A Panax quinquefolius-Based Preparation Prevents the Impact of 5-FU on Activity/Exploration Behaviors and Not on Cognitive Functions Mitigating Gut Microbiota and Inflammation in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
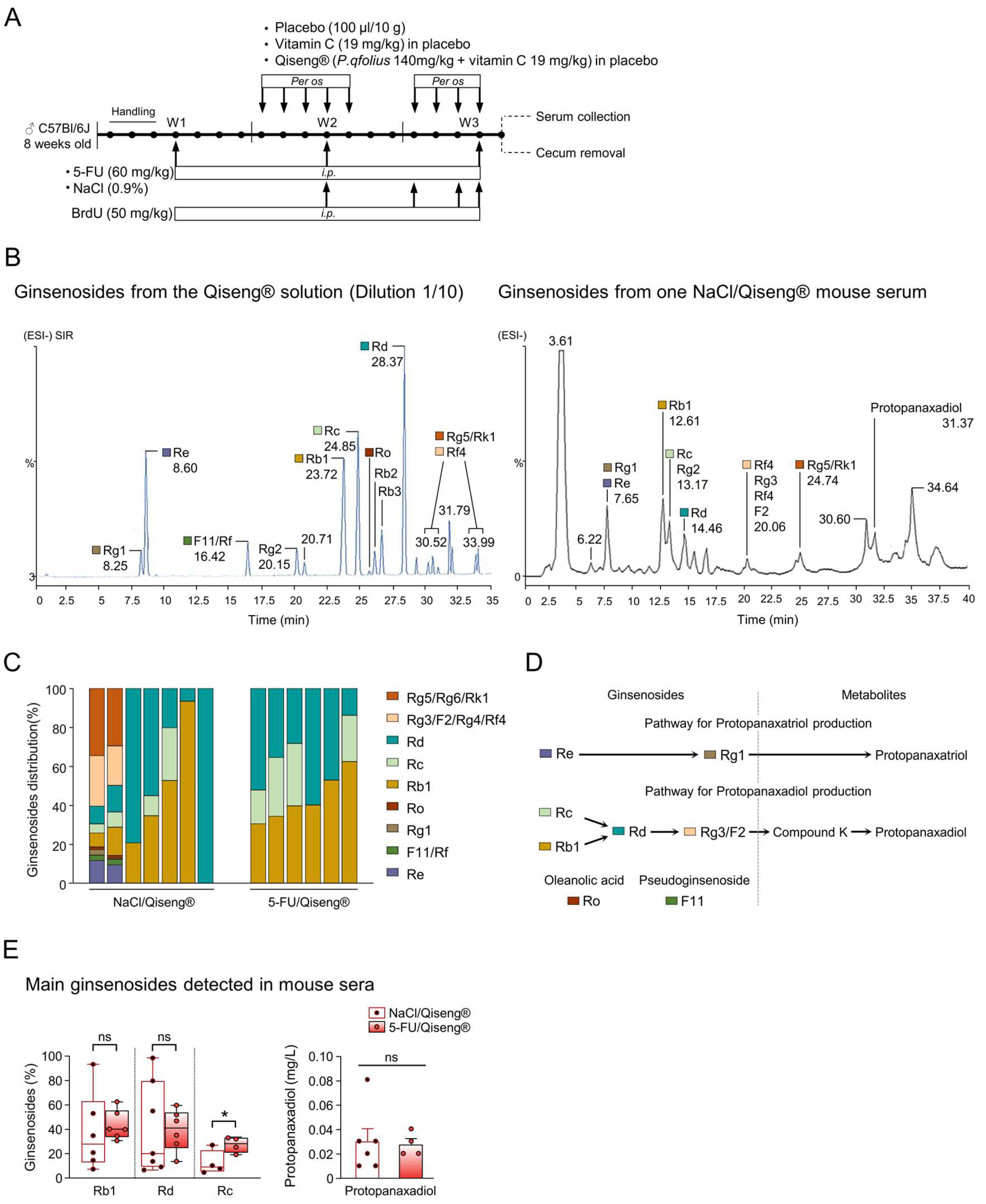
2.2. Treatments and Experimental Procedures
2.3. Behavioral Assessments
2.4. Open Field Test—OFT
2.5. Elevated plus Maze—EPM
2.6. Light/Dark Box—LDB
2.7. Tail Suspension Test—TST and Forced Swim Test—FST
2.8. Morris Water Maze Test—MWMT
2.9. Spontaneous Activity, Nycthemeral Cycle and Anhedonia
2.10. Preparations of Tissue Samples
2.11. Gut Microbiota Analyses
2.12. Ginsenosides HPLC Analysis
2.13. Cytokines Elisa Quantification
2.14. Histological Analysis of Intestinal Sections
2.15. Immunohistochemical Analysis of Brain and Intestinal Tissues
2.16. Statistical Analysis
3. Results
3.1. Deleterious Impact of 5-FU and Qiseng® on Spontaneous Activity, Nycthemeral Activity and Exploration
3.2. No Effect of Treatments on the Emotional Reactivity of Chemotherapy Mice
3.3. Impact of 5-FU Chemotherapy on Spatial Learning, Memory and Flexibility
3.4. Impact of 5-FU Chemotherapy on Hippocampal Neural Stem Cells Proliferation and on Neurogenesis
3.5. Detection of Serum Ginsenosides in Mice Receiving Qiseng®
3.6. Impact of 5-FU Chemotherapy and Qiseng® on the Intestinal Microbiota
3.7. Impact of Chemotherapy and Qiseng® on Intestinal Integrity
3.8. Impact of Chemotherapy and Qiseng® on Pro-Inflammatory, Pluripotent, Chemotactic and Leukocyte Growth Cytokines
3.9. Correlation Analysis between Neuronal Proliferation, Hippocampus and Systemic Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joly, F.; Heutte, N.; Duclos, B.; Noal, S.; Léger-Hardy, I.; Dauchy, S.; Longato, N.; Desrues, L.; Houede, N.; Lange, M.; et al. Prospective Evaluation of the Impact of Antiangiogenic Treatment on Cognitive Functions in Metastatic Renal Cancer. Eur. Urol. Focus 2016, 2, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Rigal, O.; Clarisse, B.; Giffard, B.; Sevin, E.; Barillet, M.; Eustache, F.; Joly, F. Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer Treat. Rev. 2014, 40, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.J.; McDonald, B.C.; Saykin, A.J.; Ahles, T.A. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3866–3870. [Google Scholar] [CrossRef]
- Silverman, D.H.S.; Dy, C.J.; Castellon, S.A.; Lai, J.; Pio, B.S.; Abraham, L.; Waddell, K.; Petersen, L.; Phelps, M.E.; Ganz, P.A. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res. Treat. 2007, 103, 303–311. [Google Scholar] [CrossRef]
