Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Histopathologic Evaluation and FISH-Based Classification
2.3. HER2 mRNA Expression by qRT-PCR
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
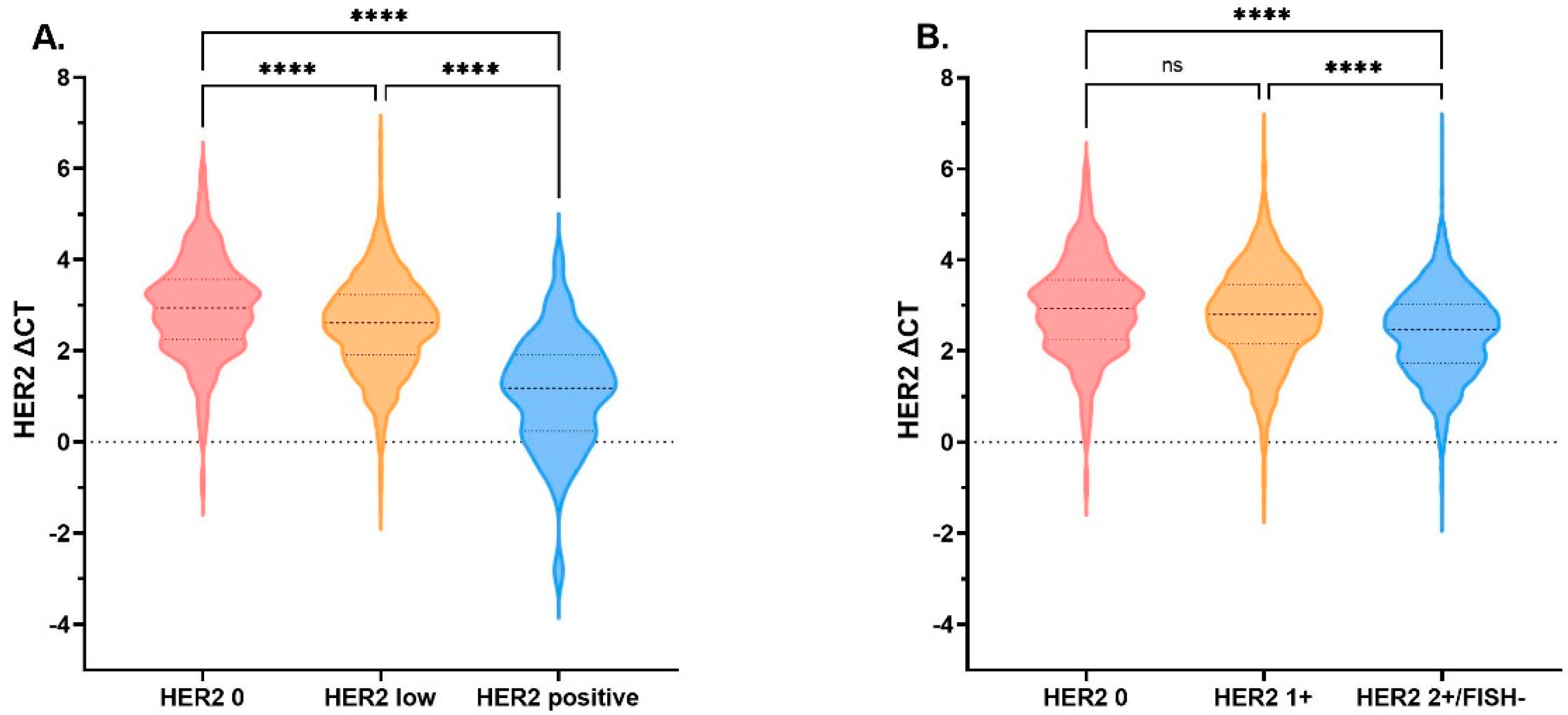
3.2. HER2 mRNA Expression Comparison by HER2 IHC/ FISH Status
3.3. Concordance between IHC/FISH and qRT-PCR for HER2 Assessment
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Gelber, R.D.; Piccart-Gebhart, M.J.; de Azambuja, E.; Procter, M.; Suter, T.M.; Jackisch, C.; Cameron, D.; Weber, H.A.; Heinzmann, D.; et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet 2013, 382, 1021–1028. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Deimling, D.; Kaltz, C.; Sendelhofert, A.; Iff, A.; Luthardt, B.; Untch, M.; Lohrs, U. Her-2/neu analysis in archival tissue samples of human breast cancer: Comparison of immunohistochemistry and fluorescence in situ hybridization. J. Clin. Oncol. 2001, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Sauter, G.; Bernstein, L.; Villalobos, I.E.; Mirlacher, M.; Zhou, J.Y.; Wardeh, R.; Li, Y.T.; Guzman, R.; Ma, Y.; et al. Diagnostic evaluation of HER-2 as a molecular target: An assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin. Cancer Res. 2005, 11, 6598–6607. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Braso-Maristany, F.; Pare, L.; Pascual, T.; Conte, B.; Martinez-Saez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Marchio, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, C.; Li, C.; Wang, Y.; Chen, B.; Wen, L.; Jia, M.; Li, K.; Mok, H.; Cao, L.; et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022, 20, 142. [Google Scholar] [CrossRef]
- Eiger, D.; Agostinetto, E.; Saude-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Lambein, K.; Van Bockstal, M.; Vandemaele, L.; Geenen, S.; Rottiers, I.; Nuyts, A.; Matthys, B.; Praet, M.; Denys, H.; Libbrecht, L. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: Clinical and pathobiological relevance. Am. J. Clin. Pathol. 2013, 140, 561–566. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Gong, Y.; Yan, K.; Lin, F.; Anderson, K.; Sotiriou, C.; Andre, F.; Holmes, F.A.; Valero, V.; Booser, D.; Pippen, J.E., Jr.; et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: A gene-expression profiling study. Lancet Oncol. 2007, 8, 203–211. [Google Scholar] [CrossRef]
- Wasserman, B.E.; Carvajal-Hausdorf, D.E.; Ho, K.; Wong, W.; Wu, N.; Chu, V.C.; Lai, E.W.; Weidler, J.M.; Bates, M.; Neumeister, V.; et al. High concordance of a closed-system, RT-qPCR breast cancer assay for HER2 mRNA, compared to clinically determined immunohistochemistry, fluorescence in situ hybridization, and quantitative immunofluorescence. Lab. Investig. 2017, 97, 1521–1526. [Google Scholar] [CrossRef]
- Baehner, F.L.; Achacoso, N.; Maddala, T.; Shak, S.; Quesenberry, C.P., Jr.; Goldstein, L.C.; Gown, A.M.; Habel, L.A. Human epidermal growth factor receptor 2 assessment in a case-control study: Comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J. Clin. Oncol. 2010, 28, 4300–4306. [Google Scholar] [CrossRef]
