Functionalized Hybrid Iron Oxide–Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hybrid Fe3O4-Au Nanoparticles (FeAuNPs)
2.2. Cell Culture
2.3. Reagents
2.4. Assessment of the Hsp70 Expression on the Cell Membrane
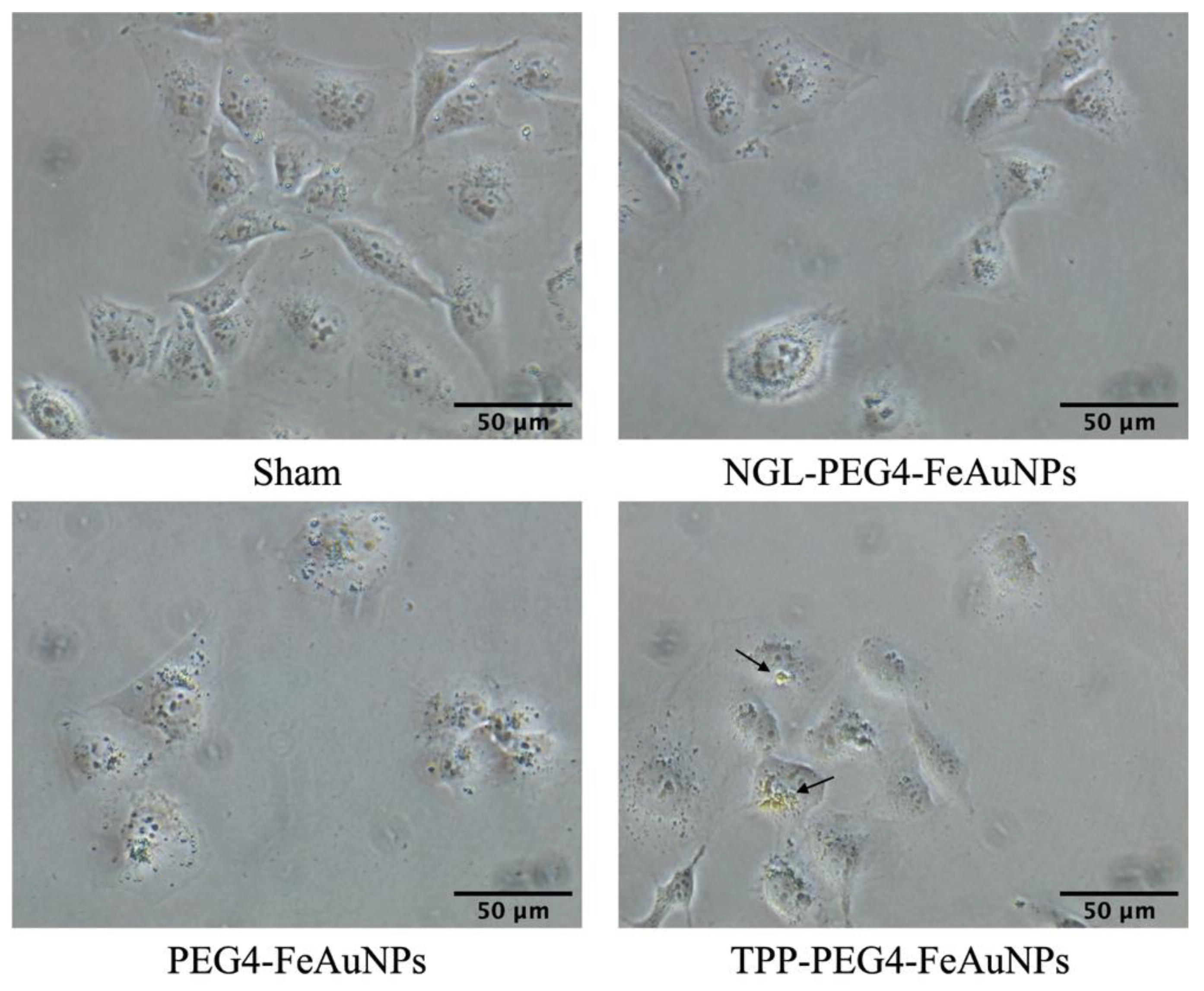
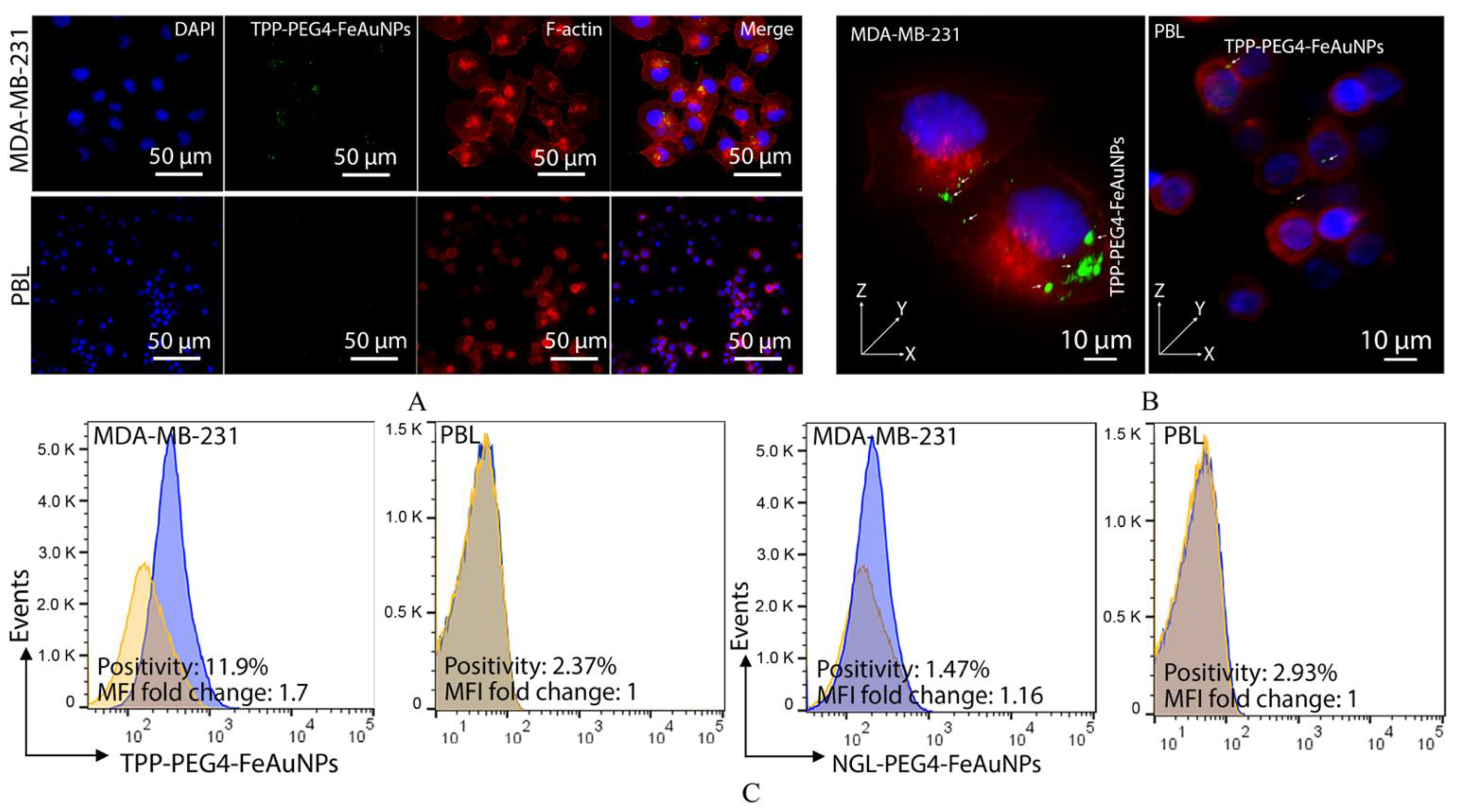
2.5. Assessment of the Uptake of TPP-Functionalized Nanoparticles into Tumor, but Not Normal, Cells
2.6. Visualization of the Internalization of TPP-Functionalized Nanoparticles into Tumor, but Not Normal, Cells
2.7. Clonogenic Colony Formation Assay (CFA)
2.8. Measurement of the Cytotoxicity of Hybrid FeAuNPs in PBL by the CCK-8 Assay
2.9. Cell Cycle Analysis
2.10. Annexin V/Propidium Iodide Apoptosis Assay
2.11. Cellular Reactive Oxygen Species (ROS) Assay
2.12. Western Blot
2.13. Statistical Analysis
3. Results
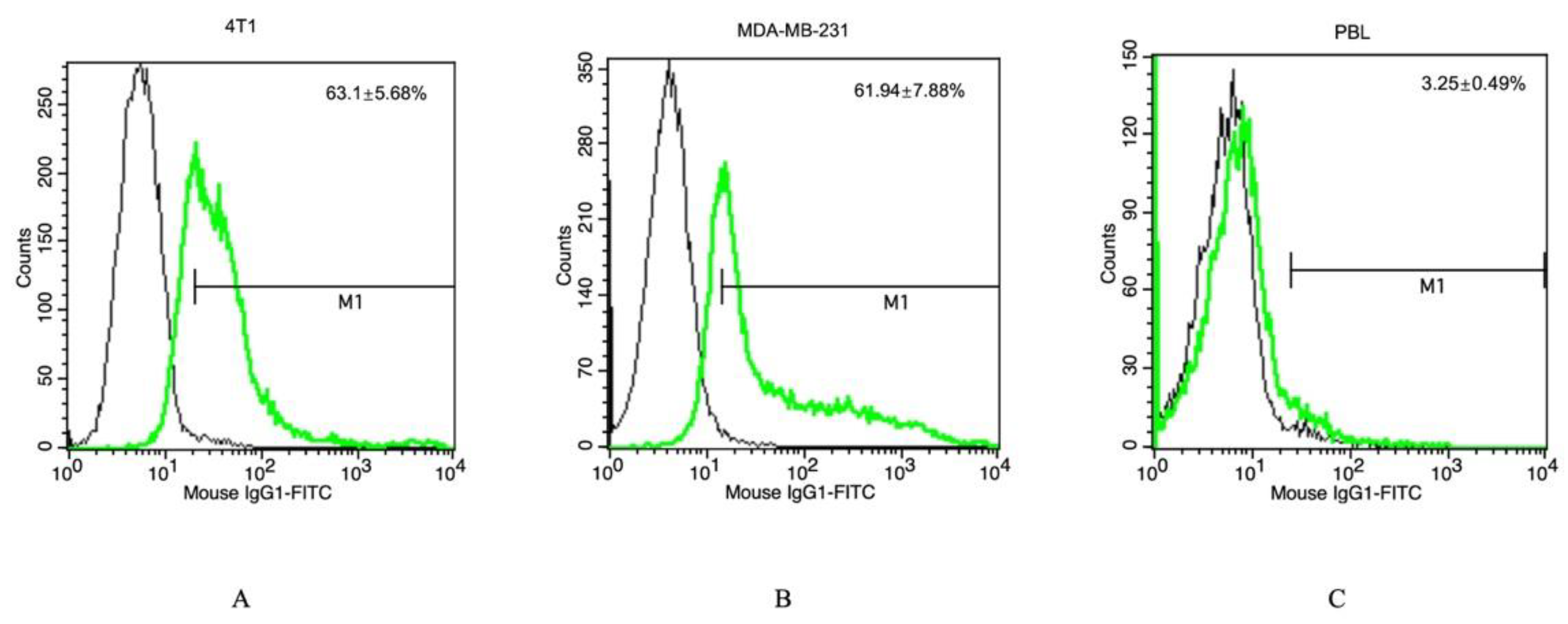
3.1. TPP Peptide Increases TNBC’s Affinity to FeAuNPs
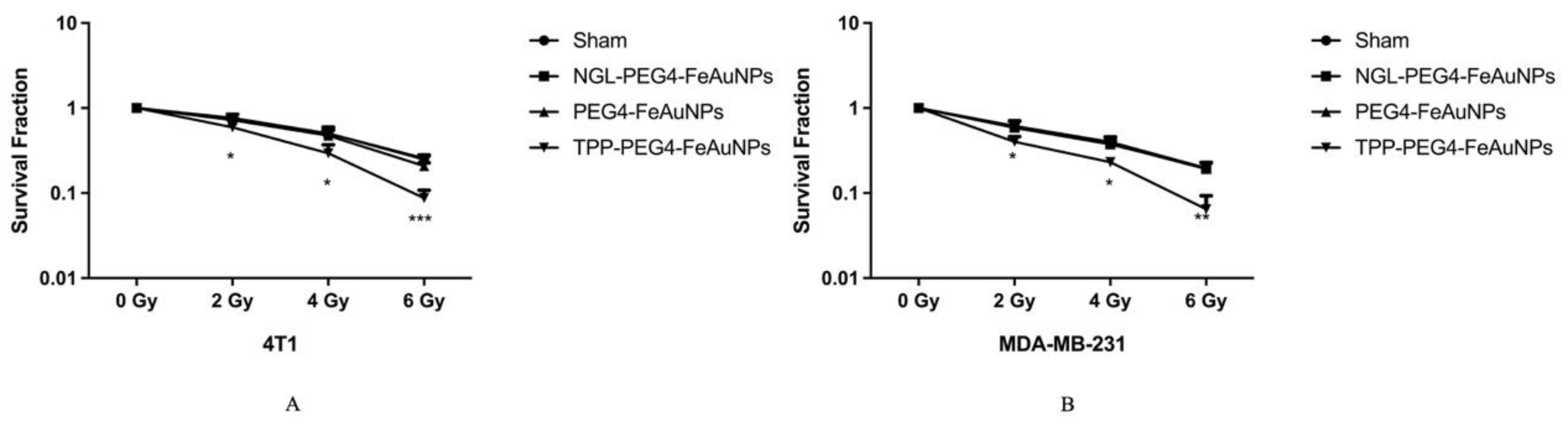
3.2. TPP-PEG4-FeAuNPs Radiosensitize TNBCs
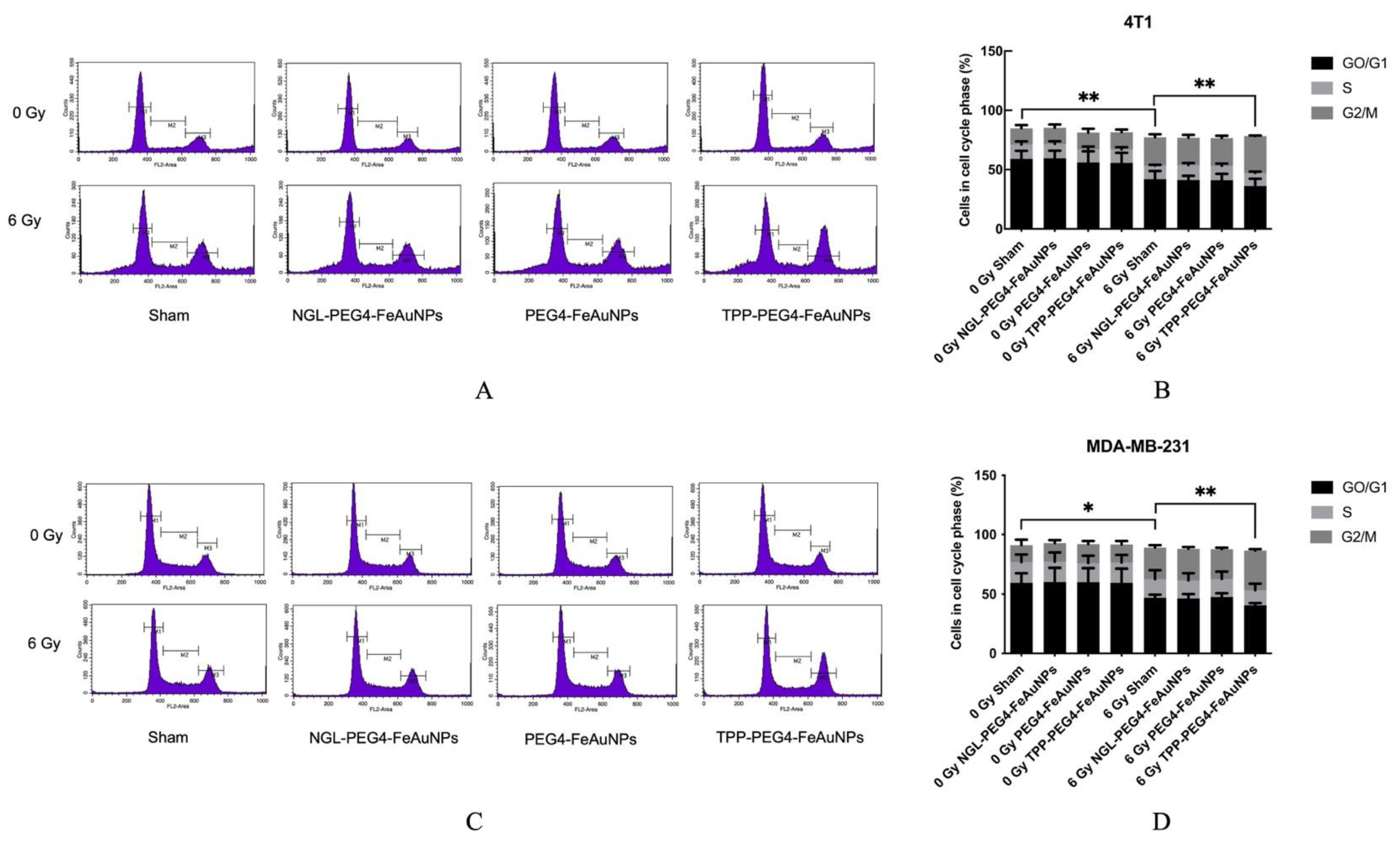
3.3. TPP-PEG4-FeAuNPs Induce Cell Cycle Arrest at G2/M in TNBCs
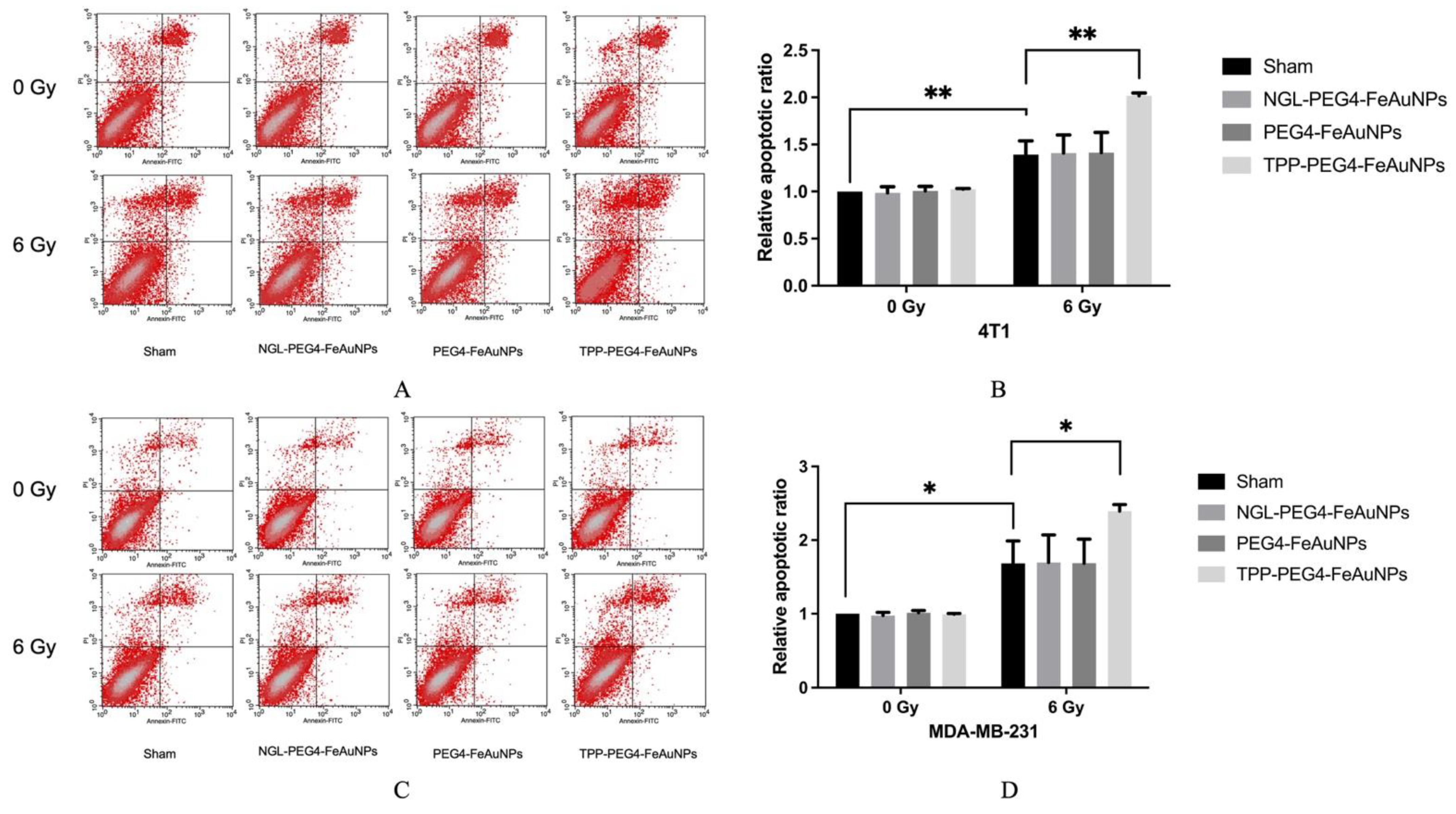
3.4. TPP-PEG4-FeAuNPs Induce Apoptosis in TNBCs
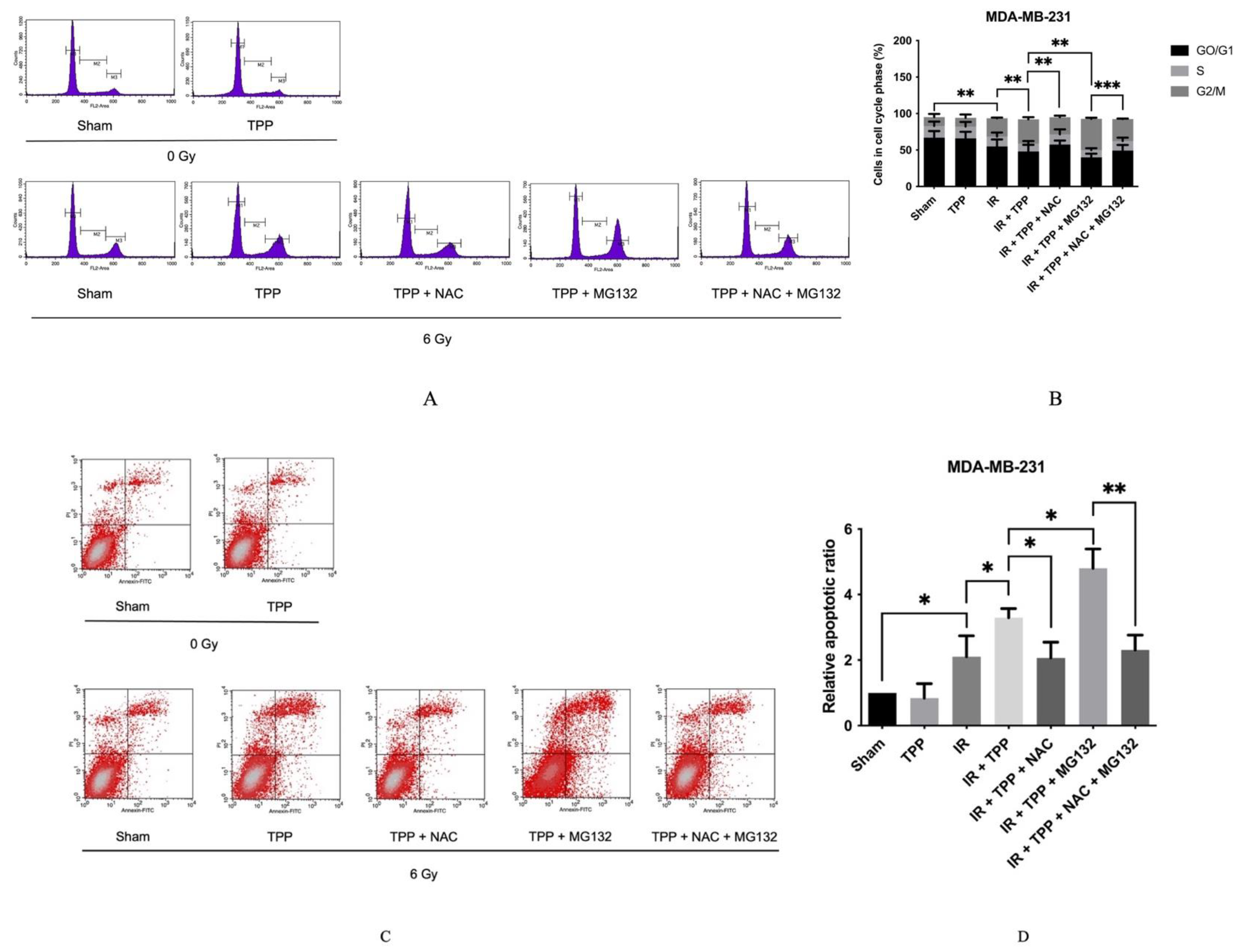
3.5. TPP-PEG4-FeAuNPs Induce Oxidative Stress in TNBCs
3.6. TPP-PEG4-FeAuNPs Induce DNA Double-Strand Breaks in TNBCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bareche, Y.; Buisseret, L.; Gruosso, T.; Girard, E.; Venet, D.; Dupont, F.; Desmedt, C.; Larsimont, D.; Park, M.; Rothe, F.; et al. Unraveling Triple-Negative Breast Cancer Tumor Microenvironment Heterogeneity: Towards an Optimized Treatment Approach. J. Natl. Cancer Inst. 2020, 112, 708–719. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- He, M.Y.; Rancoule, C.; Rehailia-Blanchard, A.; Espenel, S.; Trone, J.C.; Bernichon, E.; Guillaume, E.; Vallard, A.; Magne, N. Radiotherapy in triple-negative breast cancer: Current situation and upcoming strategies. Crit. Rev. Oncol. Hematol. 2018, 131, 96–101. [Google Scholar] [CrossRef]
