MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnosis of High-Risk Neuroblastoma
Circulating Free DNA and Circulating Free Cells
3. Current Therapies of High-Risk Neuroblastoma
3.1. Surgical Resection
3.2. Multi-Agent Chemotherapy
3.3. Autologous Stem Cell Transplantation
3.4. Radiation Therapy
3.5. Anti-GD2 Immunotherapy
3.6. Isotretinoin
4. MYCN as Prognostic Indicator in High-Risk Neuroblastoma
5. MYCN Determines High-Risk Neuroblastoma
6. MYCN as Therapeutic Target
6.1. BET Inhibitors
6.2. HDACs Inhibitors
6.3. PI3K/mTOR Inhibitors
6.4. Aurora Kinase-A Inhibitors
6.5. MDM2 Inhibitors
6.6. MYCN Direct Inhibitor
7. Challenge
7.1. Rarity of This Cancer
7.2. Diverse Prognosis
7.3. Initial Response Rates Are Not Optimal
7.4. Risk of Relapse
7.5. Measurement of Disease Extent
7.6. CNS Relapse
7.7. Minimizing Treatment-Related Morbidity
7.8. Distribution of Age of Patients
7.9. Access to New Drugs
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef]
- Ambros, P.F.; Ambros, I.M.; Brodeur, G.M.; Haber, M.; Khan, J.; Nakagawara, A.; Schleiermacher, G.; Speleman, F.; Spitz, R.; London, W.B.; et al. International Consensus for Neuroblastoma Molecular Diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Cancer 2009, 100, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Mayfield, J.R.; Raca, G.; Applebaum, M.A.; Chlenski, A.; Sukhanova, M.; Bagatell, R.; Irwin, M.S.; Little, A.; Rawwas, J.; et al. Segmental Chromosomal Aberrations in Localized Neuroblastoma Can Be Detected in Formalin-Fixed Paraffin-Embedded Tissue Samples and are Associated with Recurrence: Segmental Chromosomal Aberrations in Localized Neuroblastoma. Pediatr. Blood Cancer 2016, 63, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.; Desai, A. The Evolution of Risk Classification for Neuroblastoma. Children 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) Classification System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Rubie, H.; Hartmann, O.; Michon, J.; Frappaz, D.; Coze, C.; Chastagner, P.; Baranzelli, M.C.; Plantaz, D.; Avet-Loiseau, H.; Bénard, J.; et al. N-Myc Gene Amplification is a Major Prognostic Factor in Localized Neuroblastoma: Results of the French NBL 90 Study. Neuroblastoma Study Group of the Société Francaise d’Oncologie Pédiatrique. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 1171–1182. [Google Scholar] [CrossRef]
- Tang, X.X.; Zhao, H.; Kung, B.; Kim, D.Y.; Hicks, S.L.; Cohn, S.L.; Cheung, N.-K.; Seeger, R.C.; Evans, A.E.; Ikegaki, N. The MYCN Enigma: Significance of MYCN Expression in Neuroblastoma. Cancer Res. 2006, 66, 2826–2833. [Google Scholar] [CrossRef]
- Nisen, P.D.; Waber, P.G.; Rich, M.A.; Pierce, S.; Garvin, J.R.; Gilbert, F.; Lanzkowsky, P. N-Myc Oncogene RNA Expression in Neuroblastoma. J. Natl. Cancer Inst. 1988, 80, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.F.M.; van Bokhoven, H.; van Leeuwen, F.N.; Hulsbergen-van de Kaa, C.A.; de Vries, I.J.M.; Adema, G.J.; Hoogerbrugge, P.M.; de Brouwer, A.P.M. Regulation of MYCN Expression in Human Neuroblastoma Cells. BMC Cancer 2009, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Elsden, J.; Pearson, A.; Lunec, J. Genes Co-Amplified with MYCN in Neuroblastoma: Silent Passengers or Co-Determinants of Phenotype? Cancer Lett. 2003, 197, 81–86. [Google Scholar] [CrossRef]
- Schwab, M. MYCN in Neuronal Tumours. Cancer Lett. 2004, 204, 179–187. [Google Scholar] [CrossRef]
- Gherardi, S.; Valli, E.; Erriquez, D.; Perini, G. MYCN-Mediated Transcriptional Repression in Neuroblastoma: The Other Side of the Coin. Front. Oncol. 2013, 3, 42. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the P53-MDM2 Pathway for Neuroblastoma Therapy: Rays of Hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, A.J.; Bayliss, R.; Büchel, G.; Dang, C.V.; Eilers, M.; Gustafson, W.C.; Hansen, G.H.; Jura, N.; Knapp, S.; Lemmon, M.A.; et al. Drugging the “Undruggable” MYCN Oncogenic Transcription Factor: Overcoming Previous Obstacles to Impact Childhood Cancers. Cancer Res. 2021, 81, 1627–1632. [Google Scholar] [CrossRef]
- Yue, Z.-X.; Huang, C.; Gao, C.; Xing, T.-Y.; Liu, S.-G.; Li, X.-J.; Zhao, Q.; Wang, X.-S.; Zhao, W.; Jin, M.; et al. MYCN Amplification Predicts Poor Prognosis Based on Interphase Fluorescence in Situ Hybridization Analysis of Bone Marrow Cells in Bone Marrow Metastases of Neuroblastoma. Cancer Cell Int. 2017, 17, 43. [Google Scholar] [CrossRef]
- Campbell, K.; Gastier-Foster, J.M.; Mann, M.; Naranjo, A.H.; van Ryn, C.; Bagatell, R.; Matthay, K.K.; London, W.B.; Irwin, M.S.; Shimada, H.; et al. Association of MYCN Copy Number with Clinical Features, Tumor Biology, and Outcomes in Neuroblastoma: A Report from the Children’s Oncology Group. Cancer 2017, 123, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Swift, C.C.; Eklund, M.J.; Kraveka, J.M.; Alazraki, A.L. Updates in Diagnosis, Management, and Treatment of Neuroblastoma. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2018, 38, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Shi, S.; Ehlerding, E.B.; Graves, S.A.; Goel, S.; Engle, J.W.; Liang, J.; Tian, J.; Cai, W. Radiolabeled, Antibody-Conjugated Manganese Oxide Nanoparticles for Tumor Vasculature Targeted Positron Emission Tomography and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 38304–38312. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sever, Z.; Biassoni, L.; Shulkin, B.; Kong, G.; Hofman, M.S.; Lopci, E.; Manea, I.; Koziorowski, J.; Castellani, R.; Boubaker, A.; et al. Guidelines on Nuclear Medicine Imaging in Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2009–2024. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.S. Current Status of Functional Imaging in Neuroblastoma, Pheochromocytoma, and Paraganglioma Disease. Wien. Med. Wochenschr. 2019, 169, 25–32. [Google Scholar] [CrossRef]
- Sarioglu, F.C.; Salman, M.; Guleryuz, H.; Ozer, E.; Cecen, E.; Ince, D.; Olgun, N. Radiological Staging in Neuroblastoma: Computed Tomography or Magnetic Resonance Imaging? Pol. J. Radiol. 2019, 84, e46–e53. [Google Scholar] [CrossRef]
- Sofka, C.M.; Semelka, R.C.; Kelekis, N.L.; Worawattanakul, S.; Chung, C.J.; Gold, S.; Fordham, L.A. Magnetic Resonance Imaging of Neuroblastoma Using Current Techniques. Magn. Reson. Imaging 1999, 17, 193–198. [Google Scholar] [CrossRef]
- Wu, H.; Wu, C.; Zheng, H.; Wang, L.; Guan, W.; Duan, S.; Wang, D. Radiogenomics of Neuroblastoma in Pediatric Patients: CT-Based Radiomics Signature in Predicting MYCN Amplification. Eur. Radiol. 2021, 31, 3080–3089. [Google Scholar] [CrossRef]
- Sharp, S.E.; Parisi, M.T.; Gelfand, M.J.; Yanik, G.A.; Shulkin, B.L. Functional-Metabolic Imaging of Neuroblastoma. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 6–20. [Google Scholar]
- Campbell, K.; Shyr, D.; Bagatell, R.; Fischer, M.; Nakagawara, A.; Nieto, A.C.; Brodeur, G.M.; Matthay, K.K.; London, W.B.; DuBois, S.G. Comprehensive Evaluation of Context Dependence of the Prognostic Impact of MYCN Amplification in Neuroblastoma: A Report from the International Neuroblastoma Risk Group (INRG) Project. Pediatr. Blood Cancer 2019, 66, e27819. [Google Scholar] [CrossRef]
- Yanishevski, D.; McCarville, M.B.; Doubrovin, M.; Spiegl, H.R.; Zhao, X.; Lu, Z.; Federico, S.M.; Furman, W.L.; Murphy, A.J.; Davidoff, A.M. Impact of MYCN Status on Response of High-Risk Neuroblastoma to Neoadjuvant Chemotherapy. J. Pediatr. Surg. 2020, 55, 130–134. [Google Scholar] [CrossRef]
- Chan, H.S.; Gallie, B.L.; DeBoer, G.; Haddad, G.; Ikegaki, N.; Dimitroulakos, J.; Yeger, H.; Ling, V. MYCN Protein Expression as a Predictor of Neuroblastoma Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 1699–1706. [Google Scholar]
- Van Heerden, J.; Esterhuizen, T.M.; Hendricks, M.; Poole, J.; Büchner, A.; Naidu, G.; du Plessis, J.; van Emmenes, B.; Uys, R.; Hadley, G.P.; et al. Age at Diagnosis as a Prognostic Factor in South African Children with Neuroblastoma. Pediatr. Blood Cancer 2021, 68, e28878. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.; Desai, A.V.; Applebaum, M.A.; Valteau-Couanet, D.; Park, J.R.; Pearson, A.D.J.; Schleiermacher, G.; Irwin, M.S.; Hogarty, M.; Naranjo, A.; et al. Age, Diagnostic Category, Tumor Grade, and Mitosis-Karyorrhexis Index are Independently Prognostic in Neuroblastoma: An INRG Project. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Valentine, M.B.; Bowman, L.C.; Rowe, S.T.; Nash, M.B.; Valentine, V.A.; Cohn, S.L.; Castleberry, R.P.; Brodeur, G.M.; Look, A.T. Detection of MYCN Gene Amplification in Neuroblastoma by Fluorescence in Situ Hybridization: A Pediatric Oncology Group Study. Neoplasia 2001, 3, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.A.; Thorner, P.A.; Marrano, P.A.; Parkinson, D.I.; Ng, Y.K.; Gerrie, B.L.; Chilton-Macneill, S.; Zielenska, M. Identification of MYCN Copy Number Heterogeneity by Direct FISH Analysis of Neuroblastoma Preparations. Mol. Diagn. 1996, 1, 281–289. [Google Scholar] [CrossRef]
- Marrano, P.; Irwin, M.S.; Thorner, P.S. Heterogeneity of MYCN Amplification in Neuroblastoma at Diagnosis, Treatment, Relapse, and Metastasis. Genes Chromosom. Cancer 2017, 56, 28–41. [Google Scholar] [CrossRef]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Namløs, H.M.; Boye, K.; Mishkin, S.J.; Barøy, T.; Lorenz, S.; Bjerkehagen, B.; Stratford, E.W.; Munthe, E.; Kudlow, B.A.; Myklebost, O.; et al. Noninvasive Detection of CtDNA Reveals Intratumor Heterogeneity and is Associated with Tumor Burden in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2018, 17, 2473–2480. [Google Scholar] [CrossRef]
- Combaret, V.; Audoynaud, C.; Iacono, I.; Favrot, M.-C.; Schell, M.; Bergeron, C.; Puisieux, A. Circulating MYCN DNA as a Tumor-Specific Marker in Neuroblastoma Patients. Cancer Res. 2002, 62, 3646–3648. [Google Scholar]
- Combaret, V.; Bergeron, C.; Noguera, R.; Iacono, I.; Puisieux, A. Circulating MYCN DNA Predicts MYCN-Amplification in Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8919–8920; author reply 8920. [Google Scholar] [CrossRef]