- Noske, A.; Loibl, S.; Darb-Esfahani, S.; Roller, M.; Kronenwett, R.; Muller, B.M.; Steffen, J.; von Toerne, C.; Wirtz, R.; Baumann, I.; et al. Comparison of different approaches for assessment of HER2 expression on protein and mRNA level: Prediction of chemotherapy response in the neoadjuvant GeparTrio trial (NCT00544765). Breast Cancer Res. Treat. 2011, 126, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Chen, X.; Fei, X.; Lin, L.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; Li, Y.; et al. Can breast cancer patients with HER2 dual-equivocal tumours be managed as HER2-negative disease? Eur. J. Cancer 2018, 89, 9–18. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Li, Y.; Chen, W.; Fei, X.; Chen, X.; Shen, K. Clinicopathological Features and Disease Outcome in Breast Cancer Patients with Hormonal Receptor Discordance between Core Needle Biopsy and Following Surgical Sample. Ann. Surg. Oncol. 2019, 26, 2779–2786. [Google Scholar] [CrossRef]
- Gao, W.; Wu, J.; Chen, X.; Lin, L.; Fei, X.; Shen, K.; Huang, O. Clinical validation of Ki67 by quantitative reverse transcription-polymerase chain reaction (RT-PCR) in HR+/HER2- early breast cancer. J. Cancer 2019, 10, 1110–1116. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, m. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Wu, J.; Fang, Y.; Lin, L.; Fei, X.; Gao, W.; Zhu, S.; Zong, Y.; Chen, X.; Huang, O.; He, J.; et al. Distribution patterns of 21-gene recurrence score in 980 Chinese estrogen receptor-positive, HER2-negative early breast cancer patients. Oncotarget 2017, 8, 38706–38716. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Chen, X.; Fei, C.; Lin, L.; Chen, W.; Huang, O.; Zhu, S.; He, J.; Li, Y.; et al. Prognostic value of the 21-gene recurrence score in ER-positive, HER2-negative, node-positive breast cancer was similar in node-negative diseases: A single-center study of 800 patients. Front Med. 2021, 15, 621–628. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Garrett-Mayer, E.; White, J.; Blinder, V.S.; Foster, J.C.; Amiri-Kordestani, L.; Hwang, E.S.; Bliss, J.M.; Rakovitch, E.; Perlmutter, J.; et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in Adjuvant Breast Cancer Clinical Trials: STEEP Version 2.0. J. Clin. Oncol. 2021, 39, 2720–2731. [Google Scholar] [CrossRef]
- Perez, J.; Garrigos, L.; Gion, M.; Janne, P.A.; Shitara, K.; Siena, S.; Cortes, J. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer and beyond. Expert Opin. Biol. Ther. 2021, 21, 811–824. [Google Scholar] [CrossRef]
- Modi, S.; Ohtani, S.; Lee, C.; Wang, Y.; Saxena, K.; Cameron, D.A. A phase 3, multicenter, randomized, open-label trial of trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice in HER2-low breast cancer (DESTINY-Breast04). Cancer Res. 2020, 80. [Google Scholar] [CrossRef]
- Enhertu Significantly Improved Both Progression-Free and Overall Survival in DESTINY-Breast04 Trial in Patients with HER2-Low Metastatic Breast Cancer. Available online: https://www.astrazeneca.com/media-centre/press-releases/2022/enhertu-improves-pfs-and-os-in-her2-low-bc.html (accessed on 9 August 2022).
