Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls Enrollment
2.2. Blood and Primary Tissue Samples
2.3. Microbial DNA Amplification
2.4. Toll-Like Receptor (TLR) and Vitamin D Receptor (VDR) Genotyping
2.5. Mutational Analysis
3. Results
3.1. Patients and Healthy Donors Characteristics
3.2. Detection of Microbial DNA Fragments
3.3. TLR and VDR Genetic Variants Analysis and Clinical Outcoume
3.4. Correlation of Microbial DNA Fragments with TLR and VDR Genetic Variants Analysis
3.5. Association of Tumor Mutations and MSI Status with Microbial DNA Fragments, TLR and VDR Polymorphisms
3.6. Univariate and Multivariate Analysis for Cox Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.H.; Shi, Q.; Alberts, S.R.; Goldberg, R.M.; Thibodeau, S.N.; Sargent, D.J.; Sinicrope, F.A. Racial differences in braf/kras mutation rates and survival in stage iii colon cancer patients. J. Natl. Cancer Inst. 2015, 107, djv186. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Shi, Q.; Smyrk, T.C.; Thibodeau, S.N.; Dienstmann, R.; Guinney, J.; Bot, B.M.; Tejpar, S.; Delorenzi, M.; Goldberg, R.M.; et al. Molecular markers identify subtypes of stage iii colon cancer associated with patient outcomes. Gastroenterology 2015, 148, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The role of the gut microbiota in colorectal cancer causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef]
- Messaritakis, I.; Vogiatzoglou, K.; Tsantaki, K.; Ntretaki, A.; Sfakianaki, M.; Koulouridi, A.; Tsiaoussis, J.; Mavroudis, D.; Souglakos, J. The prognostic value of the detection of microbial translocation in the blood of colorectal cancer patients. Cancers 2020, 12, 1058. [Google Scholar] [CrossRef]
- Koulouridi, A.; Messaritakis, I.; Gouvas, N.; Tsiaoussis, J.; Souglakos, J. Immunotherapy in solid tumors and gut microbiota: The correlation-a special reference to colorectal cancer. Cancers 2020, 13, 43. [Google Scholar] [CrossRef]
- Zeromski, J.; Kaczmarek, M.; Boruczkowski, M.; Kierepa, A.; Kowala-Piaskowska, A.; Mozer-Lisewska, I. Significance and role of pattern recognition receptors in malignancy. Arch. Immunol. Ther. Exp. 2019, 67, 133–141. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.; Wu, Q.; Fang, H.; Shi, C.; Li, Z.; Lin, C.; Tang, D.; Wang, D. Intestinal microbiota: A new force in cancer immunotherapy. Cell Commun. Signal. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-like receptors (tlrs): Structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. Biomed. Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- Pradere, J.P.; Dapito, D.H.; Schwabe, R.F. The yin and yang of toll-like receptors in cancer. Oncogene 2014, 33, 3485–3495. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin d signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin d in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin d receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Palmer, H.G.; Larriba, M.J.; Garcia, J.M.; Ordonez-Moran, P.; Pena, C.; Peiro, S.; Puig, I.; Rodriguez, R.; de la Fuente, R.; Bernad, A.; et al. The transcription factor snail represses vitamin d receptor expression and responsiveness in human colon cancer. Nat. Med. 2004, 10, 917–919. [Google Scholar] [CrossRef]
