Impact of SSTR PET on Inter-Observer Variability of Target Delineation of Meningioma and the Possibility of Using Threshold-Based Segmentations in Radiation Oncology
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. MRI and PET/CT Imaging
2.3. Target Delineation/Treatment Planning
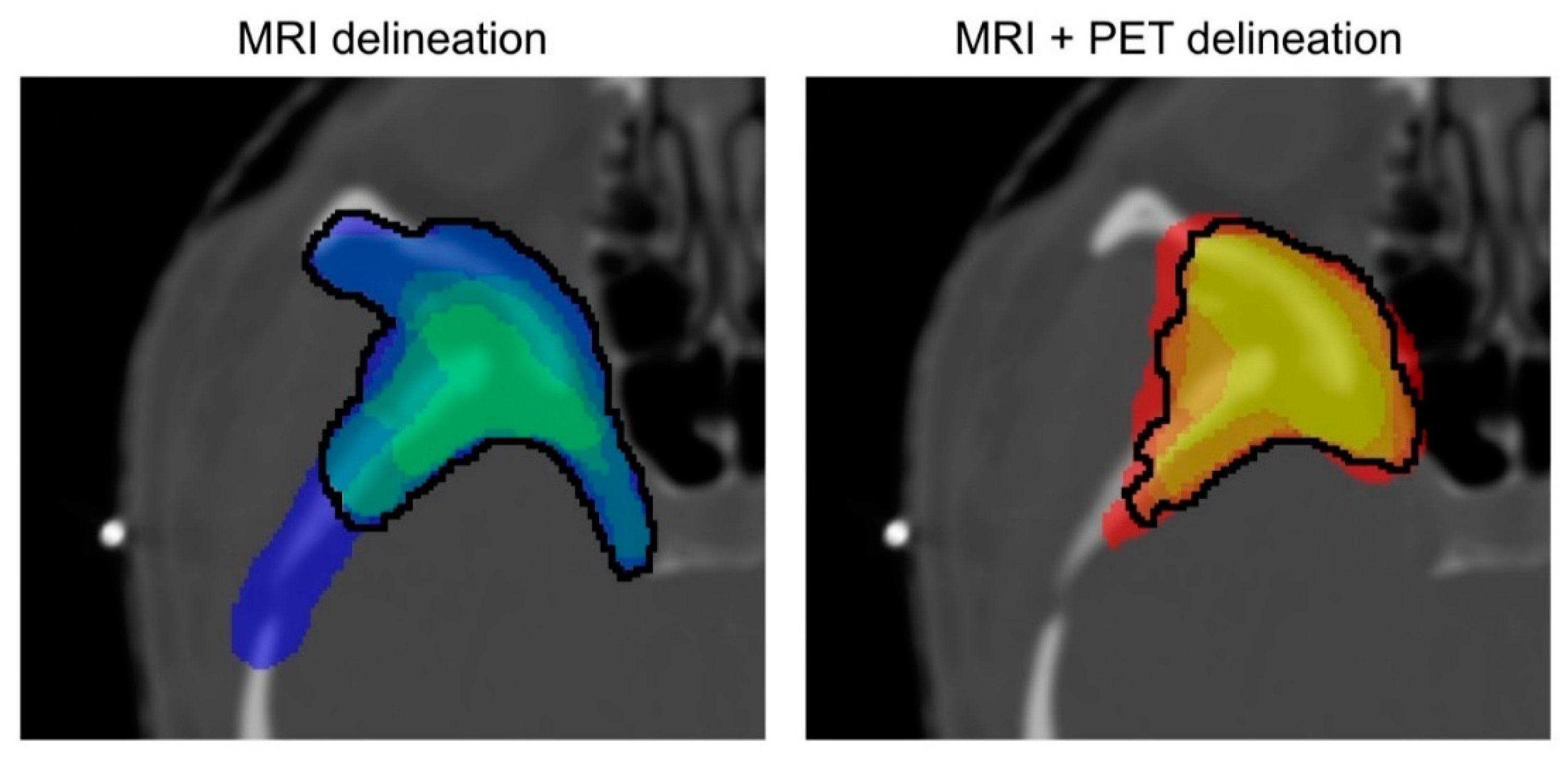
2.4. Evaluation of Inter-Observer Variability and Influence of Including PET Information
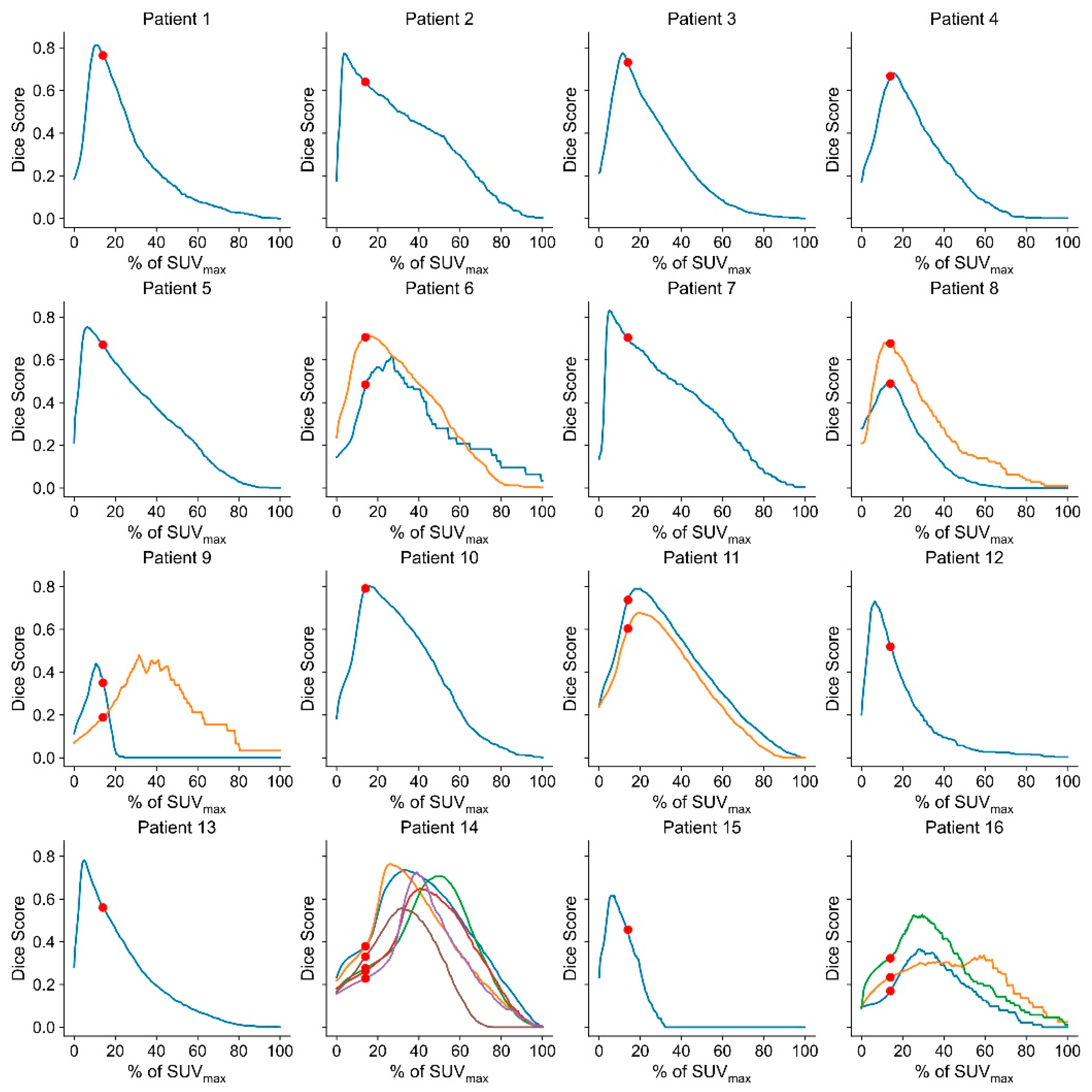
2.5. Assessment of a Thresholding Approach for GTV Definition/Lesion Delineation
2.6. Statistical Analysis
3. Results
Inter-Observer Variability
Threshold-Based Delineation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO Guidelines for the Diagnosis and Treatment of Meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Dutour, A.; Kumar, U.; Panetta, R.; Ouafik, L.; Fina, F.; Sasi, R.; Patel, Y.C. Expression of Somatostatin Receptor Subtypes in Human Brain Tumors. Int. J. Cancer 1998, 76, 620–627. [Google Scholar] [CrossRef]
- Menke, J.R.; Raleigh, D.R.; Gown, A.M.; Thomas, S.; Perry, A.; Tihan, T. Somatostatin Receptor 2a Is a More Sensitive Diagnostic Marker of Meningioma than Epithelial Membrane Antigen. Acta Neuropathol. 2015, 130, 441–443. [Google Scholar] [CrossRef]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Poschl, J.; Schuller, U.; Schichor, C.; Thon, N.; Tonn, J.-C. Increased 68Ga-DOTATATE Uptake in PET Imaging Discriminates Meningioma and Tumor-Free Tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Giesel, F.L.; Linhart, H.G.; Haberkorn, U.; Haufe, S.; Combs, S.E.; Podlesek, D.; Eisenhut, M.; Kratochwil, C. Detection of Cranial Meningiomas: Comparison of 68Ga-DOTATOC PET/CT and Contrast-Enhanced MRI. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1409–1415. [Google Scholar] [CrossRef]