- Bardia, A.; Barrios, C.; Dent, R.; Hu, X.C.; O’Shaughnessy, J.; Yonemori, K.; Darilay, A.; Boston, S.; Liu, Y.F.; Patel, G.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: A randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021, 81. [Google Scholar] [CrossRef]
- Dieras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.Y.; Viret, F.; Levy, C.; Salabert, L.; Le Du, F.; et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82, PD8-02. [Google Scholar] [CrossRef]
- Mosele, F.; Lusque, A.; Dieras, V.; Deluche, E.; Ducoulombier, A.; Pistill, B.; Bachelot, T. Unraveling the mechanism of action and resistance to Tratuzumab deruxtecan (T-Dxd): Biomarker analyses from patients from DAISY trail. In Proceedings of the ESMO Breast Cancer Annual Congress, Brelin, Germany, 3–5 May 2022. [Google Scholar]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; et al. RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with HER2-Positive and HER2-Low Expressing Advanced or Metastatic Breast Cancer: A Pooled Analysis of Two Studies. Available online: https://meetinglibrary.asco.org/record/197094/abstract (accessed on 9 August 2022).
- Ruschoff, J.; Lebeau, A.; Kreipe, H.; Sinn, P.; Gerharz, C.D.; Koch, W.; Morris, S.; Ammann, J.; Untch, M.; Nicht-interventionelle Untersuchung, H.E.R.S.G. Assessing HER2 testing quality in breast cancer: Variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod. Pathol. 2017, 30, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Pfitzner, B.M.; Lederer, B.; Lindner, J.; Solbach, C.; Engels, K.; Rezai, M.; Dohnal, K.; Tesch, H.; Hansmann, M.L.; Salat, C.; et al. Clinical relevance and concordance of HER2 status in local and central testing-an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod. Pathol. 2018, 31, 607–615. [Google Scholar] [CrossRef]
- Penault-Llorca, F. Is HER2 low breast cancer a real entity? In Proceedings of the ESMO Breast Cancer, Berlin, Germany, 3–5 May 2022. [Google Scholar]
- Zhang, H.; Katerji, H.; Turner, B.M.; Audeh, W.; Hicks, D.G. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. 2022, 35, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Bayani, J.; Mallon, E.; Pond, G.R.; Piper, T.; Hasenburg, A.; Markopoulos, C.J.; Dirix, L.; Seynaeve, C.M.; van de Velde, C.J.H.; et al. Discordance between Immunohistochemistry and ERBB2 mRNA to Determine HER2 Low Status for Breast Cancer. J. Mol. Diagn. 2022, 24, 775–783. [Google Scholar] [CrossRef]



| Characteristics | Total n = 2296 (%) | HER2 0 n = 368 (%) | HER2 1+ n = 911 (%) | HER2 2+/FISH− n = 1017 (%) | p-Value |
|---|---|---|---|---|---|
| Age (y/o) | 0.798 | ||||
| <60 | 1270 (55.3) | 204 (55.4) | 511 (56.1) | 555 (54.6) | |
| ≥60 | 1026 (44.7) | 164 (44.6) | 400 (43.9) | 462 (45.4) | |
| Gender | 0.806 | ||||
| Female | 2277 (99.2) | 366 (99.5) | 903 (99.1) | 1008 (99.1) | |
| Male | 19 (0.8) | 2 (0.5) | 8 (0.9) | 9 (0.9) | |
| Histology | 0.004 | ||||
| IDC | 1935 (84.3) | 295 (80.2) | 756 (83.0) | 884 (86.9) | |
| Non-IDC | 361 (15.7) | 73 (19.8) | 155 (17.0) | 133 (13.1) | |