- Larriba, M.J.; Bonilla, F.; Munoz, A. The transcription factors snail1 and snail2 repress vitamin d receptor during colon cancer progression. J. Steroid Biochem. Mol. Biol. 2010, 121, 106–109. [Google Scholar] [CrossRef]
- Dou, R.; Ng, K.; Giovannucci, E.L.; Manson, J.E.; Qian, Z.R.; Ogino, S. Vitamin d and colorectal cancer: Molecular, epidemiological and clinical evidence. Br. J. Nutr. 2016, 115, 1643–1660. [Google Scholar] [CrossRef]
- Messaritakis, I.; Stogiannitsi, M.; Koulouridi, A.; Sfakianaki, M.; Voutsina, A.; Sotiriou, A.; Athanasakis, E.; Xynos, E.; Mavroudis, D.; Tzardi, M.; et al. Evaluation of the detection of toll-like receptors (tlrs) in cancer development and progression in patients with colorectal cancer. PLoS ONE 2018, 13, e0197327. [Google Scholar] [CrossRef]
- Messaritakis, I.; Koulouridi, A.; Sfakianaki, M.; Vogiatzoglou, K.; Gouvas, N.; Athanasakis, E.; Tsiaoussis, J.; Xynos, E.; Mavroudis, D.; Tzardi, M.; et al. The role of vitamin d receptor gene polymorphisms in colorectal cancer risk. Cancers 2020, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Harsch, M.; Bendrat, K.; Hofmeier, G.; Branscheid, D.; Niendorf, A. A new method for histological microdissection utilizing an ultrasonically oscillating needle: Demonstrated by differential mrna expression in human lung carcinoma tissue. Am. J. Pathol. 2001, 158, 1985–1990. [Google Scholar] [CrossRef]
- Koulouridi, A.; Messaritakis, I.; Theodorakis, E.; Chondrozoumaki, M.; Sfakianaki, M.; Gouvas, N.; Tsiaoussis, J.; Mavroudis, D.; Tzardi, M.; Souglakos, J. Detection of circulating tumor cells and microbial DNA fragments in stage iii colorectal cancer patients under three versus six months of adjuvant treatment. Cancers 2021, 13, 3552. [Google Scholar] [CrossRef]
- Tahara, T.; Arisawa, T.; Wang, F.; Shibata, T.; Nakamura, M.; Sakata, M.; Hirata, I.; Nakano, H. Toll-like receptor 2 -196 to 174del polymorphism influences the susceptibility of japanese people to gastric cancer. Cancer Sci. 2007, 98, 1790–1794. [Google Scholar] [CrossRef]
- Souglakos, J.; Philips, J.; Wang, R.; Marwah, S.; Silver, M.; Tzardi, M.; Silver, J.; Ogino, S.; Hooshmand, S.; Kwak, E.; et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br. J. Cancer 2009, 101, 465–472. [Google Scholar] [CrossRef]