- Gibson, E.M.; Nagaraja, S.; Ocampo, A.; Tam, L.T.; Wood, L.S.; Pallegar, P.N.; Greene, J.J.; Geraghty, A.C.; Goldstein, A.K.; Ni, L.; et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019, 176, 43–55.e13. [Google Scholar] [CrossRef]
- Castel, H.; Denouel, A.; Lange, M.; Tonon, M.-C.; Dubois, M.; Joly, F. Biomarkers Associated with Cognitive Impairment in Treated Cancer Patients: Potential Predisposition and Risk Factors. Front. Pharmacol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Naser, A.Y.; Hameed, A.N.; Mustafa, N.; Alwafi, H.; Dahmash, E.Z.; Alyami, H.S.; Khalil, H. Depression and Anxiety in Patients with Cancer: A Cross-Sectional Study. Front. Psychol. 2021, 12, 585534. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels after Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [Green Version]
- Scuric, Z.; Carroll, J.E.; Bower, J.E.; Ramos-Perlberg, S.; Petersen, L.; Esquivel, S.; Hogan, M.; Chapman, A.M.; Irwin, M.R.; Breen, E.C.; et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer 2017, 3, 50. [Google Scholar] [CrossRef]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: A randomized, double-blind trial, N07C2. J. Natl. Cancer Inst. 2013, 105, 1230–1238. [Google Scholar] [CrossRef]
- Barton, D.L.; Soori, G.S.; Bauer, B.A.; Sloan, J.A.; Johnson, P.A.; Figueras, C.; Duane, S.; Mattar, B.; Liu, H.; Atherton, P.J.; et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: A randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2010, 18, 179–187. [Google Scholar] [CrossRef]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Skelly, D.T.; Hennessy, E.; Dansereau, M.-A.; Cunningham, C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57BL/6 mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Sonis, S.T.; O’Donnell, K.E.; Popat, R.; Bragdon, C.; Phelan, S.; Cocks, D.; Epstein, J.B. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004, 40, 170–176. [Google Scholar] [CrossRef]
- Iop, A.; Manfredi, A.M.; Bonura, S. Fatigue in cancer patients receiving chemotherapy: An analysis of published studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2004, 15, 712–720. [Google Scholar] [CrossRef]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B. Ginseng: Potential for the enhancement of cognitive performance and mood. Pharmacol. Biochem. Behav. 2003, 75, 687–700. [Google Scholar] [CrossRef]
