HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Types of HER2 Alterations
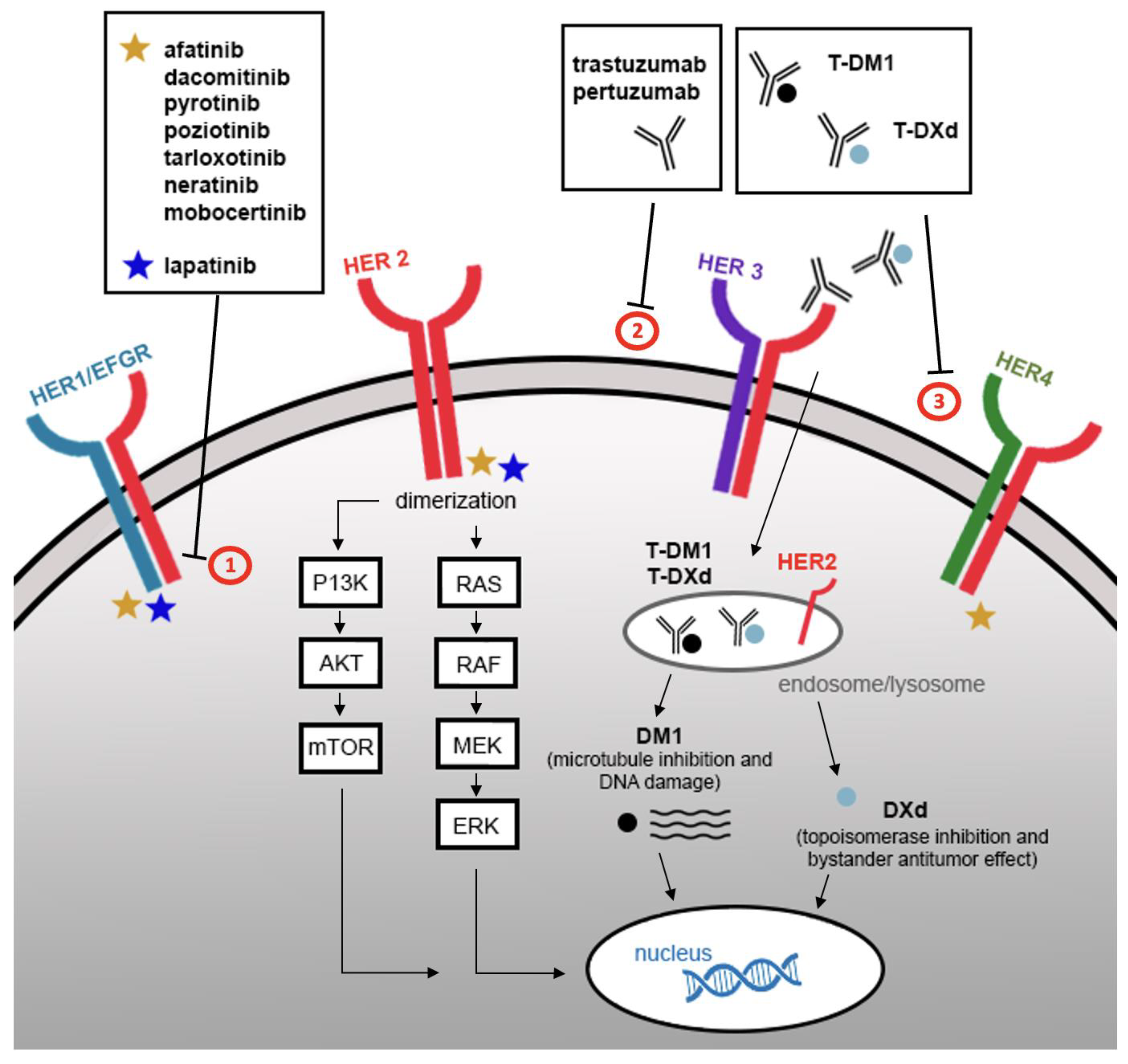
3. Treatment of HER2-Altered NSCLC
3.1. Antibody-Drug Conjugates
3.1.1. Trastuzumab–Deruxtecan (T-DXd)
3.1.2. Ado Trastuzumab–Emtansine (T-DM1)
3.1.3. Summary of Evidence regarding HER2 ADC
3.2. Monoclonal Antibodies
3.2.1. Trastuzumab
| Drug | Trial | NSCLC Population (n) | Overall Response Rate | Median PFS (Months) | Median OS (Months) | Ref |
|---|---|---|---|---|---|---|
| trastuzumab | phase II (HOT1303-B) | HER2 IHC 2/3+ or mutation (n = 10) | 0% | 5.2 | n/a | [24] |
| trastuzumab ± docetaxel | phase II | HER2 IHC 2/3+ (n = 13) | Trastuzumab: 0% Trastuzumab + docetaxel: 0% | n/a a | 5.7 | [25] |
| trastuzumab + cisplatin/ gemcitabine | phase II | HER2 IHC 1+ or HER2 shed antigen level >15 ng/mL by ELISA (n = 21) | 38% | 36 weeks | n/a | [27] |
| trastuzumab + paclitaxel/ carboplatin | phase II (ECOG 2598) | HER2 IHC ≥ 1+ (n = 56) | 24.5% | 3.3 | 10.1 | [26] |
| gemcitabine/cisplatin ± trastuzumab | phase II | HER2 IHC 2/3+, HER2/CEP17 ratio ≥ 2, Serum HER2 ECD >15 ng/mL by ELISA (n = 101) | Control arm: 41% (50% in HER2 IHC 3+) Trastuzumab arm: 36% (83% in HER2 IHC 3+) | Control arm: 7.0 Trastuzumab arm: 6.1 | Control arm: n/r Trastuzumab arm: 12.2 | [28] |
| pertuzumab + trastuzumab + docetaxel | phase II (IFCT-1703 R2D2) | HER2 exon 20 mutation (n = 45) | 29% | 6.8 | n/a | [29] |
3.2.2. Pertuzumab
3.2.3. Summary of Evidence regarding HER2 Monoclonal Antibodies
3.3. Tyrosine Kinase Inhibitors
3.3.1. Pyrotinib
3.3.2. Poziotinib
3.3.3. Mobocertinib
3.3.4. Tarloxotinib
3.3.5. Afatinib
3.3.6. Neratinib
3.3.7. Dacomitinib
3.3.8. Summary of Evidence regarding HER2 TKIs
3.4. Immunotherapy
3.5. Cytotoxic Chemotherapy
4. Practical Treatment Considerations
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Arcila, M.E.; Chaft, J.E.; Nafa, K.; Roy-Chowdhuri, S.; Lau, C.; Zaidinski, M.; Paik, P.K.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 2012, 18, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, M.; Sullivan, I.; Abdayem, P.; Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021, 6, 100260. [Google Scholar] [CrossRef]
- Mazieres, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, W.; Pan, H.; Yang, Y.; Liang, Y.; Yang, L.; Dong, X.; Zhan, J.; Wang, K.; Zhang, L. Conformational Landscapes of HER2 Exon 20 Insertions Explain Their Sensitivity to Kinase Inhibitors in Lung Adenocarcinoma. J. Thorac. Oncol. 2020, 15, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Jebbink, M.; de Langen, A.J.; Boelens, M.C.; Monkhorst, K.; Smit, E.F. The force of HER2—A druggable target in NSCLC? Cancer Treat. Rev. 2020, 86, 101996. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, Y. Targeting HER2 Alterations in Non-Small-Cell Lung Cancer: A Comprehensive Review. JCO Precis. Oncol. 