- Weidle, U.H.; Maisel, D.; Klostermann, S.; Schiller, C.; Weiss, E.H. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody-related agents. Cancer Genom. Proteom. 2011, 8, 49–63. [Google Scholar]
- Dezfouli, A.B.; Yazdi, M.; Benmebarek, M.-R.; Schwab, M.; Michaelides, S.; Miccichè, A.; Geerts, D.; Stangl, S.; Klapproth, S.; Wagner, E. CAR T cells targeting membrane-bound Hsp70 on tumor cells mimic Hsp70-primed NK cells. Front. Immunol. 2022, 13. [Google Scholar]
- Bashiri Dezfouli, A.; Yazdi, M.; Pockley, A.G.; Khosravi, M.; Kobold, S.; Wagner, E.; Multhoff, G. NK cells armed with chimeric antigen receptors (CAR): Roadblocks to successful development. Cells 2021, 10, 3390. [Google Scholar] [CrossRef]
- Vulczak, A.; Catalao, C.H.R.; Freitas, L.A.P.; Rocha, M.J.A. HSP-Target of Therapeutic Agents in Sepsis Treatment. Int. J. Mol. Sci. 2019, 20, 4255. [Google Scholar] [CrossRef]
- Kimm, M.A.; Shevtsov, M.; Werner, C.; Sievert, W.; Zhiyuan, W.; Schoppe, O.; Menze, B.H.; Rummeny, E.J.; Proksa, R.; Bystrova, O.; et al. Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors. Cancers 2020, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Muller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef]
- Gehrmann, M.; Specht, H.M.; Bayer, C.; Brandstetter, M.; Chizzali, B.; Duma, M.; Breuninger, S.; Hube, K.; Lehnerer, S.; van Phi, V.; et al. Hsp70--a biomarker for tumor detection and monitoring of outcome of radiation therapy in patients with squamous cell carcinoma of the head and neck. Radiat. Oncol. 2014, 9, 131. [Google Scholar] [CrossRef]
- Huwaidi, A.; Kumari, B.; Robert, G.; Guerin, B.; Sanche, L.; Wagner, J.R. Profiling DNA Damage Induced by the Irradiation of DNA with Gold Nanoparticles. J. Phys. Chem. Lett. 2021, 12, 9947–9954. [Google Scholar] [CrossRef]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Garnica-Garza, H.M. Microdosimetry of X-ray-irradiated gold nanoparticles. Radiat. Prot. Dosimetry 2013, 155, 59–63. [Google Scholar] [CrossRef]