- Henze, M.; Schuhmacher, J.; Hipp, P.; Kowalski, J.; Becker, D.W.; Doll, J.; Mäcke, H.R.; Hofmann, M.; Debus, J.; Haberkorn, U. PET Imaging of Somatostatin Receptors Using [68GA]DOTA-D-Phe1-Tyr3-Octreotide: First Results in Patients with Meningiomas. J. Nucl. Med. 2001, 42, 1053–1056. [Google Scholar]
- Milker-Zabel, S.; Zabel-du Bois, A.; Henze, M.; Huber, P.; Schulz-Ertner, D.; Hoess, A.; Haberkorn, U.; Debus, J. Improved Target Volume Definition for Fractionated Stereotactic Radiotherapy in Patients with Intracranial Meningiomas by Correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int. J. Radiat. Oncol. 2006, 65, 222–227. [Google Scholar] [CrossRef]
- Nyuyki, F.; Plotkin, M.; Graf, R.; Michel, R.; Steffen, I.; Denecke, T.; Geworski, L.; Fahdt, D.; Brenner, W.; Wurm, R. Potential Impact of 68Ga-DOTATOC PET/CT on Stereotactic Radiotherapy Planning of Meningiomas. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 310–318. [Google Scholar] [CrossRef]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using 68Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef]
- Perlow, H.K.; Siedow, M.; Gokun, Y.; McElroy, J.; Matsui, J.; Zoller, W.; Beyer, S.; Arnett, A.; Blakaj, D.; Boulter, D.; et al. 68Ga-DOTATATE PET-Based Radiation Contouring Creates More Precise Radiation Volumes for Meningioma Patients. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 859–865. [Google Scholar] [CrossRef]
- Maclean, J.; Fersht, N.; Sullivan, K.; Kayani, I.; Bomanji, J.; Dickson, J.; O’Meara, C.; Short, S. Simultaneous 68Ga DOTATATE Positron Emission Tomography/Magnetic Resonance Imaging in Meningioma Target Contouring: Feasibility and Impact Upon Interobserver Variability Versus Positron Emission Tomography/Computed Tomography and Computed Tomography/Magnetic Resonance Imaging. Clin. Oncol. 2017, 29, 448–458. [Google Scholar] [CrossRef]