2020, 4, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Feldman, D.; Ni, A.; Myers, M.L.; Lai, W.V.; Pentsova, E.; Boire, A.; Daras, M.; Jordan, E.J.; Solit, D.B.; et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer 2019, 125, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Zhao, C.; Li, X.; Liu, Q.; Mao, S.; Liu, Y.; Yu, X.; Wang, W.; Tian, Q.; et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl. Lung Cancer Res. 2021, 10, 753–765. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, K.A.; Lee, C.Y.; Shim, H.S. The frequency and clinical impact of HER2 alterations in lung adenocarcinoma. PLoS ONE 2017, 12, e0171280. [Google Scholar] [CrossRef]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Brabender, J.; Danenberg, K.D.; Metzger, R.; Schneider, P.M.; Park, J.; Salonga, D.; Holscher, A.H.; Danenberg, P.V. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin. Cancer Res. 2001, 7, 1850–1855. [Google Scholar]
- Vallbohmer, D.; Brabender, J.; Yang, D.Y.; Danenberg, K.; Schneider, P.M.; Metzger, R.; Holscher, A.H.; Danenberg, P.V. Sex differences in the predictive power of the molecular prognostic factor HER2/neu in patients with non-small-cell lung cancer. Clin. Lung Cancer 2006, 7, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Davies, A.M.; Natale, R.B.; Dang, T.P.; Schiller, J.H.; Garland, L.L.; Miller, V.A.; Mendelson, D.; Van den Abbeele, A.D.; Melenevsky, Y.; et al. Efficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin. Cancer Res. 2007, 13, 6175–6181. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Mileshkin, L.; Townley, P.; Gitlitz, B.; Eaton, K.; Mitchell, P.; Hicks, R.; Wood, K.; Amler, L.; Fine, B.M.; et al. Pertuzumab and erlotinib in patients with relapsed non-small cell lung cancer: A phase II study using 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging. Oncologist 2014, 19, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Janne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nagasaka, M.; Felip, E.; Pacheco, J.; Baik, C.; Goto, Y.; Saltos, A.; Li, B.; Udagawa, H.; Gadgeel, S.; et al. Trastuzumab Deruxtecan in HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Interim Results of DESTINY-Lung01. J. Thorac. Oncol. 2021, 16, S109–S110. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients with HER2-Mutant Lung Cancers: Results from a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Iwama, E.; Zenke, Y.; Sugawara, S.; Daga, H.; Morise, M.; Yanagitani, N.; Sakamoto, T.; Murakami, H.; Kishimoto, J.; Matsumoto, S.; et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur. J. Cancer 2022, 162, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, I.; Goda, T.; Watanabe, K.; Maemondo, M.; Oizumi, S.; Amano, T.; Hatanaka, Y.; Matsuno, Y.; Nishihara, H.; Asahina, H.; et al. A phase II study of trastuzumab monotherapy in pretreated patients with non-small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303-B trial. Ann. Oncol. 2018, 29, viii540. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Laptalo, L.; Longmate, J.; Lau, D.H.; Gandour-Edwards, R.; Gumerlock, P.H.; Doroshow, J.H.; Gandara, D.R. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: A California Cancer Consortium screening and phase II trial. Clin. Lung Cancer 2004, 5, 231–236. [Google Scholar] [CrossRef]