- Durand, M.; Lelievre, E.; Chateau, A.; Berquand, A.; Laurent, G.; Carl, P.; Roux, S.; Chazee, L.; Bazzi, R.; Eghiaian, F.; et al. The detrimental invasiveness of glioma cells controlled by gadolinium chelate-coated gold nanoparticles. Nanoscale 2021, 13, 9236–9251. [Google Scholar] [CrossRef]
- Bhagat, S.; Srikanth Vallabani, N.V.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J. Colloid Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Sun, Y.; Huo, B.; Mao, Z.; Wang, X.; Li, S.; Lu, R.; Li, S.; Liang, J.; Gao, Z. Development of Fe3O4@Au nanoparticles coupled to Au@Ag core-shell nanoparticles for the sensitive detection of zearalenone. Anal. Chim. Acta 2021, 1180, 338888. [Google Scholar] [CrossRef]
- Akasaka, M.; Nishi, T.; Niidome, Y. Gold-Silver and Gold-Palladium Alloy Nanoparticles as Mass-Probes for Immunosensing. Anal Sci. 2021, 37, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Miao, Y.Q.; Li, L.; Fan, H.M. Facile Preparation of Gold-Decorated Fe(3)O(4) Nanoparticles for CT and MR Dual-Modal Imaging. Int. J. Mol. Sci. 2018, 19, 4049. [Google Scholar] [CrossRef]
- Kang, N.; Xu, D.; Han, Y.; Lv, X.; Chen, Z.; Zhou, T.; Ren, L.; Zhou, X. Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 98, 545–549. [Google Scholar] [CrossRef]
- Ngwa, W.; Makrigiorgos, G.M.; Berbeco, R.I. Gold nanoparticle-aided brachytherapy with vascular dose painting: Estimation of dose enhancement to the tumor endothelial cell nucleus. Med. Phys. 2012, 39, 392–398. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, H.; Li, L.; Feng, Y.; Xu, S.; Wang, Z. Trem2 mediated Syk-dependent ROS amplification is essential for osteoclastogenesis in periodontitis microenvironment. Redox Biol. 2021, 40, 101849. [Google Scholar] [CrossRef]