- Trigg, R.M.; Turner, S.D.; Shaw, J.A.; Jahangiri, L. Diagnostic Accuracy of Circulating-Free DNA for the Determination of MYCN Amplification Status in Advanced-Stage Neuroblastoma: A Systematic Review and Meta-Analysis. Br. J. Cancer 2020, 122, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Hosoi, H.; Iehara, T.; Kuwahara, Y.; Osone, S.; Tsuchiya, K.; Ohira, M.; Nakagawara, A.; Kuroda, H.; Sugimoto, T. Prediction of MYCN Amplification in Neuroblastoma Using Serum DNA and Real-Time Quantitative Polymerase Chain Reaction. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Iehara, T.; Yagyu, S.; Gotoh, T.; Ouchi, K.; Yoshida, H.; Miyachi, M.; Kikuchi, K.; Sugimoto, T.; Hosoi, H. A Prospective Evaluation of Liquid Biopsy for Detecting MYCN Amplification in Neuroblastoma Patients. Jpn. J. Clin. Oncol. 2019, 49, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, P.; D’Argenio, V.; del Re, M.; Pellegrini, C.; Cucchiara, F.; Salvianti, F.; Galbiati, S. Updates on Liquid Biopsy: Current Trends and Future Perspectives for Clinical Application in Solid Tumors. Clin. Chem. Lab. Med. 2021, 59, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Rifatbegovic, F.; Frech, C.; Abbasi, M.R.; Taschner-Mandl, S.; Weiss, T.; Schmidt, W.M.; Schmidt, I.; Ladenstein, R.; Ambros, I.M.; Ambros, P.F. Neuroblastoma Cells Undergo Transcriptomic Alterations upon Dissemination into the Bone Marrow and Subsequent Tumor Progression. Int. J. Cancer 2018, 142, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Reza, K.K.; Dey, S.; Wuethrich, A.; Wang, J.; Behren, A.; Antaw, F.; Wang, Y.; Sina, A.A.I.; Trau, M. In Situ Single Cell Proteomics Reveals Circulating Tumor Cell Heterogeneity during Treatment. ACS Nano 2021, 15, 11231–11243. [Google Scholar] [CrossRef] [PubMed]
- Lodrini, M.; Wünschel, J.; Thole-Kliesch, T.M.; Grimaldi, M.; Sprüssel, A.; Linke, R.B.; Hollander, J.F.; Tiburtius, D.; Künkele, A.; Schulte, J.H.; et al. Circulating Cell-Free DNA Assessment in Biofluids from Children with Neuroblastoma Demonstrates Feasibility and Potential for Minimally Invasive Molecular Diagnostics. Cancers 2022, 14, 2080. [Google Scholar] [CrossRef]
- Beltran, H.; Jendrisak, A.; Landers, M.; Mosquera, J.M.; Kossai, M.; Louw, J.; Krupa, R.; Graf, R.P.; Schreiber, N.A.; Nanus, D.M.; et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1510–1519. [Google Scholar] [CrossRef]
- Shaw, J.A.; Guttery, D.S.; Hills, A.; Fernandez-Garcia, D.; Page, K.; Rosales, B.M.; Goddard, K.S.; Hastings, R.K.; Luo, J.; Ogle, O.; et al. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 88–96. [Google Scholar] [CrossRef]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef]
- DuBois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and Biological Approach to Risk Stratification and Treatment. Cell Tissue Res. 2018, 372, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Schmidt, M.L.; Cohn, S.L.; Maris, J.M.; London, W.B.; Buxton, A.; Stram, D.; Castleberry, R.P.; Shimada, H.; Sandler, A.; et al. Outcome after Reduced Chemotherapy for Intermediate-Risk Neuroblastoma. N. Engl. J. Med. 2010, 363, 1313–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubie, H.; de Bernardi, B.; Gerrard, M.; Canete, A.; Ladenstein, R.; Couturier, J.; Ambros, P.; Munzer, C.; Pearson, A.D.J.; Garaventa, A.; et al. Excellent Outcome with Reduced Treatment in Infants with Nonmetastatic and Unresectable Neuroblastoma without MYCN Amplification: Results of the Prospective INES 99.1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 449–455. [Google Scholar] [CrossRef]
- Strother, D.R.; London, W.B.; Schmidt, M.L.; Brodeur, G.M.; Shimada, H.; Thorner, P.; Collins, M.H.; Tagge, E.; Adkins, S.; Reynolds, C.P.; et al. Outcome after Surgery Alone or with Restricted Use of Chemotherapy for Patients with Low-Risk Neuroblastoma: Results of Children’s Oncology Group Study P9641. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1842–1848. [Google Scholar] [CrossRef]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of High-Risk Neuroblastoma with Intensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-Cis-Retinoic Acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Yanik, G.; Naranjo, A.; Parisi, M.T.; Shulkin, B.L.; Nadel, H.; Gelfand, M.J.; Ladenstein, R.; Boubaker, A.; Poetschger, U.; Valteau-Couanet, D.; et al. Impact of Post-Induction Curie Scores in High-Risk Neuroblastoma. Biol. Blood Marrow Transplant. 2015, 21, S107. [Google Scholar] [CrossRef]
- Yanik, G.A.; Parisi, M.T.; Naranjo, A.; Nadel, H.; Gelfand, M.J.; Park, J.R.; Ladenstein, R.L.; Poetschger, U.; Boubaker, A.; Valteau-Couanet, D.; et al. Validation of Postinduction Curie Scores in High-Risk Neuroblastoma: A Children’s Oncology Group and SIOPEN Group Report on SIOPEN/HR-NBL1. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 502–508. [Google Scholar] [CrossRef]
- Rojas, Y.; Jaramillo, S.; Lyons, K.; Mahmood, N.; Wu, M.-F.; Liu, H.; Vasudevan, S.A.; Guillerman, R.P.; Louis, C.U.; Russell, H.V.; et al. The Optimal Timing of Surgical Resection in High-Risk Neuroblastoma. J. Pediatr. Surg. 2016, 51, 1665–1669. [Google Scholar] [CrossRef]
- Vollmer, K.; Gfroerer, S.; Theilen, T.-M.; Bochennek, K.; Klingebiel, T.; Rolle, U.; Fiegel, H. Radical Surgery Improves Survival in Patients with Stage 4 Neuroblastoma. World J. Surg. 2018, 42, 1877–1884. [Google Scholar] [CrossRef]
- Englum, B.R.; Rialon, K.L.; Speicher, P.J.; Gulack, B.; Driscoll, T.A.; Kreissman, S.G.; Rice, H.E. Value of Surgical Resection in Children with High-Risk Neuroblastoma. Pediatr. Blood Cancer 2015, 62, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhan, J. Roles of Surgery in the Treatment of Patients with High-Risk Neuroblastoma in the Children Oncology Group Study: A Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Von Allmen, D.; Davidoff, A.M.; London, W.B.; van Ryn, C.; Haas-Kogan, D.A.; Kreissman, S.G.; Khanna, G.; Rosen, N.; Park, J.R.; la Quaglia, M.P. Impact of Extent of Resection on Local Control and Survival in Patients from the COG A3973 Study with High-Risk Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, G.M.; Seeger, R.C.; Barrett, A.; Berthold, F.; Castleberry, R.P.; D’Angio, G.; de Bernardi, B.; Evans, A.E.; Favrot, M.; Freeman, A.I. International Criteria for Diagnosis, Staging, and Response to Treatment in Patients with Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1988, 6, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.L.; Akinkuotu, A.; Pierro, A.; Morgenstern, D.A.; Irwin, M.S. The Role of Surgery in High-Risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2020, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; de Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the International Criteria for Neuroblastoma Diagnosis, Staging, and Response to Treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Chui, C. Effects of Preoperative Chemotherapy on Neuroblastoma with MYCN Amplification: A Surgeon’s Perspective. World J. Pediatr. Surg. 2020, 3, e000129. [Google Scholar] [CrossRef]
- Adkins, E.S.; Sawin, R.; Gerbing, R.B.; London, W.B.; Matthay, K.K.; Haase, G.M. Efficacy of Complete Resection for High-Risk Neuroblastoma: A Children’s Cancer Group Study. J. Pediatr. Surg. 2004, 39, 931–936. [Google Scholar] [CrossRef]
- Varan, A.; Ali, V.; Kesik, V.; Vural, K.; Şenocak, M.E.; Emin, Ş.M.; Kale, G.; Gulsev, K.; Akyüz, C.; Canan, A.; et al. The Efficacy of Delayed Surgery in Children with High-Risk Neuroblastoma. J. Cancer Res. Ther. 2015, 11, 268–271. [Google Scholar] [CrossRef]
- Fischer, J.; Pohl, A.; Volland, R.; Hero, B.; Dübbers, M.; Cernaianu, G.; Berthold, F.; von Schweinitz, D.; Simon, T. Complete Surgical Resection Improves Outcome in INRG High-Risk Patients with Localized Neuroblastoma Older than 18 Months. BMC Cancer 2017, 17, 520. [Google Scholar] [CrossRef]
- Peinemann, F.; Tushabe, D.A.; van Dalen, E.C.; Berthold, F. Rapid COJEC versus Standard Induction Therapies for High-Risk Neuroblastoma. Cochrane Database Syst. Rev. 2015, 5, CD010774. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.D.J.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D.; European Neuroblastoma Study Group; Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group). High-Dose Rapid and Standard Induction Chemotherapy for Patients Aged over 1 Year with Stage 4 Neuroblastoma: A Randomised Trial. Lancet Oncol. 2008, 9, 247–256. [Google Scholar] [CrossRef]
- Hobbie, W.L.; Li, Y.; Carlson, C.; Goldfarb, S.; Laskin, B.; Denburg, M.; Goldmuntz, E.; Mostoufi-Moab, S.; Wilkes, J.; Smith, K.; et al. Late Effects in Survivors of High-Risk Neuroblastoma Following Stem Cell Transplant with and without Total Body Irradiation. Pediatr. Blood Cancer 2022, 69, e29537. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.E.; Danner-Koptik, K.; Golden, S.; Schneiderman, J.; Kletzel, M.; Reichek, J.; Gosiengfiao, Y. Late Effects in Pediatric High-Risk Neuroblastoma Survivors After Intensive Induction Chemotherapy Followed by Myeloablative Consolidation Chemotherapy and Triple Autologous Stem Cell Transplants. J. Pediatr. Hematol. Oncol. 2018, 40, 31–35. [Google Scholar] [CrossRef]
- Bertolini, P.; Lassalle, M.; Mercier, G.; Raquin, M.A.; Izzi, G.; Corradini, N.; Hartmann, O. Platinum Compound-Related Ototoxicity in Children: Long-Term Follow-up Reveals Continuous Worsening of Hearing Loss. J. Pediatr. Hematol. Oncol. 2004, 26, 649–655. [Google Scholar] [CrossRef]
- Gurney, J.G.; Tersak, J.M.; Ness, K.K.; Landier, W.; Matthay, K.K.; Schmidt, M.L.; Children’s Oncology Group. Hearing Loss, Quality of Life, and Academic Problems in Long-Term Neuroblastoma Survivors: A Report from the Children’s Oncology Group. Pediatrics 2007, 120, e1229–e1236. [Google Scholar] [CrossRef]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature Menopause in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Laverdière, C.; Cheung, N.-K.V.; Kushner, B.H.; Kramer, K.; Modak, S.; LaQuaglia, M.P.; Wolden, S.; Ness, K.K.; Gurney, J.G.; Sklar, C.A. Long-Term Complications in Survivors of Advanced Stage Neuroblastoma. Pediatr. Blood Cancer 2005, 45, 324–332. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Armstrong, G.T.; Huang, S.; Ness, K.K.; Ehrhardt, M.J.; Joshi, V.M.; Plana, J.C.; Soliman, E.Z.; Green, D.M.; Srivastava, D.; et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-Sectional Study. Ann. Intern. Med. 2016, 164, 93–101. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events among Adult Survivors of Childhood Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Applebaum, M.A.; Vaksman, Z.; Lee, S.M.; Hungate, E.A.; Henderson, T.O.; London, W.B.; Pinto, N.; Volchenboum, S.L.; Park, J.R.; Naranjo, A.; et al. Neuroblastoma Survivors are at Increased Risk for Second Malignancies: A Report from the International Neuroblastoma Risk Group Project. Eur. J. Cancer 2017, 72, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.M.; Allewelt, H.B.; Spunt, S.L.; Hudson, M.M.; Wu, J.; Billups, C.A.; Jenkins, J.; Santana, V.M.; Furman, W.L.; McGregor, L.M. Subsequent Malignant Neoplasms in Pediatric Patients Initially Diagnosed with Neuroblastoma. J. Pediatr. Hematol. Oncol. 2015, 37, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.J.; Krull, K.R.; Chen, Y.; Diller, L.; Yasui, Y.; Leisenring, W.; Brouwers, P.; Howell, R.; Lai, J.-S.; Balsamo, L.; et al. Long-Term Psychological and Educational Outcomes for Survivors of Neuroblastoma: A Report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 3220–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.E.; Gordon, J.H.; Popovsky, E.Y.; Gunawardene, S.; Duffey-Lind, E.; Lehmann, L.E.; Diller, L.R. Late Effects in Children Treated with Intensive Multimodal Therapy for High-Risk Neuroblastoma: High Incidence of Endocrine and Growth Problems. Bone Marrow Transplant. 2014, 49, 502–508. [Google Scholar] [CrossRef]