- Langer, C.; Stephenson, P.; Thor, A.; Vangel, M.; Johnson, D.H. Trastuzumab in the Treatment of Advanced Non-Small-Cell Lung Cancer: Is There a Role? Focus on Eastern Cooperative Oncology Group Study 2598. Lung Cancer 2004, 22, 1180–1187. [Google Scholar] [CrossRef]
- Zinner, R.G.; Glisson, B.S.; Fossella, F.V.; Pisters, K.M.; Kies, M.S.; Lee, P.M.; Massarelli, E.; Sabloff, B.; Fritsche, H.A., Jr.; Ro, J.Y.; et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: Report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 2004, 44, 99–110. [Google Scholar]
- Gatzemeier, U.; Groth, G.; Butts, C.; Van Zandwijk, N.; Shepherd, F.; Ardizzoni, A.; Barton, C.; Ghahramani, P.; Hirsh, V. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann. Oncol. 2004, 15, 19–27. [Google Scholar] [CrossRef]
- Mazieres, J.; Lafitte, C.; Ricordel, C.; Greillier, L.; Pujol, J.-L.; Zalcman, G.; Domblides, C.; Madelaine, J.; Bennouna, J.; Mascaux, C.; et al. Combination of trastuzumab, pertuzumab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC) harboring HER2 mutation: Final results from the IFCT-1703 R2D2 trial. J. Clin. Oncol. 2021, 39, 9015. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, T.; Qin, Z.; Jiang, J.; Wang, Q.; Yang, S.; Rivard, C.; Gao, G.; Ng, T.L.; Tu, M.M.; et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann. Oncol. 2019, 30, 447–455. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma after Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Y.; Chen, S.; Ying, S.; Xu, S.; Huang, J.; Wu, D.; Lv, D.; Bei, T.; Liu, S.; et al. Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: A multicenter, single-arm, phase II trial. BMC Med. 2022, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lv, D.; Chen, S.Q.; Huang, J.; Li, Y.; Ying, S.; Wu, X.; Hua, F.; Wang, W.; Xu, C.; et al. Pyrotinib in Patients with HER2-Amplified Advanced Non-Small Cell Lung Cancer: A Prospective, Multicenter, Single-Arm Trial. Clin. Cancer Res. 2022, 28, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; Altan, M.; Gibbons, D.L.; Fossella, F.V.; Lam, V.K.; Patel, A.B.; Negrao, M.V.; Le, X.; et al. Poziotinib for Patients with HER2 Exon 20 Mutant Non-Small-Cell Lung Cancer: Results from a Phase II Trial. J. Clin. Oncol. 2022, 40, 702–709. [Google Scholar] [CrossRef]
- Le, X.; Cornelissen, R.; Garassino, M.; Clarke, J.M.; Tchekmedyian, N.; Goldman, J.W.; Leu, S.Y.; Bhat, G.; Lebel, F.; Heymach, J.V.; et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations after Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Liu, S.V.; Villaruz, L.C.; Lee, V.H.F.; Zhu, V.W.; Baik, C.S.; Sacher, A.; McCoach, C.E.; Nguyen, D.; Li, J.Y.-C.; Pacheco, J.M.; et al. First analysis of RAIN-701: Study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2-activating mutations & other solid tumours with NRG1/ERBB gene fusions. Ann. Oncol. 2020, 31, S1189. [Google Scholar]
