On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Patients’ baseline characteristics (age, gender, race, body mass index (BMI), American Society of Anesthesiologists (ASA) score, solitary kidney status).
- Perioperative variables (clamping technique, warm ischemia time (WIT), operation time (OT), length of hospital stay (LOS), postoperative complications (stratified according to the Clavien–Dindo (CD) system [11]; those ≥ grade III were defined as “major complications”. All the cases requiring intra-/postoperative blood transfusion and/or renal artery embolization were considered “major bleeding events” (MBE)).
- Functional data (pre- and postoperative estimated glomerular filtration rate (eGFR) were calculated by the Modification of Diet in Renal Disease formula [13] and stratified according to the National Kidney Foundation (NKF) and its Kidney Disease Outcomes Quality Initiative [14]. According to the NKF and the US Food and Drug Administration, a significant renal function deterioration (sRFD) was defined as a >30% postoperative eGFR reduction [15]. Postoperative blood tests were performed before hospital discharge).
2.1. Study Objective
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.W.W.; Linxweiler, J.; Terwey, S.; Rugge, S.; Ohlmann, C.-H.; Becker, F.; Thomas, C.; Neisius, A.; Thüroff, J.W.; Siemer, S.; et al. Survival outcomes in patients with large (≥7 cm) clear cell renal cell carcinomas treated with nephron-sparing surgery versus radical nephrectomy: Results of a multicenter cohort with long-term follow-up. PLoS ONE 2018, 13, e0196427. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, R.; Autorino, R.; Simone, G.; Derweesh, I.; Garisto, J.D.; Minervini, A.; Eun, D.; Perdona, S.; Porter, J.; Rha, K.H.; et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T2 Renal Tumors: A Multicenter Analysis (ROSULA Collaborative Group). Eur. Urol. 2018, 74, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Gill, T.; Medina, L.; Ashrafi, A.; Winter, M.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of Host Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.; Abreu, A.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of Surgical Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 258–274. [Google Scholar] [CrossRef]
- Pak, J.S.; Lee, J.J.; Bilal, K.; Finkelstein, M.; Palese, M.A. Utilization trends and outcomes up to 3 months of open, laparoscopic, and robotic partial nephrectomy. J. Robot. Surg. 2017, 11, 223–229. [Google Scholar] [CrossRef]