- Smith, I.; Williamson, E.M.; Putnam, S.; Farrimond, J.; Whalley, B.J. Effects and mechanisms of ginseng and ginsenosides on cognition. Nutr. Rev. 2014, 72, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-L.; Yang, C.-X.; Xu, P.; Gao, X.-Q.; Deng, L.; Chen, P.; Sun, Z.-L.; Chen, Q.-Y. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007, 1167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Li, N.; Han, J.; Kong, X.; Cao, R.; Rao, Z.; Zhao, G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci. Res. 2009, 64, 306–310. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Li, H.; Zhang, J.; Ci, Y.; Han, M. Ginsenoside Rb1 can ameliorate the key inflammatory cytokines TNF-α and IL-6 in a cancer cachexia mouse model. BMC Complement. Med. Ther. 2020, 20, 11. [Google Scholar] [CrossRef]
- Hussien, M.; Yousef, M.I. Impact of ginseng on neurotoxicity induced by cisplatin in rats. Environ. Sci. Pollut. Res. 2022, 29, 62042–62054. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-Like Behavior in Mice. J. Vis. Exp. JoVE 2015, 96, e52434. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef]
- Hascoët, M.; Bourin, M.; Nic Dhonnchadha, B.A. The mouse light-dark paradigm: A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 141–166. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Graziano, A.; Petrosini, L.; Bartoletti, A. Automatic recognition of explorative strategies in the Morris water maze. J. Neurosci. Methods 2003, 130, 33–44. [Google Scholar] [CrossRef]
- Higaki, A.; Mogi, M.; Iwanami, J.; Min, L.-J.; Bai, H.-Y.; Shan, B.-S.; Kan-No, H.; Ikeda, S.; Higaki, J.; Horiuchi, M. Recognition of early stage thigmotaxis in Morris water maze test with convolutional neural network. PLoS ONE 2018, 13, e0197003. [Google Scholar] [CrossRef]
- Illouz, T.; Madar, R.; Louzoun, Y.; Griffioen, K.J.; Okun, E. Corrigendum to “Unraveling cognitive traits using the Morris water maze unbiased strategy classification (MUST-C) algorithm”. Brain Behav. Immun. 2017, 61, 386. [Google Scholar] [CrossRef]
- Nicola, C.; Dubois, M.; Campart, C.; Al Sagheer, T.; Desrues, L.; Schapman, D.; Galas, L.; Lange, M.; Joly, F.; Castel, H. The Prostate Cancer Therapy Enzalutamide Compared with Abiraterone Acetate/Prednisone Impacts Motivation for Exploration, Spatial Learning and Alters Dopaminergic Transmission in Aged Castrated Mice. Cancers 2021, 13, 3518. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Dore, J.; Ehrlich, S.; Levenez, F.; Pelletier, E.; Alberti, A.; Bertrand, L. IHMS_SOP 06 V1; Standard Operating Procedure for Fecal Samples DNA Extraction, Protocol Q. International Human Microbiome Standards, 2015. Available online: http://www.human-microbiome.org/index.php?id=Sop&num=006.