- Dziadziuszko, R.; Smit, E.F.; Dafni, U.; Wolf, J.; Wasag, B.; Biernat, W.; Finn, S.P.; Kammler, R.; Tsourti, Z.; Rabaglio, M.; et al. Afatinib in NSCLC with HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J. Thorac. Oncol. 2019, 14, 1086–1094. [Google Scholar] [CrossRef]
- De Greve, J.; Moran, T.; Graas, M.P.; Galdermans, D.; Vuylsteke, P.; Canon, J.L.; Schallier, D.; Decoster, L.; Teugels, E.; Massey, D.; et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 2015, 88, 63–69. [Google Scholar] [CrossRef]
- Gandhi, L.; Besse, B.; Mazieres, J.; Waqar, S.; Cortot, A.; Barlesi, F.; Quoix, E.; Otterson, G.; Ettinger, D.; Horn, L.; et al. Neratinib ± Temsirolimus in HER2-Mutant Lung Cancers: An International, Randomized Phase II Study. J. Thorac. Oncol. 2017, 12, S358–S359. [Google Scholar] [CrossRef]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.T.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Robichaux, J.P.; Elamin, Y.Y.; Tan, Z.; Carter, B.W.; Zhang, S.; Liu, S.; Li, S.; Chen, T.; Poteete, A.; Estrada-Bernal, A.; et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat. Med. 2018, 24, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Kobayashi, Y.; Tomizawa, K.; Suda, K.; Kosaka, T.; Sesumi, Y.; Fujino, T.; Nishino, M.; Ohara, S.; Chiba, M.; et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study. Lung Cancer 2018, 126, 72–79. [Google Scholar] [CrossRef]
- Pasi, A.J.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Horn, L.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; et al. Antitumor activity of TAK-788 in NSCLC with EGFR exon 20 insertions. Ann. Oncol. 2019, 30, vi108. [Google Scholar]
- Han, H.; Li, S.; Chen, T.; Fitzgerald, M.; Liu, S.; Peng, C.; Tang, K.H.; Cao, S.; Chouitar, J.; Wu, J.; et al. Targeting HER2 Exon 20 Insertion-Mutant Lung Adenocarcinoma with a Novel Tyrosine Kinase Inhibitor Mobocertinib. Cancer Res. 2021, 81, 5311–5324. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; Bazhenova, L.; et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov. 2021, 11, 1688–1699. [Google Scholar] [CrossRef]
- Koga, T.; Suda, K.; Nishino, M.; Fujino, T.; Ohara, S.; Hamada, A.; Soh, J.; Tirunagaru, V.; Vellanki, A.; Doebele, R.C.; et al. Activity and mechanism of acquired resistance to tarloxotinib in HER2 mutant lung cancer: An in vitro study. Transl. Lung Cancer Res. 2021, 10, 3659–3670. [Google Scholar] [CrossRef]
- Nagano, M.; Kohsaka, S.; Ueno, T.; Kojima, S.; Saka, K.; Iwase, H.; Kawazu, M.; Mano, H. High-Throughput Functional Evaluation of Variants of Unknown Significance in ERBB2. Clin. Cancer Res. 2018, 24, 5112–5122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, C.; Ibrahim, Y.H.; Serra, V.; Calvo, M.T.; Guzman, M.; Grueso, J.; Aura, C.; Perez, J.; Jessen, K.; Liu, Y.; et al. Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin. Cancer Res. 2012, 18, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.A.; Li, D.; Shimamura, T.; Raso, M.G.; Ji, H.; Chen, L.; Borgman, C.L.; Zaghlul, S.; Brandstetter, K.A.; Kubo, S.; et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 474–479. [Google Scholar] [CrossRef]