- Simone, G.; Gill, I.S.; Mottrie, A.; Kutikov, A.; Patard, J.-J.; Alcaraz, A.; Rogers, C.G. Indications, Techniques, Outcomes, and Limitations for Minimally Ischemic and Off-clamp Partial Nephrectomy: A Systematic Review of the Literature. Eur. Urol. 2015, 68, 632–640. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.S.; Mendelsohn, A.; Husain, F.; Bhardwaj, L.; Artibani, W.; Sotelo, R.; Gill, I.S. Impact of Renal Hilar Control on Outcomes of Robotic Partial Nephrectomy: Systematic Review and Cumulative Meta-analysis. Eur. Urol. Focus 2019, 5, 619–635. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; Antonelli, A.; Artibani, W.; Bertini, R.; Carini, M.; Cosciani Cunico, S.; Imbimbo, C.; Longo, N.; Martignoni, G.; et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed? Eur. Urol. 2010, 58, 588–595. [Google Scholar] [CrossRef]
- Kutikov, A.; Smaldone, M.C.; Egleston, B.L.; Manley, B.J.; Canter, D.J.; Simhan, J.; Boorjian, S.A.; Viterbo, R.; Chen, D.Y.T.; Greenberg, R.E.; et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: A preoperative nomogram using the RENAL Nephrometry score. Eur. Urol. 2011, 60, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Matsushita, K.; Greene, T.; Willis, K.; Lewis, E.; de Zeeuw, D.; Cheung, A.K.; Coresh, J. GFR Decline as an End Point for Clinical Trials in CKD: A Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2014, 64, 821–835. [Google Scholar] [CrossRef]
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottire, A.; et al. Surgical quality, cancer control and functional preservation: Introducing a novel trifecta for robot-assisted partial nephrectomy. Minerva Urol. Nefrol. Ital. J. Urol. Nephrol. 2020, 72, 82–90. [Google Scholar] [CrossRef]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Colombel, M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2011, 59, 543–552. [Google Scholar] [CrossRef]
- Mir, M.C.; Derweesh, I.; Porpiglia, F.; Zargar, H.; Mottrie, A.; Autorino, R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. 2017, 71, 606–617. [Google Scholar] [CrossRef]
- Kopp, R.P.; Liss, M.A.; Mehrazin, R.; Wang, S.; Lee, H.J.; Jabaji, R.; Mirheydar, H.S.; Gillis, K.; Patel, N.; Palazzi, K.L.; et al. Analysis of Renal Functional Outcomes After Radical or Partial Nephrectomy for Renal Masses ≥7 cm Using the RENAL Score. Urology 2015, 86, 312–319. [Google Scholar] [CrossRef]
- Margulis, V.; Tamboli, P.; Jacobsohn, K.M.; Swanson, D.A.; Wood, C.G. Oncological efficacy and safety of nephron-sparing surgery for selected patients with locally advanced renal cell carcinoma. BJU Int. 2007, 100, 1235–1239. [Google Scholar] [CrossRef]