- Pelak, M.J.; Flechl, B.; Mumot, M.; Galalae, R.; Tubin, S.; Hug, E.; Lütgendorf-Caucig, C. The Value of SSTR2 Receptor-Targeted PET/CT in Proton Irradiation of Grade I Meningioma. Cancers 2021, 13, 4707. [Google Scholar] [CrossRef]
- Vinod, S.K.; Min, M.; Jameson, M.G.; Holloway, L.C. A Review of Interventions to Reduce Inter-Observer Variability in Volume Delineation in Radiation Oncology. J. Med. Imaging Radiat. Oncol. 2016, 60, 393–406. [Google Scholar] [CrossRef]
- Warfield, S.K.; Zou, K.H.; Wells, W.M. Simultaneous Truth and Performance Level Estimation (STAPLE): An Algorithm for the Validation of Image Segmentation. IEEE Trans. Med. Imaging 2004, 23, 903–921. [Google Scholar] [CrossRef]
- Gehler, B.; Paulsen, F.; Öksüz, M.Ö.; Hauser, T.-K.; Eschmann, S.M.; Bares, R.; Pfannenberg, C.; Bamberg, M.; Bartenstein, P.; Belka, C.; et al. [68Ga]-DOTATOC-PET/CT for Meningioma IMRT Treatment Planning. Radiat. Oncol. 2009, 4, 56. [Google Scholar] [CrossRef]
- Knäusl, B.; Rausch, I.F.; Bergmann, H.; Dudczak, R.; Hirtl, A.; Georg, D. Influence of PET Reconstruction Parameters on the TrueX Algorithm. A Combined Phantom and Patient Study. Nukl. Nucl. Med. 2013, 52, 28–35. [Google Scholar] [CrossRef]
- Blanc-Durand, P.; Van Der Gucht, A.; Schaefer, N.; Itti, E.; Prior, J.O. Automatic Lesion Detection and Segmentation of 18F-FET PET in Gliomas: A Full 3D U-Net Convolutional Neural Network Study. PLoS ONE 2018, 13, e0195798. [Google Scholar] [CrossRef]
- Ganem, J.; Thureau, S.; Gardin, I.; Modzelewski, R.; Hapdey, S.; Vera, P. Delineation of Lung Cancer with FDG PET/CT during Radiation Therapy. Radiat. Oncol. 2018, 13, 1–8. [Google Scholar] [CrossRef]
- Markel, D.; Caldwell, C.; Alasti, H.; Soliman, H.; Ung, Y.; Lee, J.; Sun, A. Automatic Segmentation of Lung Carcinoma Using 3D Texture Features in 18-FDG PET/CT. Int. J. Mol. Imaging 2013, 2013, 980769. [Google Scholar] [CrossRef][Green Version]