- Besse, B.; Soria, J.-C.; Yao, B.; Kris, M.; Chao, B.; Cortot, A.; Mazieres, J.; Socinski, M.; Horn, L.; Waqar, S.; et al. Neratinib (N) with or without temsirolimus (TEM) in patients (Pts) with non-small cell lung cancer (NSCLC) carrying HER2 somatic mutations: An international randomized phase II study. Ann. Oncol. 2014, 25, v1. [Google Scholar] [CrossRef]
- Janne, P.A.; Ou, S.I.; Kim, D.W.; Oxnard, G.R.; Martins, R.; Kris, M.G.; Dunphy, F.; Nishio, M.; O’Connell, J.; Paweletz, C.; et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 1433–1441. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Zhang, J.T.; Liu, S.Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.Y.; Xu, C.R.; Yan, L.X.; et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017, 6, e1356145. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Wei-Chu, V.L.; Feldman, D.L.; Buonocore, D.J.; Brzostowski, E.B.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Sabari, J.K.; Offin, M.D.; Kris, M.G.; et al. PD-L1 expression, tumor mutation burden and response to immune checkpoint blockade in patients with HER2-mutant lung cancers. J. Clin. Oncol. 2018, 36, 6090. [Google Scholar]
- Guisier, F.; Dubos-Arvis, C.; Vinas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC with BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020, 15, 628–636. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Du, Y.; Li, X.; Ying, L.; Lu, Y.; Shen, B.; Gao, X.; Yi, X.; Xia, X.; et al. Associations of HER2 Mutation with Immune-Related Features and Immunotherapy Outcomes in Solid Tumors. Front. Immunol. 2022, 13, 799988. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pan, G.; Cheng, G.; Zhang, F.; Xu, Y.; Huang, Z.; Fan, Y. Immune microenvironment features and efficacy of PD-1/PD-L1 blockade in non-small cell lung cancer patients with EGFR or HER2 exon 20 insertions. Thorac. Cancer 2021, 12, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Wu, F.; Zhao, J.; Li, X.; Zhao, C.; Ren, S.; Zhou, C. Outcomes of Pemetrexed-based chemotherapies in HER2-mutant lung cancers. BMC Cancer 2018, 18, 326. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ligorio, C.; Toschi, L.; Rossi, E.; Trisolini, R.; Paioli, D.; Magrini, E.; Finocchiaro, G.; Bartolini, S.; Cancellieri, A.; et al. EGFR and HER2 gene copy number and response to first-line chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2007, 2, 423–429. [Google Scholar] [CrossRef]
- Graziano, S.L.; Tatum, A.; Herndon, J.E., II; Box, J.; Memoli, V.; Green, M.R.; Kern, J.A. Use of neuroendocrine markers, p53, and HER2 to predict response to chemotherapy in patients with stage III non-small cell lung cancer: A Cancer and Leukemia Group B study. Lung Cancer 2001, 33, 115–123. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, N.; Xu, X.; Zhang, Y.; Ye, M.; Li, C.; Hu, J. Clinical outcomes of patients with HER2-mutant advanced lung cancer: Chemotherapies versus HER2-directed therapies. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936090. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, J.; Ying, J.; Mitsudomi, T.; Lee, D.H.; Wang, Z.; Chu, Q.; Mack, P.C.; Cheng, Y.; Duan, J.; et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef] [PubMed]