- Sfakianaki, M.; Papadaki, C.; Tzardi, M.; Trypaki, M.; Alam, S.; Lagoudaki, E.D.; Messaritakis, I.; Zoras, O.; Mavroudis, D.; Georgoulias, V.; et al. Loss of lkb1 protein expression correlates with increased risk of recurrence and death in patients with resected, stage ii or iii colon cancer. Cancer Res. Treat. 2019, 51, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Sfakianaki, M.; Papadaki, C.; Tzardi, M.; Trypaki, M.; Manolakou, S.; Messaritakis, I.; Saridaki, Z.; Athanasakis, E.; Mavroudis, D.; Tsiaoussis, J.; et al. Pkm2 expression as biomarker for resistance to oxaliplatin-based chemotherapy in colorectal cancer. Cancers 2020, 12, 2058. [Google Scholar] [CrossRef] [PubMed]
- Koulouridi, A.; Karagianni, M.; Messaritakis, I.; Sfakianaki, M.; Voutsina, A.; Trypaki, M.; Bachlitzanaki, M.; Koustas, E.; Karamouzis, M.V.; Ntavatzikos, A.; et al. Prognostic value of kras mutations in colorectal cancer patients. Cancers 2022, 14, 3320. [Google Scholar] [CrossRef]
- Kane, T.D.; Alexander, J.W.; Johannigman, J.A. The detection of microbial DNA in the blood: A sensitive method for diagnosing bacteremia and/or bacterial translocation in surgical patients. Ann. Surg. 1998, 227, 1–9. [Google Scholar] [CrossRef]
- Giordano, C.; Mojumdar, K.; Liang, F.; Lemaire, C.; Li, T.; Richardson, J.; Divangahi, M.; Qureshi, S.; Petrof, B.J. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in duchenne muscular dystrophy. Hum. Mol. Genet. 2015, 24, 2147–2162. [Google Scholar] [CrossRef] [Green Version]
- Brunner, R.; Jensen-Jarolim, E.; Pali-Scholl, I. The abc of clinical and experimental adjuvants—A brief overview. Immunol. Lett. 2010, 128, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Gonzalez, L.; Gonzalez, L.O.; Fernandez-Garcia, B.; Andicoechea, A.; Barbon, E.; Garcia-Muniz, J.L.; Vizoso, F.J. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J. Immunother. 2013, 36, 342–349. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Bondurant, K.L.; Wolff, R.K. Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int. J. Cancer 2012, 130, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Farooq, A.; Malik, A.; Trawick, B.N.; Berberich, D.W.; McClurg, J.P.; Galen, K.P.; Mehal, W. A novel small-molecule enantiomeric analogue of traditional (−)-morphinans has specific tlr9 antagonist properties and reduces sterile inflammation-induced organ damage. J. Immunol. 2013, 190, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Matsuo, K.; Goto, Y.; Naito, M.; Wakai, K.; Tajima, K.; Hamajima, N. No associations of toll-like receptor 2 (tlr2) -196 to -174del polymorphism with the risk of helicobacter pylori seropositivity, gastric atrophy, and gastric cancer in japanese. Gastric Cancer 2010, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Shi, Y.; Han, L.; Zhao, Z.; Zhao, C.; Luo, B. Toll-like receptor gene polymorphisms and susceptibility to epstein-barr virus-associated and -negative gastric carcinoma in northern china. Saudi J. Gastroenterol. 2015, 21, 95–103. [Google Scholar]
