Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Antibodies
2.3. Constructs Expressing sPTK7 and sPTK7 Domains
2.4. Expression and Purification of sPTK7 and sPTK7 Domains in HEK293 Cells
2.5. Anti-PTK7 mAbs
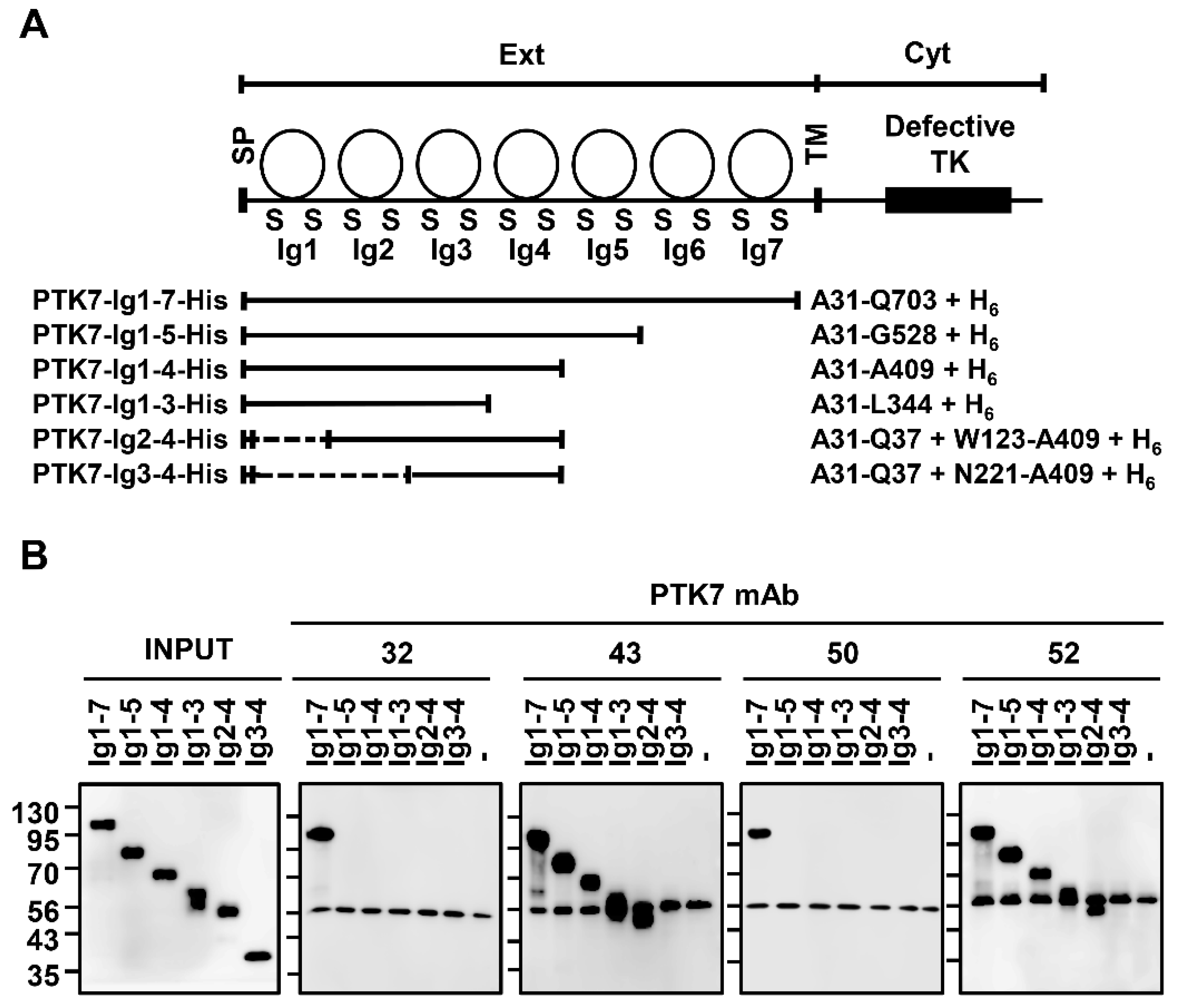
2.6. Analysis of Binding Domains of Anti-PTK7 mAbs
2.7. Western Blotting
2.8. Adhesion Assay
2.9. Wound Healing Assay