- Mack, P.C.; Banks, K.C.; Espenschied, C.R.; Burich, R.A.; Zill, O.A.; Lee, C.E.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: Analysis of over 8000 cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Waters, N.; Patel, M.R.; Schram, A.M.; Ahnert, J.R.; Jauhari, S.; Sachdev, J.C.; Zhu, V.W.; LoRusso, P.; Nguyen, D.; Hong, D.S.; et al. Clinical pharmacokinetics of bdtx-189, an inhibitor of allosteric ErbB mutations, in patients with advanced solid malignancies in MasterKey-01 study. J. Clin. Oncol. 2021, 39, 3097. [Google Scholar] [CrossRef]
- Yang, J.C.; Wang, M.; Mitchell, P.; Fang, J.; Nian, W.; Chiu, C.-H.; Zhou, J.; Zhao, Y.; Su, W.-C.; Camidge, D.R.; et al. Preliminary safety and efficacy results from phase 1 studies of DZD9008 in NSCLC patients with EGFR exon 20 insertion mutations. J. Clin. Oncol. 2021, 39, 9008. [Google Scholar] [CrossRef]
| Drug | Trial | Tumor Types | NSCLC Population (n) | Overall Response Rate | Median PFS (Months) | Median OS (Months) | Ref |
|---|---|---|---|---|---|---|---|
| T-DXd | phase I | NSCLC, colorectal, salivary gland, breast, esophageal, endometrial, Paget’s disease, biliary tract, pancreatic, cervical, extraskeletal myxoid chondrosarcoma, small intestine adenocarcinoma | HER2 IHC ≥ 1+ or HER2 mutation (NSCLC n = 18; exon 20 NSCLC n = 8) | Overall NSCLC: 55.6% HER2 mutant: 72.7% | Overall NSCLC: 11.3 HER2 mutant: 11.3 | Overall NSCLC: n/r HER2 mutant: 17.3 | [17] |
| T-Dxd | phase II (DESTINY-Lung01) | NSCLC | HER2 IHC 2/3+ (n = 49) | 24.5% | 5.4 | n/a | [18] |
| T-DXd | phase II (DESTINY-Lung01) | NSCLC | HER2 mutation (n = 91; exon 20 = 78) | 55% | 8.2 | 17.8 | [19] |
| T-DM1 | phase II | NSCLC | HER2 mutation (n = 18; exon 20 = 11) | 44% | 5.0 | n/a | [20] |
| T-DM1 | phase II | NSCLC | HER2 (IHC 3+, IHC 2+ and FISH HER2/CEP17 ratio ≥ 2, or exon 20 mutation) (n = 15, IHC/FISH+ n = 8, exon 20 n = 7) | Overall: 6.7% IHC/FISH-positive: 0% Exon 20: 14.3% | 2.0 | 10.9 | [21] |
| T-DM1 | phase II | NSCLC | HER2 IHC 2/3+ (n = 49) | IHC 2+: 0% IHC 3+: 20% | IHC 2+: 2.6 IHC 3+: 2.7 | IHC 2+: 12.2 IHC 3+: 15.3 | [22] |
| T-DM1 | phase II | NSCLC | HER2 exon 20 mutation (n = 22) | 38.1% | 2.8 | 8.1 | [23] |
| Drug | Trial | NSCLC population (n) | Overall Response Rate | Median PFS (Months) | Median OS (Months) | Ref |
|---|---|---|---|---|---|---|
| pyrotinib | phase II | HER2 exon 20 mutation (n = 15) | 53.3% | 6.4 | n/a | [30] |
| pyrotinib | phase II | HER2 mutation (n = 60; HER2 exon 20 mutation n = 56) | 30% | 6.9 | 14.4 | [31] |
| pyrotinib | phase II | HER2 mutation (n = 78, HER2 exon 20 mutation = 62) | 19.2% | 5.6 | 10.5 | [32] |
| pyrotinib | phase II | HER2 amplification (n = 27) | 22.2% | 6.3 | 12.5 | [33] |