- Schaefer, A.; Vermandel, M.; Baillet, C.; Dewalle-Vignion, A.S.; Modzelewski, R.; Vera, P.; Massoptier, L.; Parcq, C.; Gibon, D.; Fechter, T.; et al. Impact of Consensus Contours from Multiple PET Segmentation Methods on the Accuracy of Functional Volume Delineation. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 911–924. [Google Scholar] [CrossRef]
- Liberini, V.; De Santi, B.; Rampado, O.; Gallio, E.; Dionisi, B.; Ceci, F.; Polverari, G.; Thuillier, P.; Molinari, F.; Deandreis, D. Impact of Segmentation and Discretization on Radiomic Features in 68Ga-DOTA-TOC PET/CT Images of Neuroendocrine Tumor. EJNMMI Phys. 2021, 8, 21. [Google Scholar] [CrossRef] [PubMed]


| Study Group | Validation Group | |
|---|---|---|
| Number of patients | 16 | 7 |
| Age (p = 0.24) | 55.6 ± 15.2 | 61.4 ± 12.5 |
| Sex: w/m (p = 0.30) | 10/6 | 6/1 |
| Number of lesions | 27 | 7 |
| Number of lesions per patient (p = 0.97) | 1.7 ± 1.3 (range: 1–6) | 1.7 ± 1.3 (range: 1–4) |
| Location of the lesions | Skull base: 19× Falx: 3× Calvaria: 5× | Skull base: 10× Calvaria: 2× |
| Meningioma subtype | Meningothelial: 11 lesions Clear cell: 1 lesion Secretory: 1 lesion Transitional: 1 lesion Atypical: 2 lesions Anaplastic: 5 lesions Unknown: 6 lesions | Unknown: 12 lesions |
| Grading (WHO 2016) | I: 13 lesions II: 5 lesions III: 5 lesions Unknown *: 4 lesions | I: 5 lesions Unknown *: 7 lesions |
| Physician | Modality | Average Volume (cm3) | Standard Deviation (cm3) | Median Volume (cm3) | Minimum Volume (cm3) | Maximum Volume (cm3) | % Change in Volume between Modalities |
|---|---|---|---|---|---|---|---|
| CLC | MRI | 11.3 | 8.9 | 9.0 | 0.8 | 29.4 | 25.0 |
| MRI + PET | 15.1 | 14.9 | 10.2 | 1.1 | 56.8 | ||
| CW | MRI | 13.2 | 10.8 | 10.5 | 0.6 | 39.1 | 23.5 |
| MRI + PET | 17.3 | 16.0 | 11.4 | 1.1 | 56.0 | ||
| HH | MRI | 16.1 | 19.5 | 9.2 | 1.0 | 95.1 | −3.9 |
| MRI + PET | 15.5 | 14.9 | 13.2 | 1.3 | 53.1 | ||