- Beilmann-Lehtonen, I.; Hagstrom, J.; Mustonen, H.; Koskensalo, S.; Haglund, C.; Bockelman, C. High tissue tlr5 expression predicts better outcomes in colorectal cancer patients. Oncology 2021, 99, 589–600. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, L.; Ma, L.; Lv, C.; Ding, Y.; Xia, T.; Wang, J.; Dou, X. Vitamin d status and expression of vitamin d receptor and ll-37 in patients with spontaneous bacterial peritonitis. Dig. Dis. Sci. 2012, 57, 182–188. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.P.; Chang, S.C.; Linet, M.S.; Sigurdson, A.J. Sunlight, polymorphisms of vitamin d-related genes and risk of breast cancer. Anticancer Res. 2013, 33, 543–551. [Google Scholar]
- Perna, L.; Hoffmeister, M.; Schottker, B.; Arndt, V.; Haug, U.; Holleczek, B.; Burwinkel, B.; Ordonez-Mena, J.M.; Brenner, H. Vitamin d receptor polymorphism and colorectal cancer-specific and all-cause mortality. Cancer Epidemiol. 2013, 37, 905–907. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.; Pols, H.A.; Van Leeuwen, J.P. Genetics and biology of vitamin d receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Laczmanska, I.; Laczmanski, L.; Bebenek, M.; Karpinski, P.; Czemarmazowicz, H.; Ramsey, D.; Milewicz, A.; Sasiadek, M.M. Vitamin d receptor gene polymorphisms in relation to the risk of colorectal cancer in the polish population. Tumour Biol. 2014, 35, 12397–12401. [Google Scholar] [CrossRef] [Green Version]
- Sarkissyan, M.; Wu, Y.; Chen, Z.; Mishra, D.K.; Sarkissyan, S.; Giannikopoulos, I.; Vadgama, J.V. Vitamin d receptor foki gene polymorphisms may be associated with colorectal cancer among african american and hispanic participants. Cancer 2014, 120, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Lu, H.; Hong, D.; Lin, C.C.; Yu, Z.; Chen, B.C. Vitamin d receptor gene polymorphisms and colorectal cancer risk: A systematic meta-analysis. World J. Gastroenterol. 2012, 18, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; McKay, J.; Bueno-de-Mesquita, H.B.; van Duijnhoven, F.J.; Ferrari, P.; Slimani, N.; Jansen, E.H.; Pischon, T.; Rinaldi, S.; Tjonneland, A.; et al. Vitamin d receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in european populations. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2485–2491. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin d receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. The role of vitamin d and vitamin d receptors in colon cancer. Clin. Transl. Gastroenterol. 2017, 8, e103. [Google Scholar] [CrossRef] [PubMed]
- Arji, N.; Busson, M.; Iraqi, G.; Bourkadi, J.E.; Benjouad, A.; Bouayad, A.; Mariaselvam, C.; Salah, S.; Fortier, C.; Amokrane, K.; et al. Genetic diversity of tlr2, tlr4, and vdr loci and pulmonary tuberculosis in moroccan patients. J. Infect. Dev. Ctries. 2014, 8, 430–440. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin d-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Gao, L.B.; Wang, Y.Y.; Zhou, B.; Lv, M.L.; Lu, H.M.; Zhang, L. Vitamin d receptor gene polymorphisms and the risk of colorectal cancer in a chinese population. Dig. Dis. Sci. 2009, 54, 634–639. [Google Scholar] [CrossRef]