- Jeldres, C.; Patard, J.-J.; Capitanio, U.; Perrotte, P.; Suardi, N.; Crepel, M.; Ficarra, V.; Cindolo, L.; de La Taille, A.; Tostain, J.; et al. Partial Versus Radical Nephrectomy in Patients With Adverse Clinical or Pathologic Characteristics. Urology 2009, 73, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Breau, R.H.; Crispen, P.L.; Jimenez, R.E.; Lohse, C.M.; Blute, M.L.; Leibovich, B.C. Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J. Urol. 2010, 183, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.W.; Autorino, R.; Simone, G.; Yang, B.; Uzzo, R.G.; Porpiglia, F.; Capitanio, U.; Porter, J.; Bertolo, R.; Minervini, A.; et al. Robotic partial nephrectomy vs. minimally invasive radical nephrectomy for clinical T2a renal mass: A propensity score-matched comparison from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. BJU Int. 2020, 126, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Brandao, L.F.; Zargar, H.; Autorino, R.; Akca, O.; Laydner, H.; Samarasekera, D.; Krishnan, J.; Haber, G.-P.; Stein, R.J.; Kaouk, J.H. Robot-assisted partial nephrectomy for ≥7 cm renal masses: A comparative outcome analysis. Urology 2014, 84, 602–608. [Google Scholar] [CrossRef]
- Malkoc, E.; Ramirez, D.; Kara, O.; Maurice, M.J.; Nelson, R.J.; Caputo, P.A.; Kaouk, J.H. Robotic and open partial nephrectomy for localized renal tumors larger than 7 cm: A single-center experience. World J. Urol. 2017, 35, 781–787. [Google Scholar] [CrossRef]
- Mir, M.C.; Ercole, C.; Takagi, T.; Zhang, Z.; Velet, L.; Remer, E.M.; Demirjian, S.; Campbell, S.C. Decline in renal function after partial nephrectomy: Etiology and prevention. J. Urol. 2015, 193, 1889–1898. [Google Scholar] [CrossRef]
- Minervini, A.; Campi, R.; Lane, B.R.; De Cobelli, O.; Sanguedolce, F.; Hatzichristodoulou, G.; Antonelli, A.; Noyes, S.; Mari, A.; Rodriguez-Faba, O.; et al. Impact of Resection Technique on Perioperative Outcomes and Surgical Margins after Partial Nephrectomy for Localized Renal Masses: A Prospective Multicenter Study. J. Urol. 2020, 203, 496–504. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Carpanese, L.; Gallucci, M. Zero Ischemia Laparoscopic Partial Nephrectomy After Superselective Transarterial Tumor Embolization for Tumors with Moderate Nephrometry Score: Long-Term Results of a Single-Center Experience. J. Endourol. 2011, 25, 1443–1446. [Google Scholar] [CrossRef]
- Desai, M.M.; De Castro Abreu, A.L.; Leslie, S.; Cai, J.; Huang, E.Y.H.; Lewandowski, P.M.; Lee, D.; Dharmaraja, A.; Berger, A.K.; Goh, A.; et al. Robotic partial nephrectomy with superselective versus main artery clamping: A retrospective comparison. Eur. Urol. 2014, 66, 713–719. [Google Scholar] [CrossRef]
- Peyronnet, B.; Baumert, H.; Mathieu, R.; Masson-Lecomte, A.; Grassano, Y.; Roumiguié, M.; Massoud, W.; Abd El Fattah, V.; Bruyère, F.; Droupy, S.; et al. Early unclamping technique during robot-assisted laparoscopic partial nephrectomy can minimise warm ischaemia without increasing morbidity. BJU Int. 2014, 114, 741–747. [Google Scholar] [CrossRef]