| SK | MRI | 13.2 | 11.6 | 10.2 | 0.8 | 43.8 | 16.5 |
| MRI + PET | 15.8 | 15.1 | 12.8 | 1.0 | 53.4 | ||
| ULB | MRI | 11.7 | 10.8 | 8.9 | 0.5 | 44.0 | 18.0 |
| MRI + PET | 14.2 | 14.1 | 10.0 | 0.9 | 54.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriwanek, F.; Ulbrich, L.; Lechner, W.; Lütgendorf-Caucig, C.; Konrad, S.; Waldstein, C.; Herrmann, H.; Georg, D.; Widder, J.; Traub-Weidinger, T.; et al. Impact of SSTR PET on Inter-Observer Variability of Target Delineation of Meningioma and the Possibility of Using Threshold-Based Segmentations in Radiation Oncology. Cancers 2022, 14, 4435. https://doi.org/10.3390/cancers14184435
Kriwanek F, Ulbrich L, Lechner W, Lütgendorf-Caucig C, Konrad S, Waldstein C, Herrmann H, Georg D, Widder J, Traub-Weidinger T, et al. Impact of SSTR PET on Inter-Observer Variability of Target Delineation of Meningioma and the Possibility of Using Threshold-Based Segmentations in Radiation Oncology. Cancers. 2022; 14(18):4435. https://doi.org/10.3390/cancers14184435
Chicago/Turabian StyleKriwanek, Florian, Leo Ulbrich, Wolfgang Lechner, Carola Lütgendorf-Caucig, Stefan Konrad, Cora Waldstein, Harald Herrmann, Dietmar Georg, Joachim Widder, Tatjana Traub-Weidinger, and et al. 2022. "Impact of SSTR PET on Inter-Observer Variability of Target Delineation of Meningioma and the Possibility of Using Threshold-Based Segmentations in Radiation Oncology" Cancers 14, no. 18: 4435. https://doi.org/10.3390/cancers14184435
APA StyleKriwanek, F., Ulbrich, L., Lechner, W., Lütgendorf-Caucig, C., Konrad, S., Waldstein, C., Herrmann, H., Georg, D., Widder, J., Traub-Weidinger, T., & Rausch, I. (2022). Impact of SSTR PET on Inter-Observer Variability of Target Delineation of Meningioma and the Possibility of Using Threshold-Based Segmentations in Radiation Oncology. Cancers, 14(18), 4435. https://doi.org/10.3390/cancers14184435