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef]
- Mühling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008, 2, 379–392. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Hermann-Bank, M.L.; Skovgaard, K.; Stockmarr, A.; Larsen, N.; Mølbak, L. The Gut Microbiotassay: A high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genom. 2013, 14, 788. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, C.-W.; Hwang, J.-T.; Son, N.; Choi, J.H.; Shim, G.-S.; Han, C.-K. LC-MS-based metabolomic analysis of serum and livers from red ginseng-fed rats. J. Ginseng Res. 2013, 37, 371–378. [Google Scholar] [CrossRef]
- Park, H.-W.; In, G.; Han, S.-T.; Lee, M.-W.; Kim, S.-Y.; Kim, K.-T.; Cho, B.-G.; Han, G.-H.; Chang, I.-M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J. Ginseng Res. 2013, 37, 457–467. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Dubois, M.; Lapinte, N.; Villier, V.; Lecointre, C.; Roy, V.; Tonon, M.-C.; Gandolfo, P.; Joly, F.; Hilber, P.; Castel, H. Chemotherapy-induced long-term alteration of executive functions and hippocampal cell proliferation: Role of glucose as adjuvant. Neuropharmacology 2014, 79, 234–248. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 743–753. [Google Scholar] [CrossRef]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.S.; Jiang, H.; Howell, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2016, 10, 51–61. [Google Scholar] [CrossRef]
- Loos, M.; Koopmans, B.; Aarts, E.; Maroteaux, G.; Sluis, S.v.d.; Consortium, N.-B.M.P.; Verhage, M.; Smit, A.B. Sheltering Behavior and Locomotor Activity in 11 Genetically Diverse Common Inbred Mouse Strains Using Home-Cage Monitoring. PLoS ONE 2014, 9, e108563. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Hattori, S.; Yoneda, S.; Watanabe, S.; Kanemoto, E.; Sugimoto, M.; Kawai, T.; Machida, A.; Kanzaki, H.; Miyazaki, I.; et al. Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behav. Brain Res. 2015, 292, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Giffard, B.; Rigal, O.; De Ruiter, M.B.; Small, B.J.; Dubois, M.; LeFel, J.; Schagen, S.B.; Ahles, T.A.; Wefel, J.S.; et al. Impact of Cancer and Its Treatments on Cognitive Function: Advances in Research from the Paris International Cognition and Cancer Task Force Symposium and Update Since 2012. J. Pain Symptom Manag. 2015, 50, 830–841. [Google Scholar] [CrossRef] [PubMed]
- ELBeltagy, M.; Mustafa, S.; Umka, J.; Lyons, L.; Salman, A.; Dormon, K.; Allcock, C.; Bennett, G.; Wigmore, P. The effect of 5-fluorouracil on the long term survival and proliferation of cells in the rat hippocampus. Brain Res. Bull. 2012, 88, 514–518. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Kim, D.-H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.-S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515. [Google Scholar] [CrossRef]
- Rangel, I.; Ganda Mall, J.P.; Willén, R.; Sjöberg, F.; Hultgren-Hörnquist, E. Degree of colitis correlates with microbial composition and cytokine responses in colon and caecum of Gαi2-deficient mice. FEMS Microbiol. Ecol. 2016, 92, fiw098. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Neumann, E.; Sammer, G. Pro-inflammatory markers and fatigue in patients with depression: A case-control study. Sci. Rep. 2020, 10, 9494. [Google Scholar] [CrossRef]
- Bazovkina, D.V.; Tibeikina, M.A.; Kulikov, A.V.; Popova, N.K. Effects of lipopolysaccharide and interleukin-6 on cataleptic immobility and locomotor activity in mice. Neurosci. Lett. 2011, 487, 302–304. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Jiang, Y.; Lü, X.; Man, C.; Han, L.; Shan, Y.; Qu, X.; Liu, Y.; Yang, S.; Xue, Y.; Zhang, Y. Lactobacillus acidophilus Induces Cytokine and Chemokine Production via NF-κB and p38 Mitogen-Activated Protein Kinase Signaling Pathways in Intestinal Epithelial Cells. Clin. Vaccine Immunol. CVI 2012, 19, 603–608. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, S.-Y.; Cha, J.-H.; Choi, Y.-S.; Sung, K.-W.; Oh, S.T.; Kim, O.N.; Chung, J.-W.; Chun, M.-H.; Lee, S.B.; et al. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia 2003, 41, 237–246. [Google Scholar] [CrossRef]
