Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Tissue Collection
2.3. Cell Culture
2.4. Cell-Counting Kit (CCK)-8 Assay
2.5. Immunofluorescence
2.6. Flow Cytometry Analysis
2.7. Western Blotting
2.8. Real-Time Quantitative PCR
2.9. Lentiviral Vector-Mediated IDO1 Overexpression and Knockdown in ULMs
- NC-shRNA:5’-TTCTCCGAACGTGTCACGTAA-3’,
- sh1-IDO1:5’-TGCTAAACATCTGCCTGATCTCATA-3’,
- sh2-IDO1:5’CCTACTGTATTCAAGGCAATGCAAA-3’,
- sh3-IDO1: 5’-CCCTGAGGAGCTACCATCTGCAAAT-3’.
2.10. Rat Uterine Fibroid Model
2.11. Histopathological Examination of the Uterus
2.12. Transmission Electron Microscopy
2.13. Radio Immune Assay (RIA)
2.14. Immunohistochemistry
2.15. Statistical Analysis
3. Results
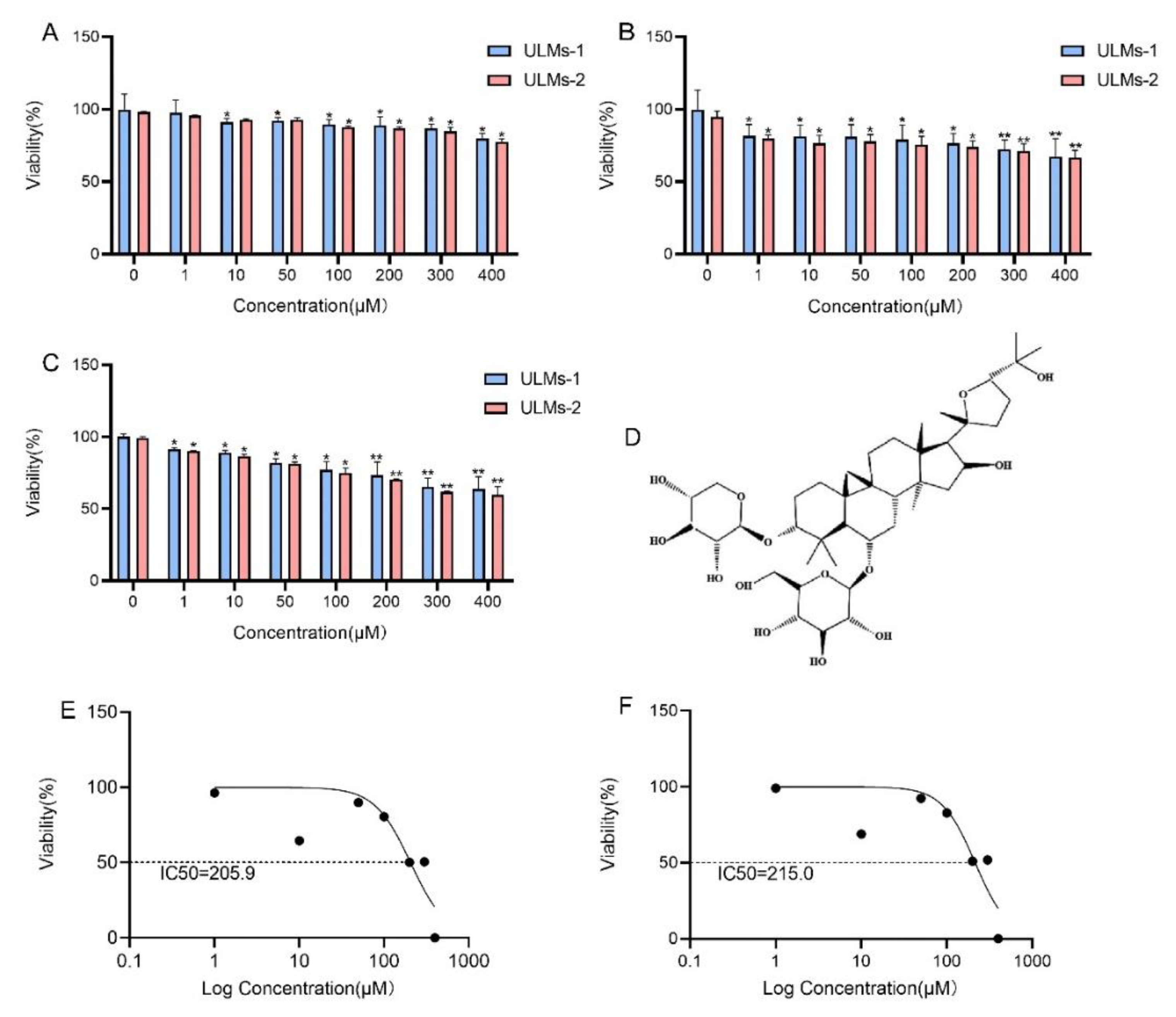
3.1. AS-IV Inhibited the Proliferation of ULM Cells
3.2. AS-IV Induced Apoptosis in ULM Cells
3.3. IDO1 Involved in the Suppressive Effect of AS-IV in ULM Cells
3.4. IDO1 Involved in the Apoptosis Induced by AS-IV in ULM Cells
3.5. Antitumor Effects of AS-IV In Vivo
3.6. Mechanism of Inhibition of ULM Growth by AS-IV In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Commandeur, A.E.; Styer, A.K.; Teixeira, J.M. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum. Reprod. Update 2015, 21, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Gorny, K.R.; Borah, B.J.; Brown, D.L.; Woodrum, D.A.; Stewart, E.A.; Hesley, G.K. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: Review of 138 patients with an average follow-up of 2.8 years. J. Vasc. Interv. Radiol. 2014, 25, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [PubMed]
- Chen, T.; Yang, P.; Jia, Y. Molecular mechanisms of astragaloside-IV in cancer therapy (Review). Int. J. Mol. Med. 2021, 47, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, Y.; Wang, Q.; Zhang, B. Astragaloside IV inhibits human colorectal cancer cell growth. Front Biosci. 2019, 24, 597–606. [Google Scholar]
- Hu, T.; Fei, Z.; Wei, N. Chemosensitive effects of Astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharm. Rep. 2017, 69, 1159–1164. [Google Scholar] [CrossRef]
- Ying, G. Astragaloside induces gastric MGC-803 cells apoptosis by inhibiting AKT and NF-KB pathway. Int. J. Lab. Med. 2018, 19, 2341–2344. [Google Scholar]