- Institute of Medicine (US); National Research Council (US); National Cancer Policy Board. Childhood Cancer Survivorship: Improving Care and Quality of Life; Hewitt, M., Weiner, S.L., Simone, J.V., Eds.; National Academies Press (US): Washington, DC, USA, 2003; ISBN 978-0-309-08898-5. [Google Scholar]
- Frei, E.; Teicher, B.A.; Holden, S.A.; Cathcart, K.N.; Wang, Y.Y. Preclinical Studies and Clinical Correlation of the Effect of Alkylating Dose. Cancer Res. 1988, 48, 6417–6423. [Google Scholar]
- Cheung, N.V.; Heller, G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 1050–1058. [Google Scholar] [CrossRef]
- Daniel, D.; Crawford, J. Myelotoxicity from Chemotherapy. Semin. Oncol. 2006, 33, 74–85. [Google Scholar] [CrossRef]
- Fish, J.; Grupp, S. Stem Cell Transplantation for Neuroblastoma. Bone Marrow Transplant. 2008, 41, 159–165. [Google Scholar] [CrossRef]
- Mora, J. Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review. Cancers 2022, 14, 2572. [Google Scholar] [CrossRef]
- Yalçin, B.; Kremer, L.C.M.; van Dalen, E.C. High-Dose Chemotherapy and Autologous Haematopoietic Stem Cell Rescue for Children with High-Risk Neuroblastoma. Cochrane Database Syst. Rev. 2015, 8, CD006301. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.L.; Bunin, N.J.; Callahan, C.; Aplenc, R.; Griffin, G.; Grupp, S.A. An Unexpectedly High Incidence of Epstein-Barr Virus Lymphoproliferative Disease after CD34+ Selected Autologous Peripheral Blood Stem Cell Transplant in Neuroblastoma. Bone Marrow Transplant. 2004, 33, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; AlSayyad, K.; Siddiqui, K.; AlAnazi, A.; AlSeraihy, A.; AlAhmari, A.; ElSolh, H.; Ghemlas, I.; AlSaedi, H.; AlJefri, A.; et al. Pediatric High Risk Neuroblastoma with Autologous Stem Cell Transplant—20 Years of Experience. Int. J. Pediatr. Adolesc. Med. 2021, 8, 253–257. [Google Scholar] [CrossRef]
- Rill, D.R.; Santana, V.M.; Roberts, W.M.; Nilson, T.; Bowman, L.C.; Krance, R.A.; Heslop, H.E.; Moen, R.C.; Ihle, J.N.; Brenner, M.K. Direct Demonstration That Autologous Bone Marrow Transplantation for Solid Tumors Can Return a Multiplicity of Tumorigenic Cells. Blood 1994, 84, 380–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.; Hero, B.; Schulte, J.H.; Deubzer, H.; Hundsdoerfer, P.; von Schweinitz, D.; Fuchs, J.; Schmidt, M.; Prasad, V.; Krug, B.; et al. 2017 GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klin. Padiatr. 2017, 229, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.E.; London, W.B.; Kreissman, S.G.; Villablanca, J.G.; Davidoff, A.M.; DeSantes, K.; Castleberry, R.P.; Murray, K.; Diller, L.; Matthay, K.; et al. Role of the Extent of Prophylactic Regional Lymph Node Radiotherapy on Survival in High-Risk Neuroblastoma: A Report from the COG A3973 Study. Pediatr. Blood Cancer 2019, 66, e27736. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Ahn, S.D.; Koh, M.; Kim, J.H.; Lee, S.-W.; Song, S.Y.; Yoon, S.M.; Kim, Y.S.; Kim, S.S.; Park, J.-H.; et al. Patterns of Recurrence after Radiation Therapy for High-Risk Neuroblastoma. Radiat. Oncol. J. 2019, 37, 224–231. [Google Scholar] [CrossRef]
- Ferris, M.J.; Danish, H.; Switchenko, J.M.; Deng, C.; George, B.A.; Goldsmith, K.C.; Wasilewski, K.J.; Cash, W.T.; Khan, M.K.; Eaton, B.R.; et al. Favorable Local Control from Consolidative Radiation Therapy in High-Risk Neuroblastoma Despite Gross Residual Disease, Positive Margins, or Nodal Involvement. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 806–812. [Google Scholar] [CrossRef]
- Jazmati, D.; Butzer, S.; Hero, B.; Doyen, J.; Ahmad Khalil, D.; Steinmeier, T.; Schulze Schleithoff, S.; Eggert, A.; Simon, T.; Timmermann, B. Long-Term Follow-up of Children with Neuroblastoma Receiving Radiotherapy to Metastatic Lesions within the German Neuroblastoma Trials NB97 and NB 2004. Strahlenther. Onkol. 2021, 197, 683–689. [Google Scholar] [CrossRef]
- Liu, K.X.; Naranjo, A.; Zhang, F.F.; DuBois, S.G.; Braunstein, S.E.; Voss, S.D.; Khanna, G.; London, W.B.; Doski, J.J.; Geiger, J.D.; et al. Prospective Evaluation of Radiation Dose Escalation in Patients with High-Risk Neuroblastoma and Gross Residual Disease After Surgery: A Report from the Children’s Oncology Group ANBL0532 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2741–2752. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Zhang, Y.; Meng, L.; Wei, J.; Wang, B.; Wang, H.; Xin, Y.; Dong, L.; Jiang, X. Role and Toxicity of Radiation Therapy in Neuroblastoma Patients: A Literature Review. Crit. Rev. Oncol. Hematol. 2020, 149, 102924. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, J.A.; Rombi, B.; Yock, T.I.; Broussard, G.; Friedmann, A.M.; Huang, M.; Chen, Y.-L.E.; Lu, H.-M.; Kooy, H.; MacDonald, S.M. Proton Radiotherapy for High-Risk Pediatric Neuroblastoma: Early Outcomes and Dose Comparison. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.D.; Yanik, G.; Naranjo, A.; Zhang, F.F.; Fitzgerald, W.; Shulkin, B.L.; Parisi, M.T.; Russell, H.; Grupp, S.; Pater, L.; et al. A Safety and Feasibility Trial of 131I-MIBG in Newly Diagnosed High-Risk Neuroblastoma: A Children’s Oncology Group Study. Pediatr. Blood Cancer 2021, 68, e29117. [Google Scholar] [CrossRef] [PubMed]
- Weyl Ben-Arush, M.; Ben Barak, A.; Bar-Deroma, R.; Ash, S.; Goldstein, G.; Golan, H.; Houri, H.; Waldman, D.; Nevo, N.; Bar Shalom, R.; et al. Targeted Therapy with Low Doses of 131I-MIBG is Effective for Disease Palliation in Highly Refractory Neuroblastoma. Isr. Med. Assoc. J. 2013, 15, 31–34. [Google Scholar]
- Genolla, J.; Rodriguez, T.; Minguez, P.; Lopez-Almaraz, R.; Llorens, V.; Echebarria, A. Dosimetry-Based High-Activity Therapy with 131I-Metaiodobenzylguanidine (131I-MIBG) and Topotecan for the Treatment of High-Risk Refractory Neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1567–1575. [Google Scholar] [CrossRef]
- Ducassou, A.; Gambart, M.; Munzer, C.; Padovani, L.; Carrie, C.; Haas-Kogan, D.; Bernier-Chastagner, V.; Demoor, C.; Claude, L.; Helfre, S.; et al. Long-Term Side Effects of Radiotherapy for Pediatric Localized Neuroblastoma: Results from Clinical Trials NB90 and NB94. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al 2015, 191, 604–612. [Google Scholar] [CrossRef]
- Yu, J.I.; Lim, D.H.; Jung, S.H.; Sung, K.W.; Yoo, S.-Y.; Nam, H. The Effects of Radiation Therapy on Height and Spine MRI Characteristics in Children with Neuroblastoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 114, 384–388. [Google Scholar] [CrossRef]
- Paulino, A.C.; Mayr, N.A.; Simon, J.H.; Buatti, J.M. Locoregional Control in Infants with Neuroblastoma: Role of Radiation Therapy and Late Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1025–1031. [Google Scholar] [CrossRef]
- Sutton, E.J.; Tong, R.T.; Gillis, A.M.; Henning, T.D.; Weinberg, V.A.; Boddington, S.; Haas-Kogan, D.A.; Matthay, K.; Sha, V.; Gooding, C.; et al. Decreased Aortic Growth and Middle Aortic Syndrome in Patients with Neuroblastoma after Radiation Therapy. Pediatr. Radiol. 2009, 39, 1194–1202. [Google Scholar] [CrossRef]
- Stauder, M.C.; Laack, N.N.I.; Moir, C.R.; Schomberg, P.J. Excellent Local Control and Survival after Intraoperative and External Beam Radiotherapy for Pediatric Solid Tumors: Long-Term Follow-up of the Mayo Clinic Experience. J. Pediatr. Hematol. Oncol. 2011, 33, 350–355. [Google Scholar] [CrossRef]
- Massimino, M.; Bode, U.; Biassoni, V.; Fleischhack, G. Nimotuzumab for Pediatric Diffuse Intrinsic Pontine Gliomas. Expert Opin. Biol. Ther. 2011, 11, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.V.; Alderete, D.; Solano-Paez, P.; Rugilo, C.; Freytes, C.; Laughlin, S.; Fonseca, A.; Bartels, U.; Tabori, U.; Bouffet, E.; et al. Bevacizumab for Pediatric Radiation Necrosis. Neuro-Oncol. Pract. 2020, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Chamberlain, E.; Kramer, K.; Modak, S. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined with Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients with Stage 4 Neuroblastoma in First Remission. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Heitzeneder, S.; Mackall, C.L. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell 2017, 31, 476–485. [Google Scholar] [CrossRef]
- Ladisch, S.; Wu, Z.L.; Feig, S.; Ulsh, L.; Schwartz, E.; Floutsis, G.; Wiley, F.; Lenarsky, C.; Seeger, R. Shedding of GD2 Ganglioside by Human Neuroblastoma. Int. J. Cancer 1987, 39, 73–76. [Google Scholar] [CrossRef]
- Sabbih, G.O.; Danquah, M.K. Neuroblastoma GD2 Expression and Computational Analysis of Aptamer-Based Bioaffinity Targeting. Int. J. Mol. Sci. 2021, 22, 9101. [Google Scholar] [CrossRef]
- Mujoo, K.; Cheresh, D.A.; Yang, H.M.; Reisfeld, R.A. Disialoganglioside GD2 on Human Neuroblastoma Cells: Target Antigen for Monoclonal Antibody-Mediated Cytolysis and Suppression of Tumor Growth. Cancer Res. 1987, 47, 1098–1104. [Google Scholar]
- Ahmed, M.; Cheung, N.-K.V. Engineering Anti-GD2 Monoclonal Antibodies for Cancer Immunotherapy. FEBS Lett. 2014, 588, 288–297. [Google Scholar] [CrossRef]
- Cheung, N.K.; Saarinen, U.M.; Neely, J.E.; Landmeier, B.; Donovan, D.; Coccia, P.F. Monoclonal Antibodies to a Glycolipid Antigen on Human Neuroblastoma Cells. Cancer Res. 1985, 45, 2642–2649. [Google Scholar]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; Mueller, B.M.; Handgretinger, R.; Herter, M.; Yu, A.L.; Reisfeld, R.A. Effect of a Chimeric Anti-Ganglioside GD2 Antibody on Cell-Mediated Lysis of Human Neuroblastoma Cells. Cancer Res. 1991, 51, 144–149. [Google Scholar] [PubMed]