- Joly, F.; Lange, M.; Dos Santos, M.; Vaz-Luis, I.; Di Meglio, A. Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors. Cancers 2019, 11, 1896. [Google Scholar] [CrossRef]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Schmits, I.C.; Verhagen, C.A.H.H.V.M.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef]
- Wolff, B.S.; Raheem, S.A.; Saligan, L.N. Comparing passive measures of fatigue-like behavior in mice. Sci. Rep. 2018, 8, 14238. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Ugalde-Morales, E.; Motola-Kuba, D.; Green, D. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br. J. Nutr. 2013, 109, 894–897. [Google Scholar] [CrossRef]
- Guindon, J.; Deng, L.; Fan, B.; Wager-Miller, J.; Hohmann, A.G. Optimization of a cisplatin model of chemotherapy-induced peripheral neuropathy in mice: Use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol. Pain 2014, 10, 56. [Google Scholar] [CrossRef]
- Du, X.F.; Jiang, C.Z.; Wu, C.F.; Won, E.K.; Choung, S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch. Pharmacal Res. 2008, 31, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Xiang, D.; Yang, J.; Liu, D.; Ren, X.; Zhang, C. Assessment of dose-response relationship of 5-fluorouracil to murine intestinal injury. Biomed. Pharmacother. 2018, 106, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.P.; Springer, D.A.; Cullen, M.J.; Gershengorn, M.C. Evaluation of the effects of chemotherapy-induced fatigue and pharmacological interventions in multiple mouse behavioral assays. Behav. Brain Res. 2019, 360, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-H.; Hung, S.-W.; Wu, C.-Y.; Chiu, C.-C.; Hong, H.-T.; Lee, G.-C.; Chen, C.-C.; Lin, J.-S.; Wu, C.-P. Supplementation of beef extract improves chemotherapy-induced fatigue and toxic effects in mice. J. Funct. Foods 2020, 75, 104232. [Google Scholar] [CrossRef]
- Arantes-Rodrigues, R.; Henriques, A.; Pinto-Leite, R.; Faustino-Rocha, A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Seixas, F.; Gama, A.; Colaço, B.; Colaço, A.; et al. The effects of repeated oral gavage on the health of male CD-1 mice. Lab. Anim. 2012, 41, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.P.; Boyd, K.L.; Wallace, J.M. Evaluation of Mice Undergoing Serial Oral Gavage While Awake or Anesthetized. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2016, 55, 805–810. [Google Scholar]
- Wu, Y.; Qin, C.; Lu, X.; Marchiori, J.; Feng, Q. North American ginseng inhibits myocardial NOX2-ERK1/2 signaling and tumor necrosis factor-α expression in endotoxemia. Pharmacol. Res. 2016, 111, 217–225. [Google Scholar] [CrossRef]
- Chanana, P.; Kumar, A. GABA-BZD Receptor Modulating Mechanism of Panax quinquefolius against 72-h Sleep Deprivation Induced Anxiety Like Behavior: Possible Roles of Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation. Front. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Akazawa, K.-h.; Cui, Y.; Tanaka, M.; Kataoka, Y.; Yoneda, Y.; Watanabe, Y. Mapping of regional brain activation in response to fatigue-load and recovery in rats with c-Fos immunohistochemistry. Neurosci. Res. 2010, 66, 372–379. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- John, J.; Kinra, M.; Mudgal, J.; Viswanatha, G.L.; Nandakumar, K. Animal models of chemotherapy-induced cognitive decline in preclinical drug development. Psychopharmacology 2021, 238, 3025–3053. [Google Scholar] [CrossRef]
- Groves, T.R.; Farris, R.; Anderson, J.E.; Alexander, T.C.; Kiffer, F.; Carter, G.; Wang, J.; Boerma, M.; Allen, A.R. 5-Fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav. Brain Res. 2017, 316, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Snyder, J.S.; Ferrante, S.C.; Cameron, H.A. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS ONE 2012, 7, e48757. [Google Scholar] [CrossRef]
