org/10.3390/cancers14174226