- Yaylim-Eraltan, I.; Arzu Ergen, H.; Arikan, S.; Okay, E.; Ozturk, O.; Bayrak, S.; Isbir, T. Investigation of the vdr gene polymorphisms association with susceptibility to colorectal cancer. Cell Biochem. Funct. 2007, 25, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Farrington, S.M.; Tenesa, A.; McNeill, G.; Cetnarskyj, R.; Barnetson, R.A.; Porteous, M.E.; Dunlop, M.G.; Campbell, H. Modification of the inverse association between dietary vitamin d intake and colorectal cancer risk by a foki variant supports a chemoprotective action of vitamin d intake mediated through vdr binding. Int. J. Cancer 2008, 123, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Gnagnarella, P.; Raimondi, S.; Gandini, S. Meta-analysis on vitamin d receptor and cancer risk: Focus on the role of taqi, apai, and cdx2 polymorphisms. Eur. J. Cancer Prev. 2016, 25, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin d receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2014, 810, 69–105. [Google Scholar]
- Luo, Q.; Zeng, L.; Tang, C.; Zhang, Z.; Chen, Y.; Zeng, C. Tlr9 induces colitis-associated colorectal carcinogenesis by regulating nf-kappab expression levels. Oncol. Lett. 2020, 20, 110. [Google Scholar] [CrossRef]
- Gao, C.; Qiao, T.; Zhang, B.; Yuan, S.; Zhuang, X.; Luo, Y. Tlr9 signaling activation at different stages in colorectal cancer and nf-kappab expression. Onco Targets Ther. 2018, 11, 5963–5971. [Google Scholar] [CrossRef]
- Furi, I.; Sipos, F.; Germann, T.M.; Kalmar, A.; Tulassay, Z.; Molnar, B.; Muzes, G. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: Clinico-pathogenic aspects. World J. Gastroenterol. 2013, 19, 4119–4126. [Google Scholar] [CrossRef] [Green Version]
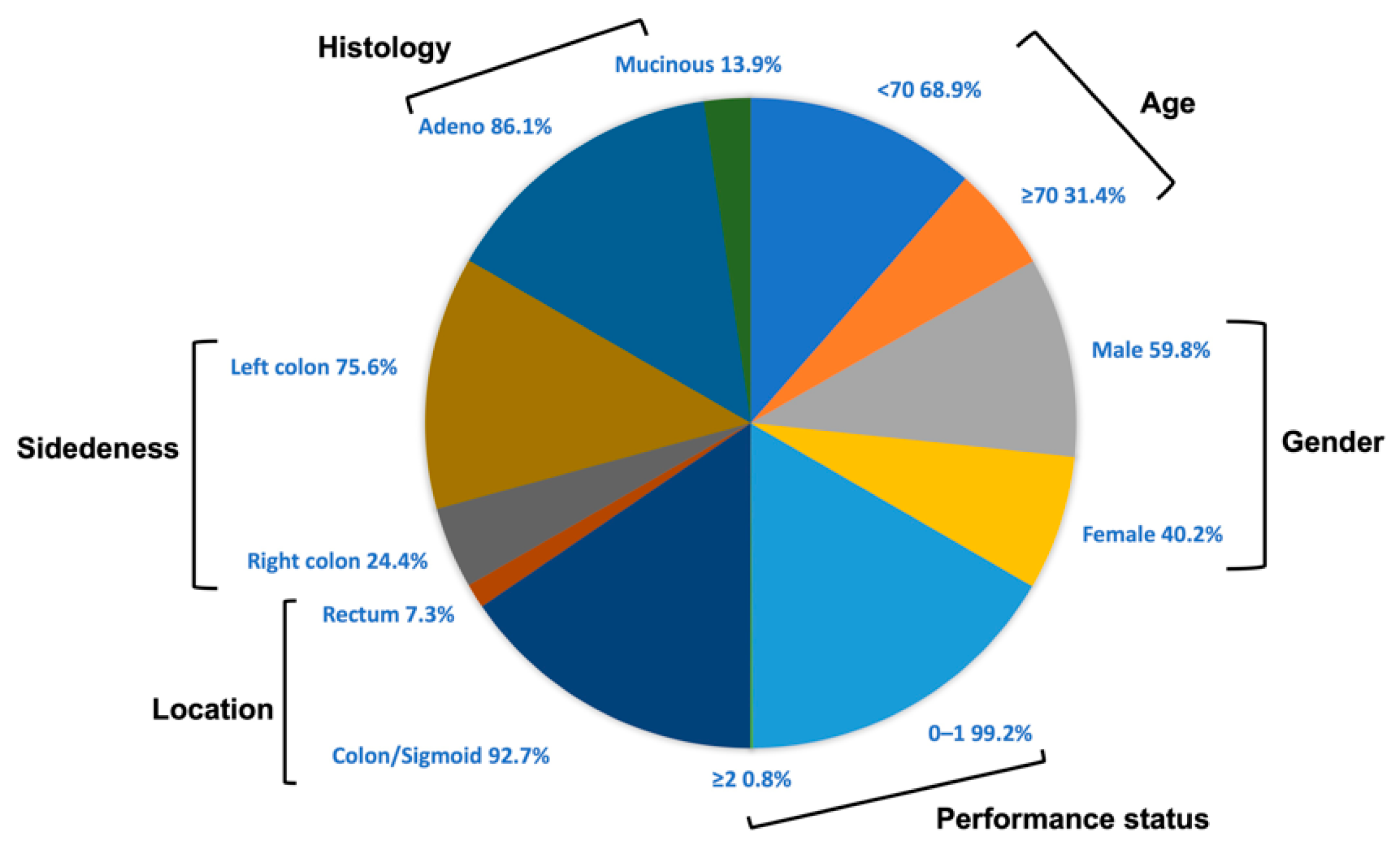

| Demographics | Patients | Healthy Controls | ||
|---|---|---|---|---|
| Characteristics | Frequency (N = 132) | % | Frequency (N = 100) | % |
| Age median (range) | 62 (36–83) | 65 (35–89) | ||
| <70 | 91 | 68.9 | 70 | 70.0 |
| ≥70 | 41 | 31.1 | 30 | 30.0 |
| Gender | ||||
| Male | 78 | 59.1 | 54 | 54.0 |
| Female | 54 | 40.9 | 46 | 46.0 |
| Performance status | ||||
| 0–1 | 131 | 99.2 | ||
| ≥2 | 1 | 0.8 | ||
| Location | ||||
| Colon/Sigmoid | 122 | 92.4 | ||
| Rectum | 10 | 7.6 | ||
| Right/Left site | ||||
| Right colon | 32 | 24.2 | ||
| Left colon | 100 | 75.8 | ||
| Histology | ||||
| Non-Mucinous | 114 | 86.4 | ||
| Mucinous | 18 | 13.9 | ||
| Regimen | ||||
| Folfox | 59 | 44.3 | ||
| Capox | 73 | 55.7 | ||
| Treatment Duration | ||||
| 3 months | 66 | 50.0 | ||
| 6 months | 66 | 50.0 | ||
| T status | ||||
| T2 | 7 | 5.3 | ||
| T3 | 95 | 72.0 | ||
| T4 | 30 | 22.7 | ||
| N status | ||||
| N0 | 22 | 16.7 | ||
| N1 | 81 | 61.4 | ||
| N2 | 29 | 21.9 | ||
| Microsatellite Instability (MSI) | ||||