- Onufriev, M.V.; Uzakov, S.S.; Freiman, S.V.; Stepanichev, M.Y.; Moiseeva, Y.V.; Lazareva, N.A.; Markevich, V.A.; Gulyaeva, N.V. The Dorsal and Ventral Hippocampus Have Different Reactivities to Proinflammatory Stress: Corticosterone Levels, Cytokine Expression, and Synaptic Plasticity. Neurosci. Behav. Physiol. 2018, 48, 1024–1031. [Google Scholar] [CrossRef]
- Komoltsev, I.G.; Tretyakova, L.V.; Frankevich, S.O.; Shirobokova, N.I.; Volkova, A.A.; Butuzov, A.V.; Novikova, M.R.; Kvichansky, A.A.; Moiseeva, Y.V.; Onufriev, M.V.; et al. Neuroinflammatory Cytokine Response, Neuronal Death and Microglial Proliferation in the Hippocampus of Rats during the Early Period after Lateral Fluid-Percussion-Induced Traumatic Injury of the Neocortex. Mol. Neurobiol. 2022, 59, 1151–1167. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.W.; Baydoun, M.; Piedalue, K.A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef]
- Tetel, M.J.; de Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018, 30, e12548. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Song, M.-Y.; Kim, B.-S.; Kim, H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef]
- Breton, J.; Tirelle, P.; Hasanat, S.; Pernot, A.; L’Huillier, C.; do Rego, J.C.; Déchelotte, P.; Coëffier, M.; Bindels, L.B.; Ribet, D. Gut microbiota alteration in a mouse model of Anorexia Nervosa. Clin. Nutr. 2021, 40, 181–189. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rath, E.; Hölzlwimmer, G.; Quintanilla-Martinez, L.; Loach, D.; Tannock, G.; Haller, D. Lactobacillus reuteri 100-23 transiently activates intestinal epithelial cells of mice that have a complex microbiota during early stages of colonization. J. Nutr. 2008, 138, 1684–1691. [Google Scholar] [CrossRef]
- Jones, R.M.; Luo, L.; Ardita, C.S.; Richardson, A.N.; Kwon, Y.M.; Mercante, J.W.; Alam, A.; Gates, C.L.; Wu, H.; Swanson, P.A.; et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013, 32, 3017–3028. [Google Scholar] [CrossRef]
- Akao, T.; Kanaoka, M.; Kobashi, K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration--measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 1998, 21, 245–249. [Google Scholar] [CrossRef]
- Sun, J.; Wu, W.; Guo, Y.; Qin, Q.; Liu, S. Pharmacokinetic study of ginsenoside Rc and simultaneous determination of its metabolites in rats using RRLC-Q-TOF-MS. J. Pharm. Biomed. Anal. 2014, 88, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Kang, K.A.; Youn, U.; Park, J.S.; Hyun, J.W. A Comparative Study of the Potential Antioxidant Activities of Ginsenosides. J. Food Biochem. 2010, 34, 31–43. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Khan, A.Q.; Al-Qasim, A.M.; Al-Yousef, Y. Ascorbic acid attenuates antineoplastic drug 5-fluorouracil induced gastrointestinal toxicity in rats by modulating the expression of inflammatory mediators. Toxicol. Rep. 2015, 2, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Moreira Lopes, T.C.; Mosser, D.M.; Gonçalves, R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 2020, 69, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 Modulation of Intestinal Epithelial Tight Junction Permeability Is Mediated by JNK Pathway Activation of Claudin-2 Gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yeom, Y.; Kim, Y.-S.; Kim, E.; Shin, J.-H.; Seok, P.R.; Woo, M.J.; Kim, Y. Effect of vitamin C on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated early colon cancer in mice. Nutr. Res. Pract. 2018, 12, 101–109. [Google Scholar] [CrossRef]
- Han, S.-K.; Joo, M.-K.; Kim, J.-K.; Jeung, W.; Kang, H.; Kim, D.-H. Bifidobacteria-Fermented Red Ginseng and Its Constituents Ginsenoside Rd and Protopanaxatriol Alleviate Anxiety/Depression in Mice by the Amelioration of Gut Dysbiosis. Nutrients 2020, 12, 901. [Google Scholar] [CrossRef]
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine Signaling Modulates Blood-Brain Barrier Function. Curr. Pharm. Des. 2011, 17, 3729–3740. [Google Scholar] [CrossRef]
- Roberts, A.J.; Khom, S.; Bajo, M.; Vlkolinsky, R.; Polis, I.; Cates-Gatto, C.; Roberto, M.; Gruol, D.L. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav. Immun. 2019, 82, 188–202. [Google Scholar] [CrossRef]
