Prospective Study of 4 Gy Radiotherapy for Orbital Mucosa-Associated Lymphoid Tissue Lymphoma (FORMAL)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
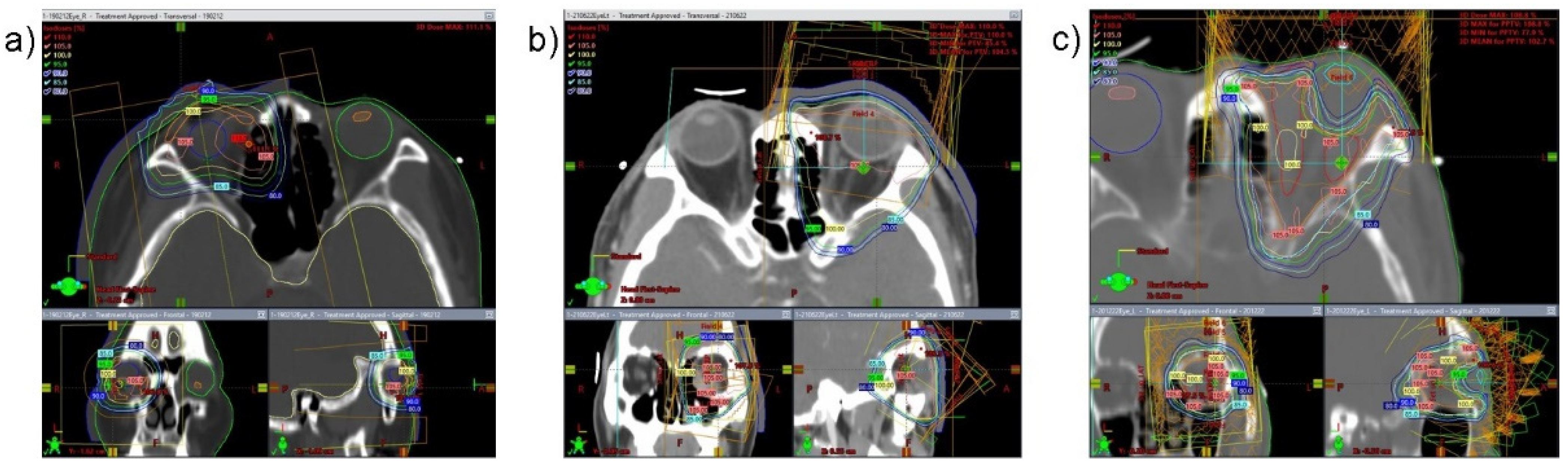
2.2. Staging Workup and Radiotherapy Technique
2.3. Assessment of Response and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Response and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raderer, M.; Kiesewetter, B.; Ferreri, A.J.M. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J. Clin. 2016, 66, 152–171. [Google Scholar] [CrossRef]
- Rossi, D.; Bertoni, F.; Zucca, E. Marginal-Zone Lymphomas. N. Engl. J. Med. 2022, 386, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, A.; Lossos, I.S. Extranodal marginal zone lymphoma of the ocular adnexa. Blood 2009, 114, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.L.; Pickles, T.; Croteau, N.S.; Wai, E.S. Treatment Outcomes of Low-grade Lymphoma of the Orbit. Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Leeson, S.; Jayamohan, J.; Vu, H.; Sundaresan, P. Examining the utility of lower dose radiotherapy for localised primary ocular adnexal MALT lymphoma. J. Med. Radiat. Sci. 2021, 68, 269–273. [Google Scholar] [CrossRef]
- Xu, L.; Tang, X.; Jiang, N.; Zhang, S.; Cao, Y.; Sun, X. Radiation Therapy Efficacy and Toxicity for Orbital and Ocular Adnexal Mucosa-Associated Lymphoid Tissue (OAMALT): A Single-Center, Retrospective Study of 32 Cases. Cancer Manag. Res. 2021, 13, 8017–8024. [Google Scholar] [CrossRef]
- Lowry, L.; Smith, P.; Qian, W.; Falk, S.; Benstead, K.; Illidge, T.; Linch, D.; Robinson, M.; Jack, A.; Hoskin, P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother. Oncol. 2011, 100, 86–92. [Google Scholar] [CrossRef]
- Hoskin, P.; Popova, B.; Schofield, O.; Brammer, C.; Robinson, M.; Brunt, A.M.; Madhavan, K.; Illidge, T.; Gallop-Evans, E.; Syndikus, I.; et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): Long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021, 22, 332–340. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Kirkwood, A.A.; Popova, B.; Smith, P.; Robinson, M.; Gallop-Evans, E.; Coltart, S.; Illidge, T.; Madhavan, K.; Brammer, C.; et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomised phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Fasola, C.E.; Jones, J.C.; Huang, D.D.; Le, Q.T.; Hoppe, R.T.; Donaldson, S.S. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 930–935. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- White, W.L.; Ferry, J.A.; Harris, N.L.; Grove, A.S., Jr. Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa-associated lymphoid tissue type. Ophthalmology 1995, 102, 1994–2006. [Google Scholar] [CrossRef]
- Goda, J.S.; Le, L.W.; Lapperriere, N.J.; Millar, B.A.; Payne, D.; Gospodarowicz, M.K.; Wells, W.; Hodgson, D.C.; Sun, A.; Simpson, R.; et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: Efficacy and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e659–e666. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Murakami, N.; Kitaguchi, M.; Sekii, S.; Takahashi, K.; Yoshio, K.; Inaba, K.; Morota, M.; Ito, Y.; Sumi, M.; et al. Localized ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiation therapy: A long-term outcome in 86 patients with 104 treated eyes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Omura, M.; Koike, I.; Tomita, N.; Iijima, Y.; Tayama, Y.; Odagiri, K.; Minagawa, Y.; Ogino, I.; Inoue, T. Treatment effects and sequelae of radiation therapy for orbital mucosa-associated lymphoid tissue lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1387–1393. [Google Scholar] [CrossRef]
- Joo, J.H.; Lee, S.W.; Huh, J.; Suh, C.; Yoon, D.H.; Ahn, S.D.; Choi, E.K.; Kim, J.H. Recurrence patterns of mucose-associated lymphoid tissue lymphoma after definitive radiation treatment: A single center experience. Hematology 2016, 21, 542–548. [Google Scholar] [CrossRef]