- Jin, T.; Wang, X.; Deng, Z.; Liu, X.; Liang, D. ROS-induced dramatic lipid changes in Arabidopsis. Redox Rep. 2021, 26, 190–196. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Wang, C.; Lu, D.F.; Zhao, X.D.; Tan, L.H.; Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging 2020, 12, 3662–3681. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ji, Y.; Li, X.; Ding, J.; Chen, L.; Huang, Y.; Wei, W. URI1 suppresses irradiation-induced reactive oxygen species (ROS) by activating autophagy in hepatocellular carcinoma cells. Int. J Biol. Sci. 2021, 17, 3091–3103. [Google Scholar] [CrossRef]
- Yalcin, Y.; Tekin, I.O.; Tigli Aydin, R.S. Ionizing radiation induced DNA damage via ROS production in nano ozonized oil treated B-16 melanoma and OV-90 ovarian cells. Biochem. Biophys. Res. Commun. 2022, 615, 143–149. [Google Scholar] [CrossRef]
- Qin, X.; Yang, C.; Xu, H.; Zhang, R.; Zhang, D.; Tu, J.; Guo, Y.; Niu, B.; Kong, L.; Zhang, Z. Cell-Derived Biogenetic Gold Nanoparticles for Sensitizing Radiotherapy and Boosting Immune Response against Cancer. Small 2021, 17, e2103984. [Google Scholar] [CrossRef]
- Stangl, S.; Tei, L.; De Rose, F.; Reder, S.; Martinelli, J.; Sievert, W.; Shevtsov, M.; Ollinger, R.; Rad, R.; Schwaiger, M.; et al. Preclinical Evaluation of the Hsp70 Peptide Tracer TPP-PEG24-DFO[(89)Zr] for Tumor-Specific PET/CT Imaging. Cancer Res. 2018, 78, 6268–6281. [Google Scholar] [CrossRef]
- Hamidi, M.; Rafiei, P.; Azadi, A. Designing PEGylated therapeutic molecules: Advantages in ADMET properties. Expert Opin. Drug Discov. 2008, 3, 1293–1307. [Google Scholar] [CrossRef]
- Xu, D.; Yao, J.; Zhang, Y.; Xiao, N.; Peng, P.; Li, Z.; Pan, Z.; Yao, Z. The Effect of PEI-Mediated E1A on the Radiosensitivity of Hepatic Carcinoma Cells. Asian Pac. J. Cancer Prev. 2020, 21, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Han, J.; Feng, J.; Guo, T.; Li, Z.; Min, F.; Jin, R.; Peng, X. N-Acetylcysteine Inhibits Patulin-Induced Apoptosis by