- Bertolo, R.; Simone, G.; Garisto, J.; Nakhoul, G.; Armanyous, S.; Agudelo, J.; Costantini, M.; Tuderti, G.; Gallucci, M.; Kaouk, J. Off-clamp vs. on-clamp robotic partial nephrectomy: Perioperative, functional and oncological outcomes from a propensity-score matching between two high-volume centers. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 1232–1237. [Google Scholar] [CrossRef]
- Fero, K.; Hamilton, Z.A.; Bindayi, A.; Murphy, J.D.; Derweesh, I.H. Utilization and quality outcomes of cT1a, cT1b and cT2a partial nephrectomy: Analysis of the national cancer database. BJU Int. 2018, 121, 565–574. [Google Scholar] [CrossRef]
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Costantini, M.; Capitanio, U.; Larcher, A.; Antonelli, A.; Mottrie, A.; et al. Comprehensive long-term assessment of outcomes following robot-assisted partial nephrectomy for renal cell carcinoma: The ROMe’s achievement and its predicting nomogram. Minerva Urol. Nefrol. Ital. J. Urol. Nephrol. 2020, 72, 482–489. [Google Scholar] [CrossRef]
- Tuderti, G.; Brassetti, A.; Mastroianni, R.; Misuraca, L.; Bove, A.; Anceschi, U.; Ferriero, M.; Guaglianone, S.; Gallucci, M.; Simone, G. Expanding the limits of nephron-sparing surgery: Surgical technique and mid-term outcomes of purely off-clamp robotic partial nephrectomy for totally endophytic renal tumors. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2022, 29, 282–288. [Google Scholar] [CrossRef]
- Anceschi, U.; Brassetti, A.; Bertolo, R.; Tuderti, G.; Ferriero, M.C.; Mastroianni, R.; Flammia, R.S.; Costantini, M.; Kaouk, J.; Leonardo, C.; et al. On-clamp versus purely off-clamp robot-assisted partial nephrectomy in solitary kidneys: Comparison of perioperative outcomes and chronic kidney disease progression at two high- volume centers. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2020, 73, 739–745. [Google Scholar] [CrossRef]
- Thompson, R.H.; Frank, I.; Lohse, C.M.; Saad, I.R.; Fergany, A.; Zincke, H.; Leibovich, B.C.; Blute, M.L.; Novick, A.C. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: A multi-institutional study. J. Urol. 2007, 177, 471–476. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Dong, W.; Remer, E.; Li, J.; Demirjian, S.; Zabell, J.; Campbell, S.C. Acute Kidney Injury after Partial Nephrectomy: Role of Parenchymal Mass Reduction and Ischemia and Impact on Subsequent Functional Recovery. Eur. Urol. 2016, 69, 745–752. [Google Scholar] [CrossRef]