| Stable | 55 | 41.7 | ||
| High | 7 | 5.3 | ||
| Unknown | 70 | 53.0 | ||
| KRAS | ||||
| Wild type | 37 | 41.7 | ||
| Mutant | 25 | 5.3 | ||
| Unknown | 70 | 53.0 | ||
| NRAS | ||||
| Wild type | 56 | 42.4 | ||
| Mutant | 1 | 0.8 | ||
| Unknown | 75 | 56.8 | ||
| BRAF | ||||
| Wild type | 49 | 41.7 | ||
| Mutant | 5 | 5.3 | ||
| Unknown | 78 | 53.0 | ||
| DNA | Gene Target | Detection | Patients | Healthy Individuals | p-Value |
|---|---|---|---|---|---|
| Microbial DNA fragments | DNA coding for 16S rRNA | Positive | 57 (43.2%) | 16 (16%) | <0.001 |
| Negative | 75 (56.8%) | 84 (84.0) | |||
| β-galactosidase gene of E. coli | Positive | 27 (20.5%) | 16 (16%) | 0.387 | |
| Negative | 105 (79.5%) | 84 (84%) | |||
| Glutamine synthase gene of B. fragilis | Positive | 42 (31.8%) | 0 (0%) | <0.001 | |
| Negative | 90 (68.2%) | 100 (100%) | |||
| DNA coding for 5.8S rRNA of C. albicans | Positive | 48 (36.4%) | 0 (0%) | <0.001 | |
| Negative | 84 (63.6%) | 100 (100%) |
| Polymorphism | Gene Target | Detection | Patients | Healthy Individuals | p-Value |
|---|---|---|---|---|---|
| VDR polymorphisms | TaqI | wild type | 55 (41.7%) | 71 (71%) | <0.001 |
| heterozygous | 57 (43.2%) | 26 (26%) | |||
| homozygous | 20 (15.2%) | 3 (3%) | |||
| ApaI | wild type | 49 (37.1%) | 52 (52%) | <0.001 | |
| heterozygous | 49 (37.1%) | 40 (40%) | |||
| homozygous | 34 (25.8%) | 8 (8%) | |||
| FokI | wild type | 41 (31.1%) | 55 (55%) | <0.001 | |
| heterozygous | 71 (53.8%) | 40 (40%) | |||
| homozygous | 20 (15.2%) | 5 (5%) | |||
| BsmI | wild type | 49 (37.1%) | 55 (55%) | <0.001 | |
| heterozygous | 66 (50%) | 43 (43%) | |||
| homozygous | 17 (12.9%) | 2 (2%) | |||
| TLR polymorphisms | TLR4—D299G | wild type | 31 (23.5%) | 100 (100%) | <0.001 |
| heterozygous | 50 (37.9%) | ||||
| homozygous | 51 (38.6%) | ||||
| TLR4—T399I | wild type | 32 (24.2%) | 100 (100%) | <0.001 | |
| heterozygous | 48 (|36.4%) | ||||
| homozygous | 52 (39.4%) | ||||
| TLR9—T1237C | wild type | 13 (9.8%) | 52 (52%) | <0.001 | |
| heterozygous | 70 (53%) | 48 (48%) | |||
| homozygous | 49 (37.1%) | ||||
| TLR9—T1486C | wild type | 13 (9.8%) | 52 (52%) | <0.001 | |
| heterozygous | 72 (54.5%) | 48 (48%) | |||
| homozygous | 47 (35.6%) | ||||
| TLR2-196 to -174bp | ins/ins | 100 (100%) | <0.001 | ||
| ins/del | 57 (43.2%) | ||||
| del/del | 75 (56.8%) |
| Target | TLR | VDR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene Target | TLR2-196 to -174bp | TLR4—D299G | TLR4—T399I | TLR9—T1237C | TLR9—T1486C | TaqI | ApaI | FokI | BsmI |
| 16S rRNA | 0.172 | 0.009 | 0.043 | 0.549 | 0.567 | <0.001 | 0.112 | <0.001 | 0.2534 |
| Escherichia coli | 0.074 | 0.091 | 0.093 | 0.617 | 0.548 | <0.001 | 0.003 | 0.0590 | 0.553 |
| Bacteroides fragilis | 0.522 | 0.025 | 0.087 | 0.229 | 0.258 | <0.001 | 0.009 | <0.001 | 0.075 |
| Candida albicans | 0.528 | 0.798 | 0.619 | 0.896 | 0.928 | <0.001 | 0.015 | 0.027 | 0.029 |