Affecting ROS-Mediated Oxidative Damage Pathway. Toxins 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Yang, Y.M.; Kim, S.Z.; Park, W.H. Attenuation of MG132-induced HeLa cell death by N-acetyl cysteine via reducing reactive oxygen species and preventing glutathione depletion. Anticancer Res. 2010, 30, 2107–2112. [Google Scholar] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef]
- Wen, K.K.; Han, S.S.; Vyas, Y.M. Wiskott-Aldrich syndrome protein senses irradiation-induced DNA damage to coordinate the cell-protective Golgi dispersal response in human T and B lymphocytes. J. Allergy Clin. Immunol. 2020, 145, 324–334. [Google Scholar] [CrossRef]
- Abdeltawab, A.A.; Ali, S.A.; Mostafa, H.G.; Hassan, M.A. Predictive Factors Increasing the Risk of Radiation Toxicity in Patients with Early Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 145–149. [Google Scholar] [CrossRef]
- Marteinsdottir, M.; Wang, C.C.; McNamara, A.; Depauw, N.; Shin, J.; Paganetti, H. The impact of variable relative biological effectiveness in proton therapy for left-sided breast cancer when estimating normal tissue complications in the heart and lung. Phys. Med. Biol. 2021, 66, 035023. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Oei, A.L.; Korangath, P.; Mulka, K.; Helenius, M.; Coulter, J.B.; Stewart, J.; Velarde, E.; Crezee, J.; Simons, B.; Stalpers, L.J.A.; et al. Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int. J. Hyperth. 2019, 36, 47–63. [Google Scholar] [CrossRef]
- Sears, J.; Swanner, J.; Fahrenholtz, C.D.; Snyder, C.; Rohde, M.; Levi-Polyachenko, N.; Singh, R. Combined Photothermal and Ionizing Radiation Sensitization of Triple-Negative Breast Cancer Using Triangular Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 851–865. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, F.Y.; Smilowitz, H.M. Iodine nanoparticle radiotherapy of human breast cancer growing in the brains of athymic mice. Sci. Rep. 2020, 10, 15627. [Google Scholar] [CrossRef]