- Gupta, R.; Tori, M.; Babitz, S.K.; Tobert, C.M.; Anema, J.G.; Noyes, S.L.; Lane, B.R. Comparison of RENAL, PADUA, CSA, and PAVP Nephrometry Scores in Predicting Functional Outcomes After Partial Nephrectomy. Urology 2019, 124, 160–167. [Google Scholar] [CrossRef]
- Ficarra, V.; Crestani, A.; Bertolo, R.; Antonelli, A.; Longo, N.; Minervini, A.; Novara, G.; Simeone, C.; Carini, M.; Mirone, V.; et al. Tumour contact surface area as a predictor of postoperative complications and renal function in patients undergoing partial nephrectomy for renal tumours. BJU Int. 2019, 123, 639–645. [Google Scholar] [CrossRef]
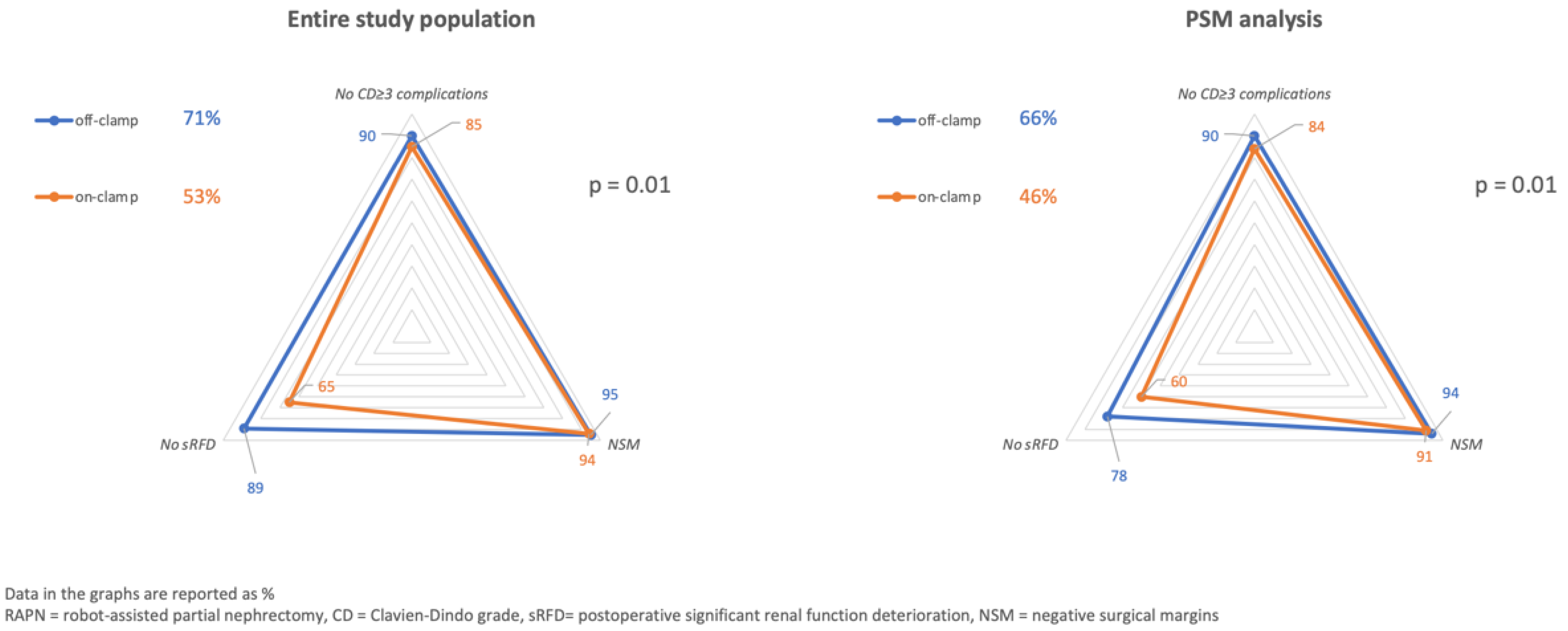
| Overall (n = 316) | On-Clamp (n = 211) | Off-Clamp (n = 105) | p | PSM On-Clamp (n = 89) | PSM Off-Clamp (n = 89) | p | |
|---|---|---|---|---|---|---|---|
| Age, years | 60 (51/67) | 60 (52/67) | 60 (46/67) | 0.21 | 59 (53/67) | 60 (48/67) | 0.58 |
| Male gender, n (%) | 202 (64%) | 145 (69%) | 57 (54%) | 0.01 | 63 (71%) | 50 (56%) | 0.04 |
| BMI | 27.1 (24.6/31.4) | 28.7 (25.3/32.5) | 25.8 (23.9/29.3) | <0.001 | 26.6 (23.9/30.4) | 26 (24.2/30) | 0.05 |
| Preop-eGFR, mL/min/1.73 m2 | 79 (64/95) | 78 (61/94.6) | 80 (66.1/96) | 0.43 | 77 (61.2/92.8) | 80.8 (66.1/95.2) | 0.49 |
| ASA score ≥ 3, n (%) | 112 (35%) | 79 (37%) | 33 (31%) | 0.29 | 37 (42%) | 29 (33%) | 0.21 |
| Solitary kidney, n | 13 (4%) | 9 (4%) | 4 (4%) | 0.848 | 3 (3%) | 4 (4%) | 0.70 |
| Clinical tumor size, mm | 80 (73/86) | 77 (73/85) | 80 (75/90) | <0.001 | 80 (75/89) | 80 (75/85) | 0.51 |
| RENAL score ≥ 10, n (%) | 117 (37%) | 66 (31%) | 51 (49%) | 0.005 | 32 (36%) | 38 (43%) | 0.09 |
| OT, min | 180 (91.5/205) | 190 (170/225) | 75 (70/110) | <0.001 | 190 (161.5/230) | 80 (70/112.5) | <0.001 |
| LOS, d | 4 (3/5) | 4 (3/5) | 4 (3/5) | 0.51 | 4 (3/5) | 4 (3/5) | 0.12 |
| Benign histology, n (%) | 58 (18%) | 36 (17%) | 22 (21%) | 0.40 | 14 (16%) | 16 (18%) | 0.69 |
| Postop-eGFR, mL/min/1.73 m2 | 68 (50.2/83.7) | 64.5 (49.2/80.7) | 73 (57/90) | 0.02 | 66 (48.8/76.5) | 70 (57/84.9) | 0.14 |
| % eGFR loss * | 12 (2/26) | 13 (2/31) | 10 (2/20) | 0.04 | 16 (0/31) | 10 (2/20) | 0.09 |
| Major bleeding events, n | 41 (13%) | 24 (11%) | 17 (16%) | 0.23 | 13 (15%) | 13 (15%) | 1.00 |
Trifecta
| 182 (58%) 18 (6%) 42 (13%) 97 (31%) | 111 (53%) 13 (6%) 31 (15%) 75 (35%) | 68 (71%) 5 (5%) 11 (10%) 22 (21%) | 0.01 0.61 0.30 0.01 | 41 (46%) 8 (9%) 14 (16%) 36 (40%) | 59 (66%) 5 (6%) 9 (10%) 20 (22%) | 0.01 0.39 0.26 0.01 |
| A | Significant (<30%) Renal Function Deterioration | |||||||
| Univariable Analysis | Multivariable Analysis | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Lower | Higher | Lower | Higher | |||||
| Age | 1.02 | 0.99 | 1.04 | 0.19 | - | - | - | - |
| Male gender | 1.32 | 0.68 | 2.58 | 0.41 | - | - | - | - |
| BMI | 1.03 | 0.97 | 1.09 | 0.39 | - | - | - | - |