- Uttenreuther-Fischer, M.M.; Huang, C.S.; Yu, A.L. Pharmacokinetics of Human-Mouse Chimeric Anti-GD2 MAb Ch14.18 in a Phase I Trial in Neuroblastoma Patients. Cancer Immunol. Immunother. 1995, 41, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Sondel, P.M.; Barfield, R.; Shulkin, B.L.; Kaufman, R.A.; Allay, J.A.; Gan, J.; Hutson, P.; Seo, S.; Kim, K.; et al. Phase I Trial of a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A, Designed to Decrease Toxicity in Children with Refractory or Recurrent Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Kushner, B.H.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K.V. Phase I Trial of Anti-GD2 Monoclonal Antibody Hu3F8 plus GM-CSF: Impact of Body Weight, Immunogenicity and Anti-GD2 Response on Pharmacokinetics and Survival. Oncoimmunology 2017, 6, e1358331. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Naxitamab: First Approval. Drugs 2021, 81, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.; Lurvink, R.; Aleven, L.; Mensink, M.; Wolfs, T.; Dierselhuis, M.; van Eijkelenburg, N.; Kraal, K.; van Noesel, M.; van Grotel, M.; et al. Treatment-Related Toxicities During Anti-GD2 Immunotherapy in High-Risk Neuroblastoma Patients. Front. Oncol. 2020, 10, 601076. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Otto, M.; Baldwin, W.M.; Vail, E.; Gillies, S.D.; Handgretinger, R.; Barfield, R.C.; Yu, H.M.; Yu, A.L. Anti-GD(2) with an FC Point Mutation Reduces Complement Fixation and Decreases Antibody-Induced Allodynia. Pain 2010, 149, 135–142. [Google Scholar] [CrossRef]
- Tse, B.C.; Navid, F.; Billups, C.A.; O’Donnell, T.; Hoehn, M.E. Ocular Abnormalities in Patients Treated with a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2015, 19, 112–115. [Google Scholar] [CrossRef]
- Yuki, N.; Yamada, M.; Tagawa, Y.; Takahashi, H.; Handa, S. Pathogenesis of the Neurotoxicity Caused by Anti-GD2 Antibody Therapy. J. Neurol. Sci. 1997, 149, 127–130. [Google Scholar] [CrossRef]
- Ceylan, K.; Jahns, L.J.; Lode, B.N.; Ehlert, K.; Kietz, S.; Troschke-Meurer, S.; Siebert, N.; Lode, H.N. Inflammatory Response and Treatment Tolerance of Long-Term Infusion of the Anti-GD2 Antibody Ch14.18/CHO in Combination with Interleukin-2 in Patients with High-Risk Neuroblastoma. Pediatr. Blood Cancer 2018, 65, e26967. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Dorvillius, M.; Cochonneau, D.; Chaumette, T.; Xiao, W.; Diccianni, M.B.; Barbet, J.; Yu, A.L.; Paris, F.; Sorkin, L.S.; et al. Chimeric Antibody c.8B6 to O-Acetyl-GD2 Mediates the Same Efficient Anti-Neuroblastoma Effects as Therapeutic Ch14.18 Antibody to GD2 without Antibody Induced Allodynia. PLoS ONE 2014, 9, e87210. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J.; Fougeray, S.; Bahri, M.; Cochonneau, D.; Clémenceau, B.; Paris, F.; Heczey, A.; Birklé, S. Targeting O-Acetyl-GD2 Ganglioside for Cancer Immunotherapy. J. Immunol. Res. 2017, 2017, 5604891. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Veal, G.J.; Errington, J.; Rowbotham, S.E.; Illingworth, N.A.; Malik, G.; Cole, M.; Daly, A.K.; Pearson, A.D.J.; Boddy, A.V. Adaptive Dosing Approaches to the Individualization of 13-Cis-Retinoic Acid (Isotretinoin) Treatment for Children with High-Risk Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 469–479. [Google Scholar] [CrossRef]
- Reynolds, C.P.; Kane, D.J.; Einhorn, P.A.; Matthay, K.K.; Crouse, V.L.; Wilbur, J.R.; Shurin, S.B.; Seeger, R.C. Response of Neuroblastoma to Retinoic Acid In Vitro and In Vivo. Prog. Clin. Biol. Res. 1991, 366, 203–211. [Google Scholar]
- Armstrong, J.L.; Ruiz, M.; Boddy, A.V.; Redfern, C.P.F.; Pearson, A.D.J.; Veal, G.J. Increasing the Intracellular Availability of All-Trans Retinoic Acid in Neuroblastoma Cells. Br. J. Cancer 2005, 92, 696–704. [Google Scholar] [CrossRef]
- Reynolds, C.P. Differentiating Agents in Pediatric Malignancies: Retinoids in Neuroblastoma. Curr. Oncol. Rep. 2000, 2, 511–518. [Google Scholar] [CrossRef]
- Matthay, K.K. Targeted Isotretinoin in Neuroblastoma: Kinetics, Genetics or Absorption. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 311–313. [Google Scholar] [CrossRef]
- Reynolds, C.P.; Lemons, R.S. Retinoid Therapy of Childhood Cancer. Hematol. Oncol. Clin. N. Am. 2001, 15, 867–910. [Google Scholar] [CrossRef]
- Pile, H.D.; Sadiq, N.M. Isotretinoin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pinto, N.; DuBois, S.G.; Marachelian, A.; Diede, S.J.; Taraseviciute, A.; Glade Bender, J.L.; Tsao-Wei, D.; Groshen, S.G.; Reid, J.M.; Haas-Kogan, D.A.; et al. Phase I Study of Vorinostat in Combination with Isotretinoin in Patients with Refractory/Recurrent Neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) Trial. Pediatr. Blood Cancer 2018, 65, e27023. [Google Scholar] [CrossRef] [PubMed]
- Pennington, B.; Ren, S.; Barton, S.; Bacelar, M.; Edwards, S.J. Dinutuximab Beta for Treating Neuroblastoma: An Evidence Review Group and Decision Support Unit Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 2019, 37, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Shusterman, S.; Naranjo, A.; van Ryn, C.; Hank, J.A.; Parisi, M.T.; Shulkin, B.L.; Servaes, S.; London, W.B.; Shimada, H.; Gan, J.; et al. Antitumor Activity and Tolerability of Hu14.18-IL2 with GMCSF and Isotretinoin in Recurrent or Refractory Neuroblastoma: A Children’s Oncology Group Phase II Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6044–6051. [Google Scholar] [CrossRef]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and Recent Advances in the Treatment of Neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, M.F.; Gilman, A.L.; London, W.B.; Naranjo, A.; Diccianni, M.B.; Tenney, S.C.; Smith, M.; Messer, K.S.; Seeger, R.; Reynolds, C.P.; et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Front. Immunol. 2018, 9, 1355. [Google Scholar] [CrossRef]
- Tonini, G.P.; Boni, L.; Pession, A.; Rogers, D.; Iolascon, A.; Basso, G.; Cordero di Montezemolo, L.; Casale, F.; Pession, A.; Perri, P.; et al. MYCN Oncogene Amplification in Neuroblastoma is Associated with Worse Prognosis, except in Stage 4s: The Italian Experience with 295 Children. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 85–93. [Google Scholar] [CrossRef]
- Brodeur, G.M. Neuroblastoma: Biological Insights into a Clinical Enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-Myc in Untreated Human Neuroblastomas Correlates with Advanced Disease Stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of Multiple Copies of the N-Myc Oncogene with Rapid Progression of Neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Lampis, S.; Raieli, S.; Montemurro, L.; Bartolucci, D.; Amadesi, C.; Bortolotti, S.; Angelucci, S.; Scardovi, A.L.; Nieddu, G.; Cerisoli, L.; et al. The MYCN Inhibitor BGA002 Restores the Retinoic Acid Response Leading to Differentiation or Apoptosis by the MTOR Block in MYCN-Amplified Neuroblastoma. J. Exp. Clin. Cancer Res. 2022, 41, 160. [Google Scholar] [CrossRef] [PubMed]
- Rickman, D.S.; Schulte, J.H.; Eilers, M. The Expanding World of N-MYC–Driven Tumors. Cancer Discov. 2018, 8, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Harashima, H.; Dissmeyer, N.; Schnittger, A. Cell Cycle Control across the Eukaryotic Kingdom. Trends Cell Biol. 2013, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.-W.; Tan, F.; Cassano, H.; Lee, J.; Lee, K.C.; Thiele, C.J. Use of RNA Interference to Elucidate the Effect of MYCN on Cell Cycle in Neuroblastoma. Pediatr. Blood Cancer 2008, 50, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Cage, T.A.; Chanthery, Y.; Chesler, L.; Grimmer, M.; Knight, Z.; Shokat, K.; Weiss, W.A.; Gustafson, W.C. Downregulation of MYCN through PI3K Inhibition in Mouse Models of Pediatric Neural Cancer. Front. Oncol. 2015, 5, 111. [Google Scholar] [CrossRef]
- Bosch, A.; Li, Z.; Bergamaschi, A.; Ellis, H.; Toska, E.; Prat, A.; Tao, J.J.; Spratt, D.E.; Viola-Villegas, N.T.; Castel, P.; et al. PI3K Inhibition Results in Enhanced Estrogen Receptor Function and Dependence in Hormone Receptor-Positive Breast Cancer. Sci. Transl. Med. 2015, 7, 283ra51. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Dittrich, O.; Kiermaier, A.; Dohmann, K.; Menkel, A.; Eilers, M.; Lüscher, B. Regulation of Cyclin D2 Gene Expression by the Myc/Max/Mad Network: Myc-Dependent TRRAP Recruitment and Histone Acetylation at the Cyclin D2 Promoter. Genes Dev. 2001, 15, 2042–2047. [Google Scholar] [CrossRef]
- Ren, B.; Cam, H.; Takahashi, Y.; Volkert, T.; Terragni, J.; Young, R.A.; Dynlacht, B.D. E2F Integrates Cell Cycle Progression with DNA Repair, Replication, and G(2)/M Checkpoints. Genes Dev. 2002, 16, 245–256. [Google Scholar] [CrossRef]
- Lasorella, A.; Stegmüller, J.; Guardavaccaro, D.; Liu, G.; Carro, M.S.; Rothschild, G.; de la Torre-Ubieta, L.; Pagano, M.; Bonni, A.; Iavarone, A. Degradation of Id2 by the Anaphase-Promoting Complex Couples Cell Cycle Exit and Axonal Growth. Nature 2006, 442, 471–474. [Google Scholar] [CrossRef]
- Kuzyk, A.; Gartner, J.; Mai, S. Identification of Neuroblastoma Subgroups Based on Three-Dimensional Telomere Organization. Transl. Oncol. 2016, 9, 348–356. [Google Scholar] [CrossRef]
- Valentijn, L.J.; Koster, J.; Zwijnenburg, D.A.; Hasselt, N.E.; van Sluis, P.; Volckmann, R.; van Noesel, M.M.; George, R.E.; Tytgat, G.A.M.; Molenaar, J.J.; et al. TERT Rearrangements are Frequent in Neuroblastoma and Identify Aggressive Tumors. Nat. Genet. 2015, 47, 1411–1414. [Google Scholar] [CrossRef]
- Ham, J.; Costa, C.; Sano, R.; Lochmann, T.L.; Sennott, E.M.; Patel, N.U.; Dastur, A.; Gomez-Caraballo, M.; Krytska, K.; Hata, A.N.; et al. Exploitation of the Apoptosis-Primed State of MYCN-Amplified Neuroblastoma to Develop a Potent and Specific Targeted Therapy Combination. Cancer Cell 2016, 29, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Iraci, N.; Gherardi, S.; Gamble, L.D.; Wood, K.M.; Perini, G.; Lunec, J.; Tweddle, D.A. P53 is a Direct Transcriptional Target of MYCN in Neuroblastoma. Cancer Res. 2010, 70, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Sun, D.; Zhang, X. The Role of MDM2 Amplification and Overexpression in Therapeutic Resistance of Malignant Tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Kracikova, M.; Akiri, G.; George, A.; Sachidanandam, R.; Aaronson, S.A. A Threshold Mechanism Mediates P53 Cell Fate Decision between Growth Arrest and Apoptosis. Cell Death Differ. 2013, 20, 576–588. [Google Scholar] [CrossRef]