- Bhattarai, S.; Mackeyev, Y.; Venkatesulu, B.P.; Krishnan, S.; Singh, P.K. CXC chemokine receptor 4 (CXCR4) targeted gold nanoparticles potently enhance radiotherapy outcomes in breast cancer. Nanoscale 2021, 13, 19056–19065. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed]
- Pullambhatla, M.; Rowe, S.P.; Lisok, A.; Wang, Y.; Todd, G.P.; Danilkovitch, A.; Pomper, M.G. Enhancement of Radiotherapy with Human Mesenchymal Stem Cells Containing Gold Nanoparticles. Tomography 2020, 6, 373–378. [Google Scholar] [CrossRef]
- Vilchis-Juarez, A.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Ocampo-Garcia, B.; Diaz-Nieto, L.; Luna-Gutierrez, M.; Jimenez-Mancilla, N.; Pedraza-Lopez, M.; Gomez-Olivan, L. Molecular targeting radiotherapy with cyclo-RGDFK(C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J. Biomed. Nanotechnol. 2014, 10, 393–404. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Li, F.; Wang, Y.; Ding, M.; Zhang, J.; Yin, H.; Zhang, R.; Ren, X. EGFR-specific single-chain variable fragment antibody-conjugated Fe3O4/Au nanoparticles as an active MRI contrast agent for NSCLC. MAGMA 2021, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.E.; Delgado, C.; Fisher, D.; Malik, F.; Agrawal, A.K. Polyethylene glycol modification: Relevance of improved methodology to tumour targeting. J. Drug. Target 1996, 3, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Dressel, R.; Alves, F.; Dullin, C.; Themelis, G.; Ntziachristos, V.; Staeblein, E.; Walch, A.; Winkelmann, I.; et al. In vivo imaging of CT26 mouse tumours by using cmHsp70.1 monoclonal antibody. J. Cell Mol. Med. 2011, 15, 874–887. [Google Scholar] [CrossRef]
- Ozcelik, S.; Pratx, G. Nuclear-targeted gold nanoparticles enhance cancer cell radiosensitization. Nanotechnology 2020, 31, 415102. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46 (Suppl. 1), 4S–12S. [Google Scholar]