| ASA score ≥ 3 | 1.28 | 0.67 | 2.45 | 0.45 | - | - | - | - |
| Solitary kidney | 0.87 | 0.16 | 4.61 | 0.87 | - | - | - | - |
| Clinical tumor size | 0.99 | 0.96 | 1.01 | 0.27 | - | - | - | - |
| RENAL score ≥ 10 | 0.99 | 0.52 | 1.90 | 0.99 | - | - | - | - |
| Preop-eGFR | 1.01 | 0.99 | 1.03 | 0.06 | - | - | - | - |
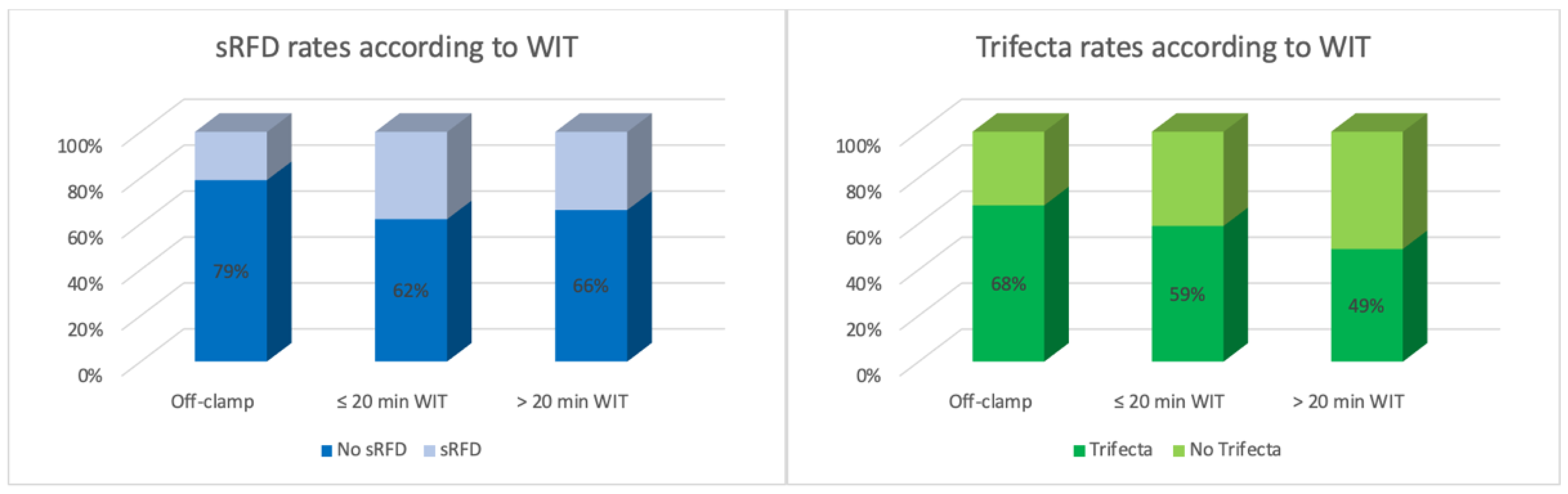
| WIT, min | 1.02 | 1.00 | 1.04 | 0.02 | - | - | - | - |
| ref | - | - | 0.38 | ||||
| 2.46 | 0.95 | 6.38 | 0.06 | ||||
| 2.30 | 1.13 | 4.64 | 0.02 | ||||
| B | Trifecta Achievement | |||||||
| Univariable Analysis | Multivariable Analysis | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Lower | Higher | Lower | Higher | |||||
| Age | 0.53 | 0.97 | 1.02 | 0.99 | - | - | - | - |
| Male gender | 0.86 | 0.47 | 1.60 | 0.64 | - | - | - | - |
| BMI | 1.008 | 0.966 | 1.051 | 0.710 | - | - | - | - |
| ASA score ≥ 3 | 0.74 | 0.40 | 1.37 | 0.34 | - | - | - | - |
| Solitary kidney | 0.30 | 0.06 | 1.58 | 0.15 | - | - | - | - |
| Clinical tumor size | 1.01 | 0.98 | 1.03 | 0.62 | - | - | - | - |
| RENAL score ≥ 10 | 1.29 | 0.70 | 2.38 | 0.409 | - | - | - | - |
| Preop-eGFR | 0.99 | 0.97 | 1.01 | 0.06 | - | - | - | - |
| WIT, min | 0.98 | 0.96 | 0.99 | 0.02 | - | - | - | - |
| ref | - | - | 0.38 | ||||
| 0.69 | 0.37 | 1.29 | 0.25 | ||||
| 0.46 | 0.27 | 0.79 | 0.004 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brassetti, A.; Cacciamani, G.E.; Mari, A.; Garisto, J.D.; Bertolo, R.; Sundaram, C.P.; Derweesh, I.; Bindayi, A.; Dasgupta, P.; Porter, J.; et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers 2022, 14, 4431. https://doi.org/10.3390/cancers14184431
Brassetti A, Cacciamani GE, Mari A, Garisto JD, Bertolo R, Sundaram CP, Derweesh I, Bindayi A, Dasgupta P, Porter J, et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers. 2022; 14(18):4431. https://doi.org/10.3390/cancers14184431
Chicago/Turabian StyleBrassetti, Aldo, Giovanni E. Cacciamani, Andrea Mari, Juan D. Garisto, Riccardo Bertolo, Chandru P. Sundaram, Ithaar Derweesh, Ahmet Bindayi, Prokar Dasgupta, James Porter, and et al. 2022. "On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis" Cancers 14, no. 18: 4431. https://doi.org/10.3390/cancers14184431
APA StyleBrassetti, A., Cacciamani, G. E., Mari, A., Garisto, J. D., Bertolo, R., Sundaram, C. P., Derweesh, I., Bindayi, A., Dasgupta, P., Porter, J., Mottrie, A., Schips, L., Rah, K. H., Chen, D. Y. T., Zhang, C., Jacobsohn, K., Anceschi, U., Bove, A. M., Costantini, M., ... Simone, G. (2022). On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for cT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers, 14(18), 4431. https://doi.org/10.3390/cancers14184431