- Muller, P.A.J.; Vousden, K.H. P53 Mutations in Cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Barbieri, E.; Mehta, P.; Chen, Z.; Zhang, L.; Slack, A.; Berg, S.; Shohet, J.M. MDM2 Inhibition Sensitizes Neuroblastoma to Chemotherapy-Induced Apoptotic Cell Death. Mol. Cancer Ther. 2006, 5, 2358–2365. [Google Scholar] [CrossRef]
- Yogev, O.; Barker, K.; Sikka, A.; Almeida, G.S.; Hallsworth, A.; Smith, L.M.; Jamin, Y.; Ruddle, R.; Koers, A.; Webber, H.T.; et al. P53 Loss in MYC-Driven Neuroblastoma Leads to Metabolic Adaptations Supporting Radioresistance. Cancer Res. 2016, 76, 3025–3035. [Google Scholar] [CrossRef]
- Qi, D.-L.; Cobrinik, D. MDM2 but Not MDM4 Promotes Retinoblastoma Cell Proliferation through P53-Independent Regulation of MYCN Translation. Oncogene 2017, 36, 1760–1769. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Diers, A.R.; Broniowska, K.A.; Chang, C.-F.; Hogg, N. Pyruvate Fuels Mitochondrial Respiration and Proliferation of Breast Cancer Cells: Effect of Monocarboxylate Transporter Inhibition. Biochem. J. 2012, 444, 561–571. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer Metabolism: Fatty Acid Oxidation in the Limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zirath, H.; Frenzel, A.; Oliynyk, G.; Segerström, L.; Westermark, U.K.; Larsson, K.; Munksgaard Persson, M.; Hultenby, K.; Lehtiö, J.; Einvik, C.; et al. MYC Inhibition Induces Metabolic Changes Leading to Accumulation of Lipid Droplets in Tumor Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10258–10263. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Qing, G.; Li, B.; Vu, A.; Skuli, N.; Walton, Z.E.; Liu, X.; Mayes, P.A.; Wise, D.R.; Thompson, C.B.; Maris, J.M.; et al. ATF4 Regulates MYC-Mediated Neuroblastoma Cell Death upon Glutamine Deprivation. Cancer Cell 2012, 22, 631–644. [Google Scholar] [CrossRef]
- Wahlström, T.; Henriksson, M.A. Impact of MYC in Regulation of Tumor Cell Metabolism. Biochim. Biophys. Acta 2015, 1849, 563–569. [Google Scholar] [CrossRef]
- Tao, L.; Mohammad, M.A.; Milazzo, G.; Moreno-Smith, M.; Patel, T.D.; Zorman, B.; Badachhape, A.; Hernandez, B.E.; Wolf, A.B.; Zeng, Z.; et al. MYCN-Driven Fatty Acid Uptake is a Metabolic Vulnerability in Neuroblastoma. Nat. Commun. 2022, 13, 3728. [Google Scholar] [CrossRef]
- Alborzinia, H.; Flórez, A.F.; Kreth, S.; Brückner, L.M.; Yildiz, U.; Gartlgruber, M.; Odoni, D.I.; Poschet, G.; Garbowicz, K.; Shao, C.; et al. MYCN Mediates Cysteine Addiction and Sensitizes Neuroblastoma to Ferroptosis. Nat. Cancer 2022, 3, 471–485. [Google Scholar] [CrossRef]
- Montemurro, L.; Raieli, S.; Angelucci, S.; Bartolucci, D.; Amadesi, C.; Lampis, S.; Scardovi, A.L.; Venturelli, L.; Nieddu, G.; Cerisoli, L.; et al. A Novel MYCN-Specific Antigene Oligonucleotide Deregulates Mitochondria and Inhibits Tumor Growth in MYCN-Amplified Neuroblastoma. Cancer Res. 2019, 79, 6166–6177. [Google Scholar] [CrossRef]
- Vaughan, L.; Clarke, P.A.; Barker, K.; Chanthery, Y.; Gustafson, C.W.; Tucker, E.; Renshaw, J.; Raynaud, F.; Li, X.; Burke, R.; et al. Inhibition of MTOR-Kinase Destabilizes MYCN and is a Potential Therapy for MYCN-Dependent Tumors. Oncotarget 2016, 7, 57525–57544. [Google Scholar] [CrossRef]
- Schramm, A.; Köster, J.; Marschall, T.; Martin, M.; Schwermer, M.; Fielitz, K.; Büchel, G.; Barann, M.; Esser, D.; Rosenstiel, P.; et al. Next-Generation RNA Sequencing Reveals Differential Expression of MYCN Target Genes and Suggests the MTOR Pathway as a Promising Therapy Target in MYCN-Amplified Neuroblastoma. Int. J. Cancer 2013, 132, E106–E115. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Jiang, J.; Gao, P.; Liu, H.; Qing, G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017, 21, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Raieli, S.; di Renzo, D.; Lampis, S.; Amadesi, C.; Montemurro, L.; Pession, A.; Hrelia, P.; Fischer, M.; Tonelli, R. MYCN Drives a Tumor Immunosuppressive Environment Which Impacts Survival in Neuroblastoma. Front. Oncol. 2021, 11, 625207. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Chava, S.; Verma, S.S.; Mishra, S.; Gorantla, S.; Coulter, D.W.; Byrareddy, S.N.; Batra, S.K.; Gupta, S.C.; Challagundla, K.B. PD-L1, Inflammation, Non-Coding RNAs, and Neuroblastoma: Immuno-Oncology Perspective. Semin. Cancer Biol. 2018, 52, 53–65. [Google Scholar] [CrossRef]
- Melaiu, O.; Mina, M.; Chierici, M.; Boldrini, R.; Jurman, G.; Romania, P.; D’Alicandro, V.; Benedetti, M.C.; Castellano, A.; Liu, T.; et al. PD-L1 is a Therapeutic Target of the Bromodomain Inhibitor JQ1 and, Combined with HLA Class I, a Promising Prognostic Biomarker in Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4462–4472. [Google Scholar] [CrossRef]
- Noguera, R.; Fredlund, E.; Piqueras, M.; Pietras, A.; Beckman, S.; Navarro, S.; Påhlman, S. HIF-1α and HIF-2α are Differentially Regulated In Vivo in Neuroblastoma: High HIF-1α Correlates Negatively to Advanced Clinical Stage and Tumor Vascularization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7130–7136. [Google Scholar] [CrossRef]
- Burr, M.L.; Sparbier, C.E.; Chan, K.L.; Chan, Y.-C.; Kersbergen, A.; Lam, E.Y.N.; Azidis-Yates, E.; Vassiliadis, D.; Bell, C.C.; Gilan, O.; et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell 2019, 36, 385–401.e8. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [Green Version]
- Majzner, R.G.; Simon, J.S.; Grosso, J.F.; Martinez, D.; Pawel, B.R.; Santi, M.; Merchant, M.S.; Geoerger, B.; Hezam, I.; Marty, V.; et al. Assessment of Programmed Death-Ligand 1 Expression and Tumor-Associated Immune Cells in Pediatric Cancer Tissues. Cancer 2017, 123, 3807–3815. [Google Scholar] [CrossRef]
- Hahn, M.; Glass, T.; Koke, J. Extracellular Matrix Effects on a Neuroblastoma Cell Line. Cytobios 2000, 102, 7–19. [Google Scholar]
- Meyer, A.; van Golen, C.M.; Kim, B.; van Golen, K.L.; Feldman, E.L. Integrin Expression Regulates Neuroblastoma Attachment and Migration. Neoplasia 2004, 6, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; McCabe, K.E.; Bartakova, A.; Delaney, J.; Pizzo, D.P.; Newbury, R.O.; Varner, J.A.; Schlaepfer, D.D.; Stupack, D.G. Integrin A4 Enhances Metastasis and May Be Associated with Poor Prognosis in MYCN-Low Neuroblastoma. PLoS ONE 2015, 10, e0120815. [Google Scholar] [CrossRef] [PubMed]
- Erdreich-Epstein, A.; Shimada, H.; Groshen, S.; Liu, M.; Metelitsa, L.S.; Kim, K.S.; Stins, M.F.; Seeger, R.C.; Durden, D.L. Integrins Alpha(v)Beta3 and Alpha(v)Beta5 are Expressed by Endothelium of High-Risk Neuroblastoma and Their Inhibition is Associated with Increased Endogenous Ceramide. Cancer Res. 2000, 60, 712–721. [Google Scholar] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Thomsen, L.B.; Bager, C.L.; Jensen, C.; Karsdal, M.A. Quantification of Altered Tissue Turnover in a Liquid Biopsy: A Proposed Precision Medicine Tool to Assess Chronic Inflammation and Desmoplasia Associated with a pro-Cancerous Niche and Response to Immuno-Therapeutic Anti-Tumor Modalities. Cancer Immunol. Immunother. 2018, 67, 1–12. [Google Scholar] [CrossRef]
- Ng, M.R.; Brugge, J.S. A Stiff Blow from the Stroma: Collagen Crosslinking Drives Tumor Progression. Cancer Cell 2009, 16, 455–457. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan Inhibits Collagen I Synthesis and Improves the Distribution and Efficacy of Nanotherapeutics in Tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Sugiura, Y.; Shimada, H.; Seeger, R.C.; Laug, W.E.; DeClerck, Y.A. Matrix Metalloproteinases-2 and -9 are Expressed in Human Neuroblastoma: Contribution of Stromal Cells to Their Production and Correlation with Metastasis. Cancer Res. 1998, 58, 2209–2216. [Google Scholar]
- Sans-Fons, M.G.; Sole, S.; Sanfeliu, C.; Planas, A.M. Matrix Metalloproteinase-9 and Cell Division in Neuroblastoma Cells and Bone Marrow Macrophages. Am. J. Pathol. 2010, 177, 2870–2885. [Google Scholar] [CrossRef]
- Ara, T.; Fukuzawa, M.; Kusafuka, T.; Komoto, Y.; Oue, T.; Inoue, M.; Okada, A. Immunohistochemical Expression of MMP-2, MMP-9, and TIMP-2 in Neuroblastoma: Association with Tumor Progression and Clinical Outcome. J. Pediatr. Surg. 1998, 33, 1272–1278. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ramani, P.; Nash, R.; Radevsky, L.; Patel, A.; Luckett, M.; Rogers, C. VEGF-C, VEGF-D and VEGFR-3 Expression in Peripheral Neuroblastic Tumours. Histopathology 2012, 61, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Chlenski, A.; Liu, S.; Cohn, S.L. The Regulation of Angiogenesis in Neuroblastoma. Cancer Lett. 2003, 197, 47–52. [Google Scholar] [CrossRef]
- Meitar, D.; Crawford, S.E.; Rademaker, A.W.; Cohn, S.L. Tumor Angiogenesis Correlates with Metastatic Disease, N-Myc Amplification, and Poor Outcome in Human Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1996, 14, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Nico, B.; de Falco, G.; Giuseppe Montaldo, P.; Ponzoni, M. Angiogenesis and Anti-Angiogenesis in Neuroblastoma. Eur. J. Cancer 2002, 38, 750–757. [Google Scholar] [CrossRef]
- Rössler, J.; Taylor, M.; Geoerger, B.; Farace, F.; Lagodny, J.; Peschka-Süss, R.; Niemeyer, C.M.; Vassal, G. Angiogenesis as a Target in Neuroblastoma. Eur. J. Cancer 2008, 44, 1645–1656. [Google Scholar] [CrossRef]
- Chanthery, Y.H.; Gustafson, W.C.; Itsara, M.; Persson, A.; Hackett, C.S.; Grimmer, M.; Charron, E.; Yakovenko, S.; Kim, G.; Matthay, K.K.; et al. Paracrine Signaling through MYCN Enhances Tumor-Vascular Interactions in Neuroblastoma. Sci. Transl. Med. 2012, 4, 115ra3. [Google Scholar] [CrossRef]
- Kang, J.; Rychahou, P.G.; Ishola, T.A.; Mourot, J.M.; Evers, B.M.; Chung, D.H. N-Myc is a Novel Regulator of PI3K-Mediated VEGF Expression in Neuroblastoma. Oncogene 2008, 27, 3999–4007. [Google Scholar] [CrossRef]