| TLR2-196 to -174bp | <0.001 | <0.001 | <0.001 | <0.001 | 0.671 | 0.399 | 0.080 | 0.004 | |
| TLR4—D299G | <0.001 | <0.001 | <0.001 | <0.001 | 0.043 | <0.001 | 0.015 | <0.001 | |
| TLR4—T399I | <0.001 | <0.001 | <0.001 | <0.001 | 0.042 | <0.001 | 0.036 | <0.001 | |
| TLR9—T1237C | <0.001 | <0.001 | <0.001 | <0.001 | 0.364 | 0.008 | 0.012 | <0.001 | |
| TLR9—T1486C | <0.001 | <0.001 | <0.001 | <0.001 | 0.423 | 0.018 | 0.029 | <0.001 | |
| TaqI | 0.671 | 0.043 | 0.042 | 0.364 | 0.423 | 0.395 | <0.001 | <0.001 | |
| ApaI | 0.399 | <0.001 | <0.001 | 0.008 | 0.018 | 0.395 | <0.001 | <0.001 | |
| FokI | 0.080 | 0.015 | 0.036 | 0.012 | 0.027 | <0.001 | <0.001 | <0.001 | |
| BsmI | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Mutation/ MSI Status | TLR9—T1237C | TLR9—T1486C | TaqI | ApaI | FokI | BsmI |
|---|---|---|---|---|---|---|
| KRAS | 0.014 | 0.006 | ||||
| BRAFV600E | 0.045 | |||||
| MSI | 0.012 | 0.025 | 0.047 | 0.001 | <0.001 |
| Factor | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| DFS | OS | DFS | OS | |||||
| Factor | HR (Range) | p-Value | HR (Range) | p-Value | HR (Range) | p-Value | HR (Range) | p-Value |
| BRAF mut vs. wt | 17.05 (2.4–123.4) | 0.005 | - | - | ||||
| B. fragilis pos vs. neg | 2.09 (1.0–4.3) | 0.047 | - | - | 33.85 (1.8–622.4) | 0.018 | - | - |
| C. albicans pos vs. neg | - | - | 3.57 (1.2–10.3) | 0.019 | ||||
| VDR—ApaI | 1.56 (1.0–2.3) | 0.031 | - | - | ||||
| Histology adeno vs. mucinus | 2.72 (1.1–6.8) | 0.031 | - | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messaritakis, I.; Koulouridi, A.; Boukla, E.; Sfakianaki, M.; Vogiatzoglou, K.; Karagianni, M.; Gouvas, N.; Tsiaoussis, J.; Xynos, E.; Athanasakis, E.; et al. Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients. Cancers 2022, 14, 4407. https://doi.org/10.3390/cancers14184407
Messaritakis I, Koulouridi A, Boukla E, Sfakianaki M, Vogiatzoglou K, Karagianni M, Gouvas N, Tsiaoussis J, Xynos E, Athanasakis E, et al. Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients. Cancers. 2022; 14(18):4407. https://doi.org/10.3390/cancers14184407
Chicago/Turabian StyleMessaritakis, Ippokratis, Asimina Koulouridi, Eleni Boukla, Maria Sfakianaki, Konstantinos Vogiatzoglou, Michaela Karagianni, Nikolaos Gouvas, John Tsiaoussis, Evangelos Xynos, Elias Athanasakis, and et al. 2022. "Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients" Cancers 14, no. 18: 4407. https://doi.org/10.3390/cancers14184407
APA StyleMessaritakis, I., Koulouridi, A., Boukla, E., Sfakianaki, M., Vogiatzoglou, K., Karagianni, M., Gouvas, N., Tsiaoussis, J., Xynos, E., Athanasakis, E., Mavroudis, D., Tzardi, M., & Souglakos, J. (2022). Investigation of Microbial Translocation, TLR and VDR Gene Polymorphisms, and Recurrence Risk in Stage III Colorectal Cancer Patients. Cancers, 14(18), 4407. https://doi.org/10.3390/cancers14184407