- Wang, S.; Mou, J.; Cui, L.; Wang, X.; Zhang, Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed. Pharmacother. 2018, 102, 1037–1044. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Qin, X.; Huang, H.; Nie, C. Astragaloside IV inhibits the invasion and metastasis of SiHa cervical cancer cells via the TGF-β1-mediated PI3K and MAPK pathways. Oncol. Rep. 2019, 41, 2975–2986. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; He, Z.; Cai, Y. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell Mol. Biol. Lett. 2020, 25, 25. [Google Scholar] [CrossRef]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef]
- Goyne, H.E.; Cannon, M.J. Dendritic cell vaccination, immune regulation, and clinical outcomes in ovarian cancer. Front Immunol. 2013, 4, 382. [Google Scholar] [CrossRef]
- Asghar, K.; Farooq, A.; Zulfiqar, B.; Rashid, M.U. Indoleamine 2,3-dioxygenase: As a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma. World J. Gastroenterol. 2017, 23, 2286–2293. [Google Scholar] [CrossRef]
- Ferns, D.M.; Kema, I.P.; Buist, M.R.; Nijman, H.W.; Kenter, G.G.; Jordanova, E.S. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology 2015, 4, e981457. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Carvajal-Hausdorf, D.; McLaughlin, J.; Altan, M.; Velcheti, V.; Gaule, P.; Sanmamed, M.F.; Chen, L.; Herbst, R.S.; Rimm, D.L. Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin. Cancer Res. 2017, 23, 370–378. [Google Scholar] [CrossRef]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005, 7, 390–396. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Investig. 2007, 117, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Thaker, A.I.; Rao, M.S.; Bishnupuri, K.S.; Kerr, T.A.; Foster, L.; Marinshaw, J.M.; Newberry, R.D.; Stenson, W.F.; Ciorba, M.A. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology 2013, 145, 416–425.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Li, D.; Liu, Y.; Wang, W.; Qiu, T. Curdione Induces Antiproliferation Effect on Human Uterine Leiomyosarcoma via Targeting IDO1. Front. Oncol. 2021, 11, 637024. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef]
- Karra, L.; Shushan, A.; Ben-Meir, A.; Rojansky, N.; Klein, B.Y.; Shveiky, D.; Levitzki, R.; Ben-Bassat, H. Changes related to phosphatidylinositol 3-kinase/Akt signaling in leiomyomas: Possible involvement of glycogen synthase kinase 3alpha and cyclin D2 in the pathophysiology. Fertil. Steril. 2010, 93, 2646–2651. [Google Scholar] [CrossRef]
- Maehama, T. PTEN: Its deregulation and tumorigenesis. Biol. Pharm. Bull. 2007, 30, 1624–1627. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Hoekstra, A.V.; Sefton, E.C.; Berry, E.; Lu, Z.; Hardt, J.; Marsh, E.; Yin, P.; Clardy, J.; Chakravarti, D.; Bulun, S.; et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J. Clin. Endocrinol. Metab. 2009, 94, 1768–1774. [Google Scholar] [CrossRef]