- Singh, A.R.; Joshi, S.; Burgoyne, A.M.; Sicklick, J.K.; Ikeda, S.; Kono, Y.; Garlich, J.R.; Morales, G.A.; Durden, D.L. Single Agent and Synergistic Activity of the “First-in-Class” Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 2553–2562. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.R.; Durden, D.L. Pan-PI-3 Kinase Inhibitor SF1126 Shows Antitumor and Antiangiogenic Activity in Renal Cell Carcinoma. Cancer Chemother. Pharmacol. 2015, 75, 595–608. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.R.; Zulcic, M.; Durden, D.L. A Macrophage-Dominant PI3K Isoform Controls Hypoxia-Induced HIF1α and HIF2α Stability and Tumor Growth, Angiogenesis, and Metastasis. Mol. Cancer Res. 2014, 12, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Joshi, S.; George, E.; Durden, D.L. Anti-Tumor Effect of a Novel PI3-Kinase Inhibitor, SF1126, in (12) V-Ha-Ras Transgenic Mouse Glioma Model. Cancer Cell Int. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Saidou, J.; Watabe, K. Cancer Associated Fibroblasts (CAFs) in Tumor Microenvironment. Front. Biosci. Landmark Ed. 2010, 15, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Yazhou, C.; Wenlv, S.; Weidong, Z.; Licun, W. Clinicopathological Significance of Stromal Myofibroblasts in Invasive Ductal Carcinoma of the Breast. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2004, 25, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2912–2923. [Google Scholar]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Silzle, T.; Kreutz, M.; Dobler, M.A.; Brockhoff, G.; Knuechel, R.; Kunz-Schughart, L.A. Tumor-Associated Fibroblasts Recruit Blood Monocytes into Tumor Tissue. Eur. J. Immunol. 2003, 33, 1311–1320. [Google Scholar] [CrossRef]
- Yingling, J.M.; Blanchard, K.L.; Sawyer, J.S. Development of TGF-Beta Signalling Inhibitors for Cancer Therapy. Nat. Rev. Drug Discov. 2004, 3, 1011–1022. [Google Scholar] [CrossRef]
- Hashimoto, O.; Yoshida, M.; Koma, Y.-I.; Yanai, T.; Hasegawa, D.; Kosaka, Y.; Nishimura, N.; Yokozaki, H. Collaboration of Cancer-Associated Fibroblasts and Tumour-Associated Macrophages for Neuroblastoma Development. J. Pathol. 2016, 240, 211–223. [Google Scholar] [CrossRef]
- Kakarla, S.; Song, X.-T.; Gottschalk, S. Cancer-Associated Fibroblasts as Targets for Immunotherapy. Immunotherapy 2012, 4, 1129–1138. [Google Scholar] [CrossRef]
- Fakhrai, H.; Dorigo, O.; Shawler, D.L.; Lin, H.; Mercola, D.; Black, K.L.; Royston, I.; Sobol, R.E. Eradication of Established Intracranial Rat Gliomas by Transforming Growth Factor Beta Antisense Gene Therapy. Proc. Natl. Acad. Sci. USA 1996, 93, 2909–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braoudaki, M.; Hatziagapiou, K.; Zaravinos, A.; Lambrou, G.I. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases 2021, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Buechner, J.; Einvik, C. N-Myc and Noncoding RNAs in Neuroblastoma. Mol. Cancer Res. 2012, 10, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; van Peer, G.; Carter, D.R.; Mets, E.; Althoff, K.; Cheung, B.B.; Schulte, J.H.; Mestdagh, P.; Vandesompele, J.; Marshall, G.M.; et al. MYCN-Targeting MiRNAs are Predominantly Downregulated during MYCN-driven Neuroblastoma Tumor Formation. Oncotarget 2015, 6, 5204–5216. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.J.; Domingo-Fernández, R.; Ebus, M.E.; Lindner, S.; Koster, J.; Drabek, K.; Mestdagh, P.; van Sluis, P.; Valentijn, L.J.; van Nes, J.; et al. LIN28B Induces Neuroblastoma and Enhances MYCN Levels via Let-7 Suppression. Nat. Genet. 2012, 44, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.T.; Tsanov, K.M.; Pearson, D.S.; Roels, F.; Spina, C.S.; Ebright, R.; Seligson, M.; de Soysa, Y.; Cahan, P.; Theißen, J.; et al. Multiple Mechanisms Disrupt the Let-7 MicroRNA Family in Neuroblastoma. Nature 2016, 535, 246–251. [Google Scholar] [CrossRef]
- Misiak, D.; Hagemann, S.; Bell, J.L.; Busch, B.; Lederer, M.; Bley, N.; Schulte, J.H.; Hüttelmaier, S. The MicroRNA Landscape of MYCN-Amplified Neuroblastoma. Front. Oncol. 2021, 11, 647737. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The MiR-17/92 Cluster: A Comprehensive Update on Its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Mestdagh, P.; Boström, A.-K.; Impens, F.; Fredlund, E.; van Peer, G.; de Antonellis, P.; von Stedingk, K.; Ghesquière, B.; Schulte, S.; Dews, M.; et al. The MiR-17-92 MicroRNA Cluster Regulates Multiple Components of the TGF-β Pathway in Neuroblastoma. Mol. Cell 2010, 40, 762–773. [Google Scholar] [CrossRef]
- Armstrong, B.C.; Krystal, G.W. Isolation and Characterization of Complementary DNA for N-Cym, a Gene Encoded by the DNA Strand Opposite to N-Myc. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1992, 3, 385–390. [Google Scholar]
- Vadie, N.; Saayman, S.; Lenox, A.; Ackley, A.; Clemson, M.; Burdach, J.; Hart, J.; Vogt, P.K.; Morris, K.V. MYCNOS Functions as an Antisense RNA Regulating MYCN. RNA Biol. 2015, 12, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suenaga, Y.; Islam, S.M.R.; Alagu, J.; Kaneko, Y.; Kato, M.; Tanaka, Y.; Kawana, H.; Hossain, S.; Matsumoto, D.; Yamamoto, M.; et al. NCYM, a Cis-Antisense Gene of MYCN, Encodes a de Novo Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas. PLoS Genet. 2014, 10, e1003996. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Selfe, J.L.; Martins, A.S.; Walters, Z.S.; Shipley, J.M. The Long Non-Coding RNA MYCNOS-01 Regulates MYCN Protein Levels and Affects Growth of MYCN-Amplified Rhabdomyosarcoma and Neuroblastoma Cells. BMC Cancer 2018, 18, 217. [Google Scholar] [CrossRef]
- Liu, P.Y.; Atmadibrata, B.; Mondal, S.; Tee, A.E.; Liu, T. NCYM is Upregulated by LncUSMycN and Modulates N-Myc Expression. Int. J. Oncol. 2016, 49, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Decock, A.; Ongenaert, M.; Vandesompele, J.; Speleman, F. Neuroblastoma Epigenetics: From Candidate Gene Approaches to Genome-Wide Screenings. Epigenetics 2011, 6, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, I.; Shi, Y.; Toskas, K.; Fell, S.M.; Li, S.; Surova, O.; Södersten, E.; Kogner, P.; Nyman, U.; Schlisio, S.; et al. Combined Epigenetic and Differentiation-Based Treatment Inhibits Neuroblastoma Tumor Growth and Links HIF2α to Tumor Suppression. Proc. Natl. Acad. Sci. USA 2017, 114, E6137–E6146. [Google Scholar] [CrossRef]
- Parodi, F.; Carosio, R.; Ragusa, M.; di Pietro, C.; Maugeri, M.; Barbagallo, D.; Sallustio, F.; Allemanni, G.; Pistillo, M.P.; Casciano, I.; et al. Epigenetic Dysregulation in Neuroblastoma: A Tale of MiRNAs and DNA Methylation. Biochim. Biophys. Acta 2016, 1859, 1502–1514. [Google Scholar] [CrossRef]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular Pathogenesis and Therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef]
- Pession, A.; Tonelli, R. The MYCN Oncogene as a Specific and Selective Drug Target for Peripheral and Central Nervous System Tumors. Curr. Cancer Drug Targets 2005, 5, 273–283. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Ziegler, D.S.; Trahair, T.N.; Marshall, G.M.; Haber, M.; Norris, M.D. Too Many Targets, Not Enough Patients: Rethinking Neuroblastoma Clinical Trials. Nat. Rev. Cancer 2018, 18, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Andresen, C.; Helander, S.; Lemak, A.; Farès, C.; Csizmok, V.; Carlsson, J.; Penn, L.Z.; Forman-Kay, J.D.; Arrowsmith, C.H.; Lundström, P.; et al. Transient Structure and Dynamics in the Disordered C-Myc Transactivation Domain Affect Bin1 Binding. Nucleic Acids Res. 2012, 40, 6353–6366. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, R.; Burgess, S.G.; Leen, E.; Richards, M.W. A Moving Target: Structure and Disorder in Pursuit of Myc Inhibitors. Biochem. Soc. Trans. 2017, 45, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Kohl, N.E.; Legouy, E.; DePinho, R.A.; Nisen, P.D.; Smith, R.K.; Gee, C.E.; Alt, F.W. Human N-Myc is Closely Related in Organization and Nucleotide Sequence to c-Myc. Nature 1986, 319, 73–77. [Google Scholar] [CrossRef]
- Esposito, M.R.; Aveic, S.; Seydel, A.; Tonini, G.P. Neuroblastoma Treatment in the Post-Genomic Era. J. Biomed. Sci. 2017, 24, 14. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T.; et al. Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell 2012, 149, 214–231. [Google Scholar] [CrossRef]
- Patel, M.C.; Debrosse, M.; Smith, M.; Dey, A.; Huynh, W.; Sarai, N.; Heightman, T.D.; Tamura, T.; Ozato, K. BRD4 Coordinates Recruitment of Pause Release Factor P-TEFb and the Pausing Complex NELF/DSIF to Regulate Transcription Elongation of Interferon-Stimulated Genes. Mol. Cell. Biol. 2013, 33, 2497–2507. [Google Scholar] [CrossRef]
- Yang, Z.; Yik, J.H.N.; Chen, R.; He, N.; Jang, M.K.; Ozato, K.; Zhou, Q. Recruitment of P-TEFb for Stimulation of Transcriptional Elongation by the Bromodomain Protein Brd4. Mol. Cell 2005, 19, 535–545. [Google Scholar] [CrossRef]
- Shapiro, G.I.; LoRusso, P.; Dowlati, A.; Do, K.T.; Jacobson, C.A.; Vaishampayan, U.; Weise, A.; Caimi, P.F.; Eder, J.P.; French, C.A.; et al. A Phase 1 Study of RO6870810, a Novel Bromodomain and Extra-Terminal Protein Inhibitor, in Patients with NUT Carcinoma, Other Solid Tumours, or Diffuse Large B-Cell Lymphoma. Br. J. Cancer 2021, 124, 744–753. [Google Scholar] [CrossRef]
- Puissant, A.; Frumm, S.M.; Alexe, G.; Bassil, C.F.; Qi, J.; Chanthery, Y.H.; Nekritz, E.A.; Zeid, R.; Gustafson, W.C.; Greninger, P.; et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013, 3, 308–323. [Google Scholar] [CrossRef]
- Jang, M.K.; Mochizuki, K.; Zhou, M.; Jeong, H.-S.; Brady, J.N.; Ozato, K. The Bromodomain Protein Brd4 is a Positive Regulatory Component of P-TEFb and Stimulates RNA Polymerase II-Dependent Transcription. Mol. Cell 2005, 19, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; et al. Selective Inhibition of BET Bromodomains. Nature 2010, 468, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.-W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of Inflammation by a Synthetic Histone Mimic. Nature 2010, 468, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Henssen, A.; Althoff, K.; Odersky, A.; Beckers, A.; Koche, R.; Speleman, F.; Schäfers, S.; Bell, E.; Nortmeyer, M.; Westermann, F.; et al. Targeting MYCN-Driven Transcription By BET-Bromodomain Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, Z.; Oh, D.-Y.; Thiele, C.J. MYCN and the Epigenome. Front. Oncol. 2013, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Diolaiti, D.; Papa, A.; Porro, A.; Valli, E.; Gherardi, S.; Herold, S.; Eilers, M.; Bernardoni, R.; della Valle, G.; et al. A SP1/MIZ1/MYCN Repression Complex Recruits HDAC1 at the TRKA and P75NTR Promoters and Affects Neuroblastoma Malignancy by Inhibiting the Cell Response to NGF. Cancer Res. 2011, 71, 404–412. [Google Scholar] [CrossRef]