- Peng, Y.; Fu, S.; Hu, W.; Qiu, Y.; Zhang, L.; Tan, R.; Sun, L.Q. Glutamine synthetase facilitates cancer cells to recover from irradiation-induced G2/M arrest. Cancer Biol. Ther. 2020, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Masoudi-Khoram, N.; Abdolmaleki, P.; Hosseinkhan, N.; Nikoofar, A.; Mowla, S.J.; Monfared, H.; Baldassarre, G. Differential miRNAs expression pattern of irradiated breast cancer cell lines is correlated with radiation sensitivity. Sci. Rep. 2020, 10, 9054. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Aberg, C.; Salvati, A.; Dawson, K.A. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 2011, 7, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, W.; Zhang, Z.; Huang, Y. Time-staggered delivery of docetaxel and H1-S6A,F8A peptide for sequential dual-strike chemotherapy through tumor priming and nuclear targeting. J. Control Release 2016, 232, 62–74. [Google Scholar] [CrossRef]
- Evan, G.I.; Brown, L.; Whyte, M.; Harrington, E. Apoptosis and the cell cycle. Curr. Opin. Cell Biol. 1995, 7, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Wang, J.; Kopecek, J. Drug-free macromolecular therapeutics exhibit amplified apoptosis in G2/M phase arrested cells. J. Drug. Target 2019, 27, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J. Mol. Med 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Kuczler, M.D.; Olseen, A.M.; Pienta, K.J.; Amend, S.R. ROS-induced cell cycle arrest as a mechanism of resistance in polyaneuploid cancer cells (PACCs). Prog. Biophys. Mol. Biol. 2021, 165, 3–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takahashi, J.; Nagasawa, S.; Doi, M.; Moriyama, A.; Iwahashi, H. DNA Strand Break Properties of Protoporphyrin IX by X-Ray Irradiation against Melanoma. Int. J. Mol. Sci. 2020, 21, 2302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Subedi, K.P.; Zheng, C.; Ambudkar, I. Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction. Sci. Rep. 2021, 11, 7690. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Influence of N-acetyl-L-cysteine against bisphenol a on the maturation of mouse oocytes and embryo development: In vitro study. BMC Pharmacol. Toxicol. 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Moon, H.J.; You, B.R.; Park, W.H. The effect of MG132, a proteasome inhibitor on HeLa cells in relation to cell growth, reactive oxygen species and GSH. Oncol. Rep. 2009, 22, 215–221. [Google Scholar] [PubMed]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, X. ROS Reduction Does Not Decrease the Anticancer Efficacy of X-Ray in Two Breast Cancer Cell Lines. Oxid. Med. Cell Longev. 2019, 2019, 3782074. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Stangl, S.; Hernandez-Schnelzer, A.; Wang, F.; Hasanzadeh Kafshgari, M.; Bashiri Dezfouli, A.; Multhoff, G. Functionalized Hybrid Iron Oxide–Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis. Cancers 2023, 15, 1167. https://doi.org/10.3390/cancers15041167
Wu Z, Stangl S, Hernandez-Schnelzer A, Wang F, Hasanzadeh Kafshgari M, Bashiri Dezfouli A, Multhoff G. Functionalized Hybrid Iron Oxide–Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis. Cancers. 2023; 15(4):1167. https://doi.org/10.3390/cancers15041167
Chicago/Turabian StyleWu, Zhiyuan, Stefan Stangl, Alicia Hernandez-Schnelzer, Fei Wang, Morteza Hasanzadeh Kafshgari, Ali Bashiri Dezfouli, and Gabriele Multhoff. 2023. "Functionalized Hybrid Iron Oxide–Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis" Cancers 15, no. 4: 1167. https://doi.org/10.3390/cancers15041167
APA StyleWu, Z., Stangl, S., Hernandez-Schnelzer, A., Wang, F., Hasanzadeh Kafshgari, M., Bashiri Dezfouli, A., & Multhoff, G. (2023). Functionalized Hybrid Iron Oxide–Gold Nanoparticles Targeting Membrane Hsp70 Radiosensitize Triple-Negative Breast Cancer Cells by ROS-Mediated Apoptosis. Cancers, 15(4), 1167. https://doi.org/10.3390/cancers15041167