- Wang, D.; Saga, Y.; Sato, N.; Nakamura, T.; Takikawa, O.; Mizukami, H.; Matsubara, S.; Fujiwara, H. The hepatocyte growth factor antagonist NK4 inhibits indoleamine-2,3-dioxygenase expression via the c-Met-phosphatidylinositol 3-kinase-AKT signaling pathway. Int. J. Oncol. 2016, 48, 2303–2309. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Cavnar, M.J.; Zeng, S.; Bamboat, Z.M.; Ocuin, L.M.; Obaid, H.; Sorenson, E.C.; Popow, R.; Ariyan, C.; Rossi, F.; et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011, 17, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Zuo, D.; Li, Z.; Liu, H.; Dai, Z.; Cai, J.; Pang, L.; Wu, Y. Astragaloside IV inhibits doxorubicin-induced cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway via activating the PI3K/Akt pathway. Chem. Pharm. Bull. 2014, 62, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.L., Jr.; Paz-Ares, P.L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clin. Cancer Res. 2019, 25, 1462–1471. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, Y.; Que, Z.; Zhang, L.; Lin, S.; Le, V.; Liu, J.; Tian, J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J. Cancer Res. Clin. Oncol. 2014, 140, 1883–1890. [Google Scholar] [CrossRef]
- Gu, T.; Rowswell-Turner, R.B.; Kilinc, M.O.; Egilmez, N.K. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010, 70, 129–138. [Google Scholar] [CrossRef]
- Nair, R.E.; Kilinc, M.O.; Jones, S.A.; Egilmez, N.K. Chronic immune therapy induces a progressive increase in intratumoral T suppressor activity and a concurrent loss of tumor-specific CD8+ T effectors in her-2/neu transgenic mice bearing advanced spontaneous tumors. J. Immunol. 2006, 176, 7325–7334. [Google Scholar] [CrossRef] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Li, D.; Liu, Y.; Ren, H.; Yang, X.; Luo, W. Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1. Cancers 2022, 14, 4424. https://doi.org/10.3390/cancers14184424
Qiu T, Li D, Liu Y, Ren H, Yang X, Luo W. Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1. Cancers. 2022; 14(18):4424. https://doi.org/10.3390/cancers14184424
Chicago/Turabian StyleQiu, Tiantian, Donghua Li, Yu Liu, Hui Ren, Xuan Yang, and Wenting Luo. 2022. "Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1" Cancers 14, no. 18: 4424. https://doi.org/10.3390/cancers14184424
APA StyleQiu, T., Li, D., Liu, Y., Ren, H., Yang, X., & Luo, W. (2022). Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1. Cancers, 14(18), 4424. https://doi.org/10.3390/cancers14184424