| poziotinib | phase II | HER2 exon 20 mutation (n = 30) | 27% | 5.5 | 15 | [34] |
| poziotinib | phase II (ZENITH20) | HER2 exon 20 mutation (n = 90) | 27.8% | 5.5 | n/a | [35] |
| tarloxotinib | phase II (RAIN-701) | EGFR Exon 20 insertion, HER2 activating mutation, or any solid tumors with NRG1, EGFR, HER2 or HER4 fusion (n = 23; HER2 n = 11) | HER2 cohort: 22% | n/a | n/a | [36] |
| afatinib | phase II (NICHE) | HER2 exon 20 mutation(n = 13) | 8% | 15.9 weeks | 56.0 weeks | [37] |
| afatinib ± paclitaxel | phase II | EGFR and HER2 (n = 41; HER2 exon 20 mutation n = 7) | afatinib HER2: 0% afatinib + paclitaxel HER2: 33.3% | afatinib HER2: 17 weeks afatinib + paclitaxel (EGFR and HER2): 6.7 weeks | n /a | [38] |
| neratinib ± TEM | phase II (PUMA-NER-4201) | HER2 exon 20 mutation (n = 60) | neratinib: 0% neratinib + TEM: 19% | neratinib: 3.0 neratinib + TEM: 4.1 | neratinib: 10.0 neratinib + TEM: 15.8 | [39] |
| dacomitinib | phase II | HER2 exon 20 mutation (n = 26) or amplification (n = 4) | HER2 exon 20 mutation: 12% HER2 amplification: 0% | HER2 exon 20 mutation: 3.0 HER2 amplification: n/a a | HER2 exon 20 mutation: 9.0 HER2 amplification: n/a a | [40] |
| Drug | Mechanism of Action | Development Phase | Sponsor | NCT Number |
|---|---|---|---|---|
| Trastuzumab Deruxtecan | ADC | phase III (DESTINY-Lung04) | AstraZeneca | NCT05048797 |
| Pertuzumab + Trastuzumab + Docetaxel | Monoclonal antibody | phase II | Intergroupe Francophone de Cancerologie Thoracique | NCT03845270 |
| Pyrotinib | TKI | phase III (PYRAMID-1) | Jiangsu HengRui Medicine Co | NCT04447118 |
| Pyrotinib + Thalidomide | TKI | phase II | Shanghai Chest Hospital | NCT04382300 |
| Pyrotinib | TKI | phase II | Tianjin Medical University Cancer Institute and Hospital | NCT04063462 |
| Poziotinib | TKI | phase III (PINNACLE) | Spectrum Pharmaceuticals | NCT05378763 |
| Poziotinib | TKI | phase II | MD Anderson Cancer Center | NCT03066206 |
| Poziotinib | TKI | phase II | Spectrum Pharmaceuticals | NCT03318939 |
| Mobocertinib | TKI | phase I/II | Takeda | NCT02716116 |
| BDTX-189 (tuxobertinib) | TKI | phase I/II(MasterKey-01) | Black Diamond Therapeutics | NCT04209465 |
| DZD9008 (sunvozertinib) | TKI | phase I/II (WU-KONG1) | Dizal Pharmaceuticals | NCT03974022 |
| AST2818 (furmonertinib) | TKI | phase I | ArriVent BioPharma | NCT05364073 |
| BAY2927088 | TKI | phase I | Bayer | NCT05099172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers 2022, 14, 4155. https://doi.org/10.3390/cancers14174155
Uy NF, Merkhofer CM, Baik CS. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers. 2022; 14(17):4155. https://doi.org/10.3390/cancers14174155
Chicago/Turabian StyleUy, Natalie F., Cristina M. Merkhofer, and Christina S. Baik. 2022. "HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies" Cancers 14, no. 17: 4155. https://doi.org/10.3390/cancers14174155
APA StyleUy, N. F., Merkhofer, C. M., & Baik, C. S. (2022). HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers, 14(17), 4155. https://doi.org/10.3390/cancers14174155