- Le, Q.T.; Eulau, S.M.; George, T.I.; Hildebrand, R.; Warnke, R.A.; Donaldson, S.S.; Hoppe, R.T. Primary radiotherapy for localized orbital MALT lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 657–663. [Google Scholar] [CrossRef]
- Suh, C.O.; Shim, S.J.; Lee, S.W.; Yang, W.I.; Lee, S.Y.; Hahn, J.S. Orbital marginal zone B-cell lymphoma of MALT: Radiotherapy results and clinical behavior. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 228–233. [Google Scholar] [CrossRef]
- Kleiman, N.J. Radiation cataract. Ann. ICRP 2012, 41, 80–97. [Google Scholar] [CrossRef]
- Tran, K.H.; Campbell, B.A.; Fua, T.; MacManus, M.; Ryan, G.; Chesson, B.; Wirth, A. Efficacy of low dose radiotherapy for primary orbital marginal zone lymphoma. Leuk Lymphoma 2013, 54, 491–496. [Google Scholar] [CrossRef]
- Sawyer, E.J.; Timothy, A.R. Low dose palliative radiotherapy in low grade non-Hodgkin’s lymphoma. Radiother. Oncol. 1997, 42, 49–51. [Google Scholar] [CrossRef]
- Chan, E.K.; Fung, S.; Gospodarowicz, M.; Hodgson, D.; Wells, W.; Sun, A.; Pintile, M.; Tsang, R.W. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e781–e786. [Google Scholar] [CrossRef] [PubMed]
- Jóhannsson, J.; Specht, L.; Mejer, J.; Jensen, B.A. Phase II study of palliative low-dose local radiotherapy in disseminated indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1466–1470. [Google Scholar] [CrossRef]
- Herfarth, K.; König, L. Radiation therapy (4 Gy vs. 24 Gy) in patients with indolent non-Hodgkins lymphoma: Results of the FORT Study. Strahlenther. Onkol. 2014, 190, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Stade, R.; Rieber, J.; Debus, J.; Herfarth, K. Radiotherapy of indolent orbital lymphomas. Strahlenther. Onkol. 2016, 192, 414–421. [Google Scholar] [CrossRef]
- Imber, B.S.; Chau, K.W.; Lee, J.; Lee, J.; Casey, D.L.; Yang, J.C.; Wijentunga, N.A.; Shepherd, A.; Hajj, C.; Qi, S.; et al. Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: Is 4 Gy suitable for curable patients? Blood Adv. 2021, 5, 4185–4197. [Google Scholar] [CrossRef]
- Kim, S.E.; Yang, H.J.; Yang, S.W. Effects of radiation therapy on the meibomian glands and dry eye in patients with ocular adnexal mucosa-associated lymphoid tissue lymphoma. BMC Ophthalmol. 2020, 20, 24. [Google Scholar] [CrossRef] [Green Version]



| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 7 | 50.0 |
| Female | 7 | 50.0 |
| Median Age | 60.5 years | |
| Laterality | ||
| Left | 6 | 42.9 |
| Right | 5 | 35.7 |
| Both | 3 | 21.4 |
| Characteristic | n | % |
|---|---|---|
| Laterality | ||
| Left | 9 | 52.9 |
| Right | 8 | 47.1 |
| Location | ||
| Conjunctiva | 14 | 82.3 |
| Retrobulbar | 1 | 5.9 |
| Conjunctiva + retrobulbar | 2 | 11.8 |
| Visible mass in CT | ||
| Yes | 4 | 23.5 |
| No | 13 | 76.5 |
| Hypermetabolic lesion in PET-CT | ||
| Yes | 5 | 29.4 |
| No | 12 | 70.6 |
| RT technique | ||
| 2D | 14 | 82.3 |
| 3D | 2 | 11.8 |
| IMRT | 1 | 5.9 |
| Additional RT | ||
| Yes | 5 | 29.4 |
| No | 12 | 70.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yea, J.W.; Oh, S.A.; Kim, M.K.; Son, J.H.; Park, J.W. Prospective Study of 4 Gy Radiotherapy for Orbital Mucosa-Associated Lymphoid Tissue Lymphoma (FORMAL). Cancers 2022, 14, 4274. https://doi.org/10.3390/cancers14174274
Park J, Yea JW, Oh SA, Kim MK, Son JH, Park JW. Prospective Study of 4 Gy Radiotherapy for Orbital Mucosa-Associated Lymphoid Tissue Lymphoma (FORMAL). Cancers. 2022; 14(17):4274. https://doi.org/10.3390/cancers14174274
Chicago/Turabian StylePark, Jaehyeon, Ji Woon Yea, Se An Oh, Min Kyoung Kim, Jun Hyuk Son, and Jae Won Park. 2022. "Prospective Study of 4 Gy Radiotherapy for Orbital Mucosa-Associated Lymphoid Tissue Lymphoma (FORMAL)" Cancers 14, no. 17: 4274. https://doi.org/10.3390/cancers14174274