- Lu, Z.; Tian, Y.; Salwen, H.R.; Chlenski, A.; Godley, L.A.; Raj, J.U.; Yang, Q. Histone Lysine Methyltransferase EHMT2 is Involved in Proliferation, Apoptosis, Cell Invasion and DNA Methylation of Human Neuroblastoma Cells. Anti-Cancer Drugs 2013, 24, 484–493. [Google Scholar] [CrossRef]
- Lodrini, M.; Oehme, I.; Schroeder, C.; Milde, T.; Schier, M.C.; Kopp-Schneider, A.; Schulte, J.H.; Fischer, M.; de Preter, K.; Pattyn, F.; et al. MYCN and HDAC2 Cooperate to Repress MiR-183 Signaling in Neuroblastoma. Nucleic Acids Res. 2013, 41, 6018–6033. [Google Scholar] [CrossRef]
- Bishayee, K.; Nazim, U.M.; Kumar, V.; Kang, J.; Kim, J.; Huh, S.-O.; Sadra, A. Reversing the HDAC-Inhibitor Mediated Metabolic Escape in MYCN-Amplified Neuroblastoma. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 113032. [Google Scholar] [CrossRef]
- West, A.C.; Johnstone, R.W. New and Emerging HDAC Inhibitors for Cancer Treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef]
- Phimmachanh, M.; Han, J.Z.R.; O’Donnell, Y.E.I.; Latham, S.L.; Croucher, D.R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Front. Cell Dev. Biol. 2020, 8, 578770. [Google Scholar] [CrossRef] [PubMed]
- Gallinari, P.; di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, Histone Deacetylation and Gene Transcription: From Molecular Biology to Cancer Therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, M.J.; Tangutur, A.D.; Manyam, R.R. Epigenetic Modulation and Understanding of HDAC Inhibitors in Cancer Therapy. Life Sci. 2021, 277, 119504. [Google Scholar] [CrossRef]
- Rettig, I.; Koeneke, E.; Trippel, F.; Mueller, W.C.; Burhenne, J.; Kopp-Schneider, A.; Fabian, J.; Schober, A.; Fernekorn, U.; von Deimling, A.; et al. Selective Inhibition of HDAC8 Decreases Neuroblastoma Growth In Vitro and In Vivo and Enhances Retinoic Acid-Mediated Differentiation. Cell Death Dis. 2015, 6, e1657. [Google Scholar] [CrossRef]
- Kenney, A.M.; Widlund, H.R.; Rowitch, D.H. Hedgehog and PI-3 Kinase Signaling Converge on Nmyc1 to Promote Cell Cycle Progression in Cerebellar Neuronal Precursors. Dev. Camb. Engl. 2004, 131, 217–228. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Gustafson, W.C.; Weiss, W.A. Myc Proteins as Therapeutic Targets. Oncogene 2010, 29, 1249–1259. [Google Scholar] [CrossRef]
- Borgenvik, A.; Čančer, M.; Hutter, S.; Swartling, F.J. Targeting MYCN in Molecularly Defined Malignant Brain Tumors. Front. Oncol. 2020, 10, 626751. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, Y. MTOR Inhibitors at a Glance. Mol. Cell. Pharmacol. 2015, 7, 15–20. [Google Scholar]
- Wu, C.-C.; Hou, S.; Orr, B.A.; Kuo, B.R.; Youn, Y.H.; Ong, T.; Roth, F.; Eberhart, C.G.; Robinson, G.W.; Solecki, D.J.; et al. MTORC1-Mediated Inhibition of 4EBP1 is Essential for Hedgehog Signaling-Driven Translation and Medulloblastoma. Dev. Cell 2017, 43, 673–688.e5. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.I.; Segerström, L.; Orrego, A.; Elfman, L.; Henriksson, M.; Kågedal, B.; Eksborg, S.; Sveinbjörnsson, B.; Kogner, P. Inhibitors of Mammalian Target of Rapamycin Downregulate MYCN Protein Expression and Inhibit Neuroblastoma Growth In Vitro and In Vivo. Oncogene 2008, 27, 2910–2922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dou, J.; Yu, Y.; Zhao, Y.; Fan, Y.; Cheng, J.; Xu, X.; Liu, W.; Guan, S.; Chen, Z.; et al. MTOR ATP-Competitive Inhibitor INK128 Inhibits Neuroblastoma Growth via Blocking MTORC Signaling. Apoptosis Int. J. Program. Cell Death 2015, 20, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Wu, Y.; Zhou, X.; Qian, J.; Zhu, W.; Shu, Y.; Liu, P. Clinical Efficacy of MTOR Inhibitors in Solid Tumors: A Systematic Review. Future Oncol. 2015, 11, 1687–1699. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting MTOR for Cancer Therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Carmena, M.; Earnshaw, W.C. The Cellular Geography of Aurora Kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Anderson, L.; Zhu, Y.; Mossie, K.; Ng, L.; Souza, B.; Schryver, B.; Flanagan, P.; Clairvoyant, F.; Ginther, C.; et al. A Homologue of Drosophila Aurora Kinase is Oncogenic and Amplified in Human Colorectal Cancers. EMBO J. 1998, 17, 3052–3065. [Google Scholar] [CrossRef]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol. Cancer 2021, 20, 15. [Google Scholar] [CrossRef]
- Otto, T.; Horn, S.; Brockmann, M.; Eilers, U.; Schüttrumpf, L.; Popov, N.; Kenney, A.M.; Schulte, J.H.; Beijersbergen, R.; Christiansen, H.; et al. Stabilization of N-Myc is a Critical Function of Aurora A in Human Neuroblastoma. Cancer Cell 2009, 15, 67–78. [Google Scholar] [CrossRef]
- Brockmann, M.; Poon, E.; Berry, T.; Carstensen, A.; Deubzer, H.E.; Rycak, L.; Jamin, Y.; Thway, K.; Robinson, S.P.; Roels, F.; et al. Small Molecule Inhibitors of Aurora-a Induce Proteasomal Degradation of N-Myc in Childhood Neuroblastoma. Cancer Cell 2013, 24, 75–89. [Google Scholar] [CrossRef]
- Gustafson, W.C.; Meyerowitz, J.G.; Nekritz, E.A.; Chen, J.; Benes, C.; Charron, E.; Simonds, E.F.; Seeger, R.; Matthay, K.K.; Hertz, N.T.; et al. Drugging MYCN through an Allosteric Transition in Aurora Kinase A. Cancer Cell 2014, 26, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, J.; Tomino, L.; Selich-Anderson, J.; Bopp, E.; Shah, N. Dual BRD4 and AURKA Inhibition is Synergistic against MYCN-Amplified and Nonamplified Neuroblastoma. Neoplasia 2018, 20, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Pastor, E.R.; Mousa, S.A. Current Management of Neuroblastoma and Future Direction. Crit. Rev. Oncol. Hematol. 2019, 138, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Čančer, M.; Drews, L.F.; Bengtsson, J.; Bolin, S.; Rosén, G.; Westermark, B.; Nelander, S.; Forsberg-Nilsson, K.; Uhrbom, L.; Weishaupt, H.; et al. BET and Aurora Kinase A Inhibitors Synergize against MYCN-Positive Human Glioblastoma Cells. Cell Death Dis. 2019, 10, 881. [Google Scholar] [CrossRef]
- Du, J.; Yan, L.; Torres, R.; Gong, X.; Bian, H.; Marugán, C.; Boehnke, K.; Baquero, C.; Hui, Y.-H.; Chapman, S.C.; et al. Aurora A-Selective Inhibitor LY3295668 Leads to Dominant Mitotic Arrest, Apoptosis in Cancer Cells, and Shows Potent Preclinical Antitumor Efficacy. Mol. Cancer Ther. 2019, 18, 2207–2219. [Google Scholar] [CrossRef]
- Bálint, E.; Vousden, K.H. Activation and Activities of the P53 Tumour Suppressor Protein. Br. J. Cancer 2001, 85, 1813–1823. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The P53 Pathway: Positive and Negative Feedback Loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-P53 Pathway Revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar] [CrossRef]
- Bullock, A.N.; Fersht, A.R. Rescuing the Function of Mutant P53. Nat. Rev. Cancer 2001, 1, 68–76. [Google Scholar] [CrossRef]
- Hainaut, P.; Hollstein, M. P53 and Human Cancer: The First Ten Thousand Mutations. Adv. Cancer Res. 2000, 77, 81–137. [Google Scholar] [CrossRef] [PubMed]
- Vogan, K.; Bernstein, M.; Leclerc, J.M.; Brisson, L.; Brossard, J.; Brodeur, G.M.; Pelletier, J.; Gros, P. Absence of P53 Gene Mutations in Primary Neuroblastomas. Cancer Res. 1993, 53, 5269–5273. [Google Scholar] [PubMed]
- Wang, H.; Wang, X.; Xu, L.; Zhang, J. TP53 and TP53-Associated Genes are Correlated with the Prognosis of Paediatric Neuroblastoma. BMC Genom. Data 2022, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, V.; Schmelz, K.; Proba, J.; Winkler, A.; Wünschel, J.; Toedling, J.; Deubzer, H.E.; Künkele, A.; Eggert, A.; Schulte, J.H.; et al. Reactivating TP53 Signaling by the Novel MDM2 Inhibitor DS-3032b as a Therapeutic Option for High-Risk Neuroblastoma. Oncotarget 2018, 9, 2304–2319. [Google Scholar] [CrossRef]
- Corvi, R.; Savelyeva, L.; Breit, S.; Wenzel, A.; Handgretinger, R.; Barak, J.; Oren, M.; Amler, L.; Schwab, M. Non-Syntenic Amplification of MDM2 and MYCN in Human Neuroblastoma. Oncogene 1995, 10, 1081–1086. [Google Scholar]
- Cattelani, S.; Defferrari, R.; Marsilio, S.; Bussolari, R.; Candini, O.; Corradini, F.; Ferrari-Amorotti, G.; Guerzoni, C.; Pecorari, L.; Menin, C.; et al. Impact of a Single Nucleotide Polymorphism in the MDM2 Gene on Neuroblastoma Development and Aggressiveness: Results of a Pilot Study on 239 Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3248–3253. [Google Scholar] [CrossRef]
- Rayburn, E.; Zhang, R.; He, J.; Wang, H. MDM2 and Human Malignancies: Expression, Clinical Pathology, Prognostic Markers, and Implications for Chemotherapy. Curr. Cancer Drug Targets 2005, 5, 27–41. [Google Scholar] [CrossRef]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The Mdm-2 Oncogene Product Forms a Complex with the P53 Protein and Inhibits P53-Mediated Transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 Promotes the Rapid Degradation of P53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, H.; He, J.; Li, J.; Huang, M.; Zhou, M. MDM2 Regulates MYCN MRNA Stabilization and Translation in Human Neuroblastoma Cells. Oncogene 2012, 31, 1342–1353. [Google Scholar] [CrossRef]
- Slack, A.; Lozano, G.; Shohet, J.M. MDM2 as MYCN Transcriptional Target: Implications for Neuroblastoma Pathogenesis. Cancer Lett. 2005, 228, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 Inhibition: An Important Step Forward in Cancer Therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef] [PubMed]
- Pairawan, S.; Zhao, M.; Yuca, E.; Annis, A.; Evans, K.; Sutton, D.; Carvajal, L.; Ren, J.-G.; Santiago, S.; Guerlavais, V.; et al. First in Class Dual MDM2/MDMX Inhibitor ALRN-6924 Enhances Antitumor Efficacy of Chemotherapy in TP53 Wild-Type Hormone Receptor-Positive Breast Cancer Models. Breast Cancer Res. 2021, 23, 29. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.S.; Clarke, S.; Veschi, V.; Thiele, C.J. Targeting MYCN in Pediatric and Adult Cancers. Front. Oncol. 2020, 10, 623679. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Li, J.; Zhang, B.; Wang, Z.; Shaabani, S.; Ter Brake, F.; Essa, K.; Dömling, A. PROTACs—A Game-Changing Technology. Expert Opin. Drug Discov. 2019, 14, 1255–1268. [Google Scholar] [CrossRef]
- Gao, H.; Sun, X.; Rao, Y. PROTAC Technology: Opportunities and Challenges. ACS Med. Chem. Lett. 2020, 11, 237–240. [Google Scholar] [CrossRef]
- Müller, I.; Larsson, K.; Frenzel, A.; Oliynyk, G.; Zirath, H.; Prochownik, E.V.; Westwood, N.J.; Henriksson, M.A. Targeting of the MYCN Protein with Small Molecule C-MYC Inhibitors. PLoS ONE 2014, 9, e97285. [Google Scholar] [CrossRef]