| TNM stage | 0.020 | ||||
| I | 1423 (62.0) | 242 (65.8) | 588 (64.5) | 593 (58.3) | |
| II | 859 (37.4) | 123 (33.4) | 317 (34.8) | 419 (41.2) | |
| III | 14 (9.6) | 3 (0.8) | 6 (0.7) | 5 (0.5) | |
| Tumor Size | 0.050 | ||||
| <2 cm | 1227 (53.4) | 201 (54.6) | 511 (56.1) | 515 (50.6) | |
| ≥2 cm | 1069 (46.6) | 167 (45.4) | 400 (43.9) | 502 (49.4) | |
| Breast surgery | 0.372 | ||||
| BCS | 1006 (43.8) | 166 (45.1) | 411 (45.1) | 429 (42.2) | |
| Mastectomy | 1290 (56.2) | 202 (54.9) | 500 (54.9) | 588 (57.8) | |
| ALN status | 0.001 | ||||
| Negative | 1919 (83.6) | 323 (87.8) | 777 (85.3) | 819 (80.5) | |
| Positive | 377 (16.4) | 45 (12.2) | 134 (14.7) | 198 (19.5) | |
| Histological grade | 0.334 | ||||
| I-II | 1879 (82.8) | 292 (79.3) | 755 (82.9) | 832 (81.8) | |
| III | 417 (18.2) | 76 (20.7) | 156 (17.1) | 185 (18.2) | |
| ER | 0.115 | ||||
| Negative | 5 (0.2) | 1 (0.3) | 4 (0.4) | 0 (0.0) | |
| Positive | 2291 (99.8) | 367 (99.7) | 907 (99.6) | 1017 (100.0) | |
| PR | 0.037 | ||||
| Negative | 250 (10.9) | 54 (14.7) | 95 (10.4) | 101 (9.9) | |
| Positive | 2046 (89.1) | 314 (85.3) | 816 (89.6) | 916 (90.1) | |
| Ki-67 | <0.001 | ||||
| <14% | 1065 (46.4) | 178 (48.4) | 467 (51.3) | 420 (41.3) | |
| ≥14% | 1231 (53.6) | 190 (51.6) | 444 (48.7) | 597 (58.7) | |
| Molecular subtype | 0.010 | ||||
| Luminal A | 733 (31.9) | 119 (32.3) | 321 (35.2) | 293 (28.8) | |
| Luminal B (HER2-) | 1563 (68.1) | 249 (67.7) | 590 (64.8) | 724 (71.2) |
| Characteristics | HER2 0 n = 368 | HER2 1+ n = 911 | p | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | pb | ||
| Histology | 0.006 | 0.045 | 0.233 | 0.015 | ||||
| Non-IDC | 1.57 | 1.14–2.15 | 1.30 | 1.01–1.67 | ||||
| IDC | 1.00 | 1.00 | ||||||
| Tumor Size | 0.395 | 0.110 | 0.730 | 0.265 | ||||
| <2 cm | 1.11 | 0.87–1.42 | 1.16 | 0.96–1.40 | ||||
| ≥2 cm | 1.00 | 1.00 | ||||||
| ALN status | 0.012 | 0.044 | 0.264 | 0.016 | ||||
| Negative | 1.58 | 1.11–2.25 | 1.29 | 1.01–1.65 | ||||
| Positive | 1.00 | 1.00 | ||||||
| PR | 0.017 | 0.668 | 0.083 | 0.073 | ||||
| Negative | 1.54 | 1.08–2.20 | 1.07 | 0.79–1.44 | ||||
| Positive | 1.00 | 1.00 | ||||||
| Ki-67 | 0.070 | <0.001 | 0.319 | 0.025 | ||||
| <14% | 1.25 | 0.98–1.60 | 1.42 | 1.18–1.70 | ||||
| ≥14% | 1.00 | 1.00 | ||||||
| Molecular subtype | 0.452 | 0.691 | 0.686 | 0.948 | ||||
| Luminal A | 0.87 | 0.59–1.26 | 0.94 | 0.71–1.26 | ||||
| Luminal B (HER2-) | 1.00 | 1.00 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, L.; Tong, Y.; Li, Z.; Chen, X.; Shen, K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers 2022, 14, 4250. https://doi.org/10.3390/cancers14174250
Shu L, Tong Y, Li Z, Chen X, Shen K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers. 2022; 14(17):4250. https://doi.org/10.3390/cancers14174250
Chicago/Turabian StyleShu, Lan, Yiwei Tong, Zhuoxuan Li, Xiaosong Chen, and Kunwei Shen. 2022. "Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer" Cancers 14, no. 17: 4250. https://doi.org/10.3390/cancers14174250