- Haroon, F.; Drögemüller, K.; Händel, U.; Brunn, A.; Reinhold, D.; Nishanth, G.; Mueller, W.; Trautwein, C.; Ernst, M.; Deckert, M.; et al. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J. Immunol. 2011, 186, 6521–6531. [Google Scholar] [CrossRef]
- Vallières, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 486–492. [Google Scholar] [CrossRef]
- Berger, A.M.; Mitchell, S.A.; Jacobsen, P.B.; Pirl, W.F. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J. Clin. 2015, 65, 190–211. [Google Scholar] [CrossRef]
- Miller, A.H.; Jones, J.F.; Drake, D.F.; Tian, H.; Unger, E.R.; Pagnoni, G. Decreased basal ganglia activation in subjects with chronic fatigue syndrome: Association with symptoms of fatigue. PLoS ONE 2014, 9, e98156. [Google Scholar] [CrossRef] [Green Version]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front. Immunol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Dougherty, J.P.; Wolff, B.S.; Cullen, M.J.; Saligan, L.N.; Gershengorn, M.C. Taltirelin alleviates fatigue-like behavior in mouse models of cancer-related fatigue. Pharm. Res. 2017, 124, 1–8. [Google Scholar] [CrossRef]
- Zombeck, J.A.; Fey, E.G.; Lyng, G.D.; Sonis, S.T. A clinically translatable mouse model for chemotherapy-related fatigue. Comp. Med. 2013, 63, 491–497. [Google Scholar]
- Renner, M.; Feng, R.; Springer, D.; Chen, M.K.; Ntamack, A.; Espina, A.; Saligan, L.N. A murine model of peripheral irradiation-induced fatigue. Behav. Brain Res. 2016, 307, 218–226. [Google Scholar] [CrossRef]
- Perals, D. Revisiting the open-field test: What does it really tell us about animal personality? Anim. Behav. 2017, 123, 69–79. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Shao, S.; Cui, S.; Wan, Y.; Yi, M. Ventral Hippocampus Modulates Anxiety-Like Behavior in Male but Not Female C57BL/6J Mice. Neuroscience 2019, 418, 50–58. [Google Scholar] [CrossRef]
- Konsman, J.P.; Laaker, C.J.; Lloyd, K.R.; Hiltz, A.; Smith, B.L.; Smail, M.A.; Reyes, T.M. Translationally relevant mouse model of early life cancer and chemotherapy exposure results in brain and small intestine cytokine responses: A potential link to cognitive deficits. Brain Behav. Immun. 2022, 99, 192–202. [Google Scholar] [CrossRef]
- Kok, A. Cognitive control, motivation and fatigue: A cognitive neuroscience perspective. Brain Cogn. 2022, 160, 105880. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parment, R.; Dubois, M.; Desrues, L.; Mutel, A.; Dembélé, K.-P.; Belin, N.; Tron, L.; Guérin, C.; Coëffier, M.; Compère, V.; et al. A Panax quinquefolius-Based Preparation Prevents the Impact of 5-FU on Activity/Exploration Behaviors and Not on Cognitive Functions Mitigating Gut Microbiota and Inflammation in Mice. Cancers 2022, 14, 4403. https://doi.org/10.3390/cancers14184403
Parment R, Dubois M, Desrues L, Mutel A, Dembélé K-P, Belin N, Tron L, Guérin C, Coëffier M, Compère V, et al. A Panax quinquefolius-Based Preparation Prevents the Impact of 5-FU on Activity/Exploration Behaviors and Not on Cognitive Functions Mitigating Gut Microbiota and Inflammation in Mice. Cancers. 2022; 14(18):4403. https://doi.org/10.3390/cancers14184403
Chicago/Turabian StyleParment, Renaud, Martine Dubois, Laurence Desrues, Alexandre Mutel, Kléouforo-Paul Dembélé, Nicolas Belin, Laure Tron, Charlène Guérin, Moïse Coëffier, Vincent Compère, and et al. 2022. "A Panax quinquefolius-Based Preparation Prevents the Impact of 5-FU on Activity/Exploration Behaviors and Not on Cognitive Functions Mitigating Gut Microbiota and Inflammation in Mice" Cancers 14, no. 18: 4403. https://doi.org/10.3390/cancers14184403
APA StyleParment, R., Dubois, M., Desrues, L., Mutel, A., Dembélé, K.-P., Belin, N., Tron, L., Guérin, C., Coëffier, M., Compère, V., Féger, C., Joly, F., Hilber, P., Ribet, D., & Castel, H. (2022). A Panax quinquefolius-Based Preparation Prevents the Impact of 5-FU on Activity/Exploration Behaviors and Not on Cognitive Functions Mitigating Gut Microbiota and Inflammation in Mice. Cancers, 14(18), 4403. https://doi.org/10.3390/cancers14184403