- An, S.; Fu, L. Small-Molecule PROTACs: An Emerging and Promising Approach for the Development of Targeted Therapy Drugs. EBioMedicine 2018, 36, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, D.; Pession, A.; Hrelia, P.; Tonelli, R. Precision Anti-Cancer Medicines by Oligonucleotide Therapeutics in Clinical Research Targeting Undruggable Proteins and Non-Coding RNAs. Pharmaceutics 2022, 14, 1453. [Google Scholar] [CrossRef]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef]
- Kang, J.-H.; Rychahou, P.G.; Ishola, T.A.; Qiao, J.; Evers, B.M.; Chung, D.H. MYCN Silencing Induces Differentiation and Apoptosis in Human Neuroblastoma Cells. Biochem. Biophys. Res. Commun. 2006, 351, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Piacenti, V.; Langella, E.; Autiero, I.; Nolan, J.C.; Piskareva, O.; Adamo, M.F.A.; Saviano, M.; Moccia, M. A Combined Experimental and Computational Study on Peptide Nucleic Acid (PNA) Analogues of Tumor Suppressive MiRNA-34a. Bioorgan. Chem. 2019, 91, 103165. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Purgato, S.; Camerin, C.; Fronza, R.; Bologna, F.; Alboresi, S.; Franzoni, M.; Corradini, R.; Sforza, S.; Faccini, A.; et al. Anti-Gene Peptide Nucleic Acid Specifically Inhibits MYCN Expression in Human Neuroblastoma Cells Leading to Cell Growth Inhibition and Apoptosis. Mol. Cancer Ther. 2005, 4, 779–786. [Google Scholar] [CrossRef]
- Janowski, B.A.; Kaihatsu, K.; Huffman, K.E.; Schwartz, J.C.; Ram, R.; Hardy, D.; Mendelson, C.R.; Corey, D.R. Inhibiting Transcription of Chromosomal DNA with Antigene Peptide Nucleic Acids. Nat. Chem. Biol. 2005, 1, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; McIntyre, A.; Camerin, C.; Walters, Z.S.; di Leo, K.; Selfe, J.; Purgato, S.; Missiaglia, E.; Tortori, A.; Renshaw, J.; et al. Antitumor Activity of Sustained N-Myc Reduction in Rhabdomyosarcomas and Transcriptional Block by Antigene Therapy. Clin. Cancer Res. 2012, 18, 796–807. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-Selective Recognition of DNA by Strand Displacement with a Thymine-Substituted Polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Zhang, B. CRISPR/Cas Gene Therapy. J. Cell. Physiol. 2021, 236, 2459–2481. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Betge, J.; Ebert, M.P.; Boutros, M. CRISPR/Cas9 for Cancer Research and Therapy. Semin. Cancer Biol. 2019, 55, 106–119. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Hassanpour, P.; Sadeghsoltani, F.; Malakoti, F.; Alemi, F.; Qujeq, D.; Asemi, Z.; Yousefi, B. CRISPR/Cas9 Gene Editing: A New Approach for Overcoming Drug Resistance in Cancer. Cell. Mol. Biol. Lett. 2022, 27, 49. [Google Scholar] [CrossRef]
- Yoda, H.; Inoue, T.; Shinozaki, Y.; Lin, J.; Watanabe, T.; Koshikawa, N.; Takatori, A.; Nagase, H. Direct Targeting of MYCN Gene Amplification by Site-Specific DNA Alkylation in Neuroblastoma. Cancer Res. 2019, 79, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Valteau-Couanet, D.; Brock, P.; Yaniv, I.; Castel, V.; Laureys, G.; Malis, J.; Papadakis, V.; Lacerda, A.; Ruud, E.; et al. Randomized Trial of Prophylactic Granulocyte Colony-Stimulating Factor during Rapid COJEC Induction in Pediatric Patients with High-Risk Neuroblastoma: The European HR-NBL1/SIOPEN Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-Term Results for Children with High-Risk Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy Followed by 13-Cis-Retinoic Acid: A Children’s Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Berthold, F.; Borkhardt, A.; Kremens, B.; de Carolis, B.; Hero, B. Treatment and Outcomes of Patients with Relapsed, High-Risk Neuroblastoma: Results of German Trials. Pediatr. Blood Cancer 2011, 56, 578–583. [Google Scholar] [CrossRef]
- Iehara, T.; Hosoi, H.; Akazawa, K.; Matsumoto, Y.; Yamamoto, K.; Suita, S.; Tajiri, T.; Kusafuka, T.; Hiyama, E.; Kaneko, M.; et al. MYCN Gene Amplification is a Powerful Prognostic Factor Even in Infantile Neuroblastoma Detected by Mass Screening. Br. J. Cancer 2006, 94, 1510–1515. [Google Scholar] [CrossRef]
- Kushner, B.H.; LaQuaglia, M.P.; Bonilla, M.A.; Lindsley, K.; Rosenfield, N.; Yeh, S.; Eddy, J.; Gerald, W.L.; Heller, G.; Cheung, N.K. Highly Effective Induction Therapy for Stage 4 Neuroblastoma in Children over 1 Year of Age. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 2607–2613. [Google Scholar] [CrossRef]
- Beiske, K.; Burchill, S.A.; Cheung, I.Y.; Hiyama, E.; Seeger, R.C.; Cohn, S.L.; Pearson, A.D.J.; Matthay, K.K.; International neuroblastoma Risk Group Task Force. Consensus Criteria for Sensitive Detection of Minimal Neuroblastoma Cells in Bone Marrow, Blood and Stem Cell Preparations by Immunocytology and QRT-PCR: Recommendations by the International Neuroblastoma Risk Group Task Force. Br. J. Cancer 2009, 100, 1627–1637. [Google Scholar] [CrossRef]
- Garaventa, A.; Parodi, S.; de Bernardi, B.; Dau, D.; Manzitti, C.; Conte, M.; Casale, F.; Viscardi, E.; Bianchi, M.; D’Angelo, P.; et al. Outcome of Children with Neuroblastoma after Progression or Relapse. A Retrospective Study of the Italian Neuroblastoma Registry. Eur. J. Cancer 2009, 45, 2835–2842. [Google Scholar] [CrossRef]
- Laverdière, C.; Liu, Q.; Yasui, Y.; Nathan, P.C.; Gurney, J.G.; Stovall, M.; Diller, L.R.; Cheung, N.-K.; Wolden, S.; Robison, L.L.; et al. Long-Term Outcomes in Survivors of Neuroblastoma: A Report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 1131–1140. [Google Scholar] [CrossRef]
- Conte, M.; Parodi, S.; de Bernardi, B.; Milanaccio, C.; Mazzocco, K.; Angelini, P.; Viscardi, E.; di Cataldo, A.; Luksch, R.; Haupt, R. Neuroblastoma in Adolescents: The Italian Experience. Cancer 2006, 106, 1409–1417. [Google Scholar] [CrossRef]
- Castel, V.; Villamón, E.; Cañete, A.; Navarro, S.; Ruiz, A.; Melero, C.; Herrero, A.; Yáñez, Y.; Noguera, R. Neuroblastoma in Adolescents: Genetic and Clinical Characterisation. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2010, 12, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Esiashvili, N.; Goodman, M.; Ward, K.; Marcus, R.B.; Johnstone, P.A.S. Neuroblastoma in Adults: Incidence and Survival Analysis Based on SEER Data. Pediatr. Blood Cancer 2007, 49, 41–46. [Google Scholar] [CrossRef] [PubMed]
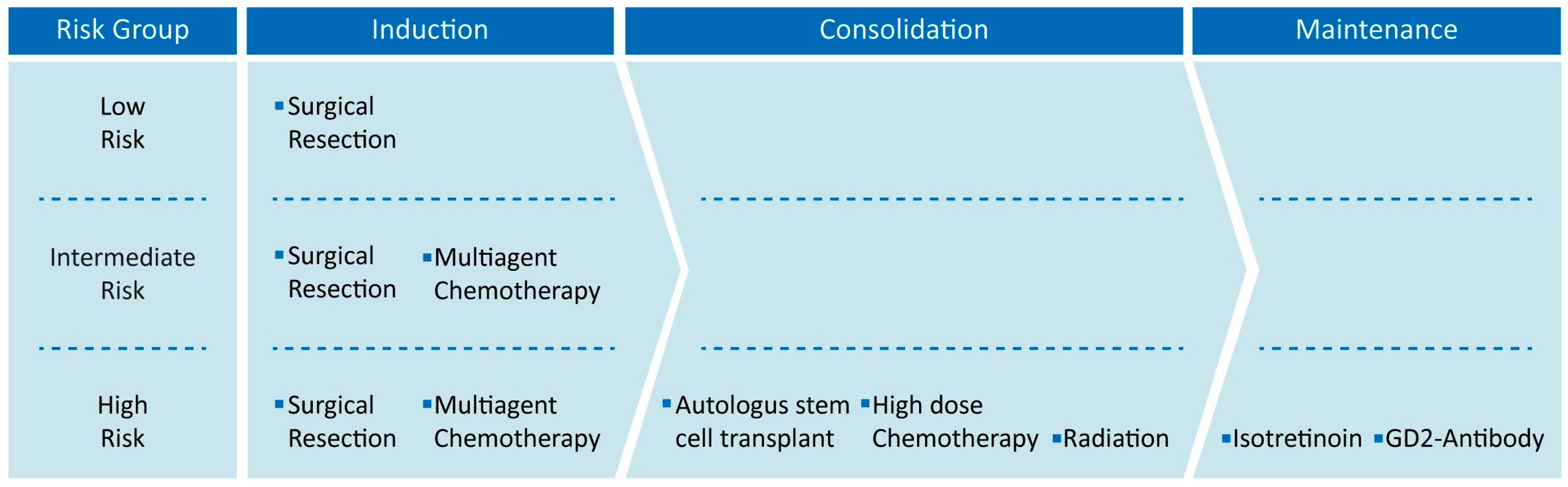

| INRGSS | Age | MYCN Amp | SCA at 1 p or 11 q | Ploidy | INPC | Differentiation | Risk Group |
|---|---|---|---|---|---|---|---|
| L1 | No | Any | Any | Any | LR | ||
| AMP | LR or HR | ||||||
| L2 | <18 months | No | Absent | DI > 1 | FH | IR | |
| Any | Any | Any | IR | ||||
| AMP | Any | Any | Any | HR | |||
| 18 months–5 years | No | Any | Any | FH | IR | ||
| UH | HR | ||||||
| ≥5 years | No | Any | Any | UH | Differentiating | IR | |
| Undifferentiated or poorly differentiated | HR | ||||||
| M | <12 months | No | Any | Any | Any | IR | |
| AMP | HR | ||||||
| 12 to <18 months | No | Absent | DI > 1 | FH | IR | ||
| Present | Any | Any | HR | ||||
| Any | DI = 1 | Any | HR | ||||
| Any | UH | HR | |||||
| Any | NA | ||||||
| At least 1 feature | Unfavorable | HR | |||||
| AMP | Any | Any | Any | HR | |||
| ≥18 months | Any | Any | Any | Any | HR | ||
| MS | <12 months | No bx | No bx | No bx | No bx | LR or IR | |
| No | Absent | DI > 1 | FH | LR or IR | |||
| Present | Any | Any | IR | ||||
| Any | DI = 1 | Any | IR | ||||
| Any | UH | IR | |||||
| AMP | Any | Any | Any | HR |
| Therapy | Therapeutic Strategy | Availability |
|---|---|---|
| Surgical Resection | Tumor mass removal by surgical resection | Standard Medical Practice |
| Multimodal Chemotherapy | Tumor cell elimination using non-specific chemical agents | Standard Medical Practice |
| Autologous stem cell transplantation | Stem cell reinfusion after high dose chemotherapy | Standard Medical Practice |
| Radiation Therapy | Tumor mass removal by radiations | Standard Medical Practice |
| Anti-GD2 Immunotherapy | Induction of immune system stimulation | Standard Medical Practice |
| Isotretinoin | Induction of tumor cell differentiation and proliferation arrest | Standard Medical Practice |
| BET Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| HDACs Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| PI3K/mTOR Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| Aurora Kinase-A Inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| MDM2 inhibitors | Inhibition of specific molecular pathway related to MYCN | Clinical Studies |
| MYCN direct inhibitor | Specific MYCN expression inhibition or N-Myc protein degradation | Preclinical Studies |
| Drug Name | Target | Clinical Phase | Status | NTC Number |
|---|---|---|---|---|
| Vorinostat | HDAC | Phase1|Phase2 | Recruiting|Completed|No longer available | NCT01019850|NCT03561259|NCT01838187|NCT01132911|NCT02559778|NCT03332667|NCT02035137|NCT01208454|NCT04308330 |
| Panobinostat | HDAC | Phase 2 | Terminated | NCT04897880 |
| BMS-986158 | BET | Phase 1 | Recruiting | NCT03936465 |
| BMS-986378 | BET | Phase1 | Recruiting | NCT03936465 |
| GSK525762 | BET | Phase1 | Completed | NCT01587703 |
| SF1126 | PI3K/mTOR | Phase 1 | Terminated | NCT02337309 |
| Samotolisib | PI3K/mTOR | Phase 2 | Recruiting | NCT03213678 |
| ALRN-6924 | Dual MDM2/MDMX | Phase 1 | Recruiting | NCT03654716 |
| LY3295668 | Aurora-A Kinase | Phase 1 | Active, no recruiting | NCT04106219 |
| Alisertib (MLN8237) | Aurora-A Kinase | Phase 1|Phase 2 | Complete | NCT02444884|NCT01601535|NCT01154816 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolucci, D.; Montemurro, L.; Raieli, S.; Lampis, S.; Pession, A.; Hrelia, P.; Tonelli, R. MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment. Cancers 2022, 14, 4421. https://doi.org/10.3390/cancers14184421
Bartolucci D, Montemurro L, Raieli S, Lampis S, Pession A, Hrelia P, Tonelli R. MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment. Cancers. 2022; 14(18):4421. https://doi.org/10.3390/cancers14184421
Chicago/Turabian StyleBartolucci, Damiano, Luca Montemurro, Salvatore Raieli, Silvia Lampis, Andrea Pession, Patrizia Hrelia, and Roberto Tonelli. 2022. "MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment" Cancers 14, no. 18: 4421. https://doi.org/10.3390/cancers14184421
APA StyleBartolucci, D., Montemurro, L., Raieli, S., Lampis, S., Pession, A., Hrelia, P., & Tonelli, R. (2022). MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment. Cancers, 14(18), 4421. https://doi.org/10.3390/cancers14184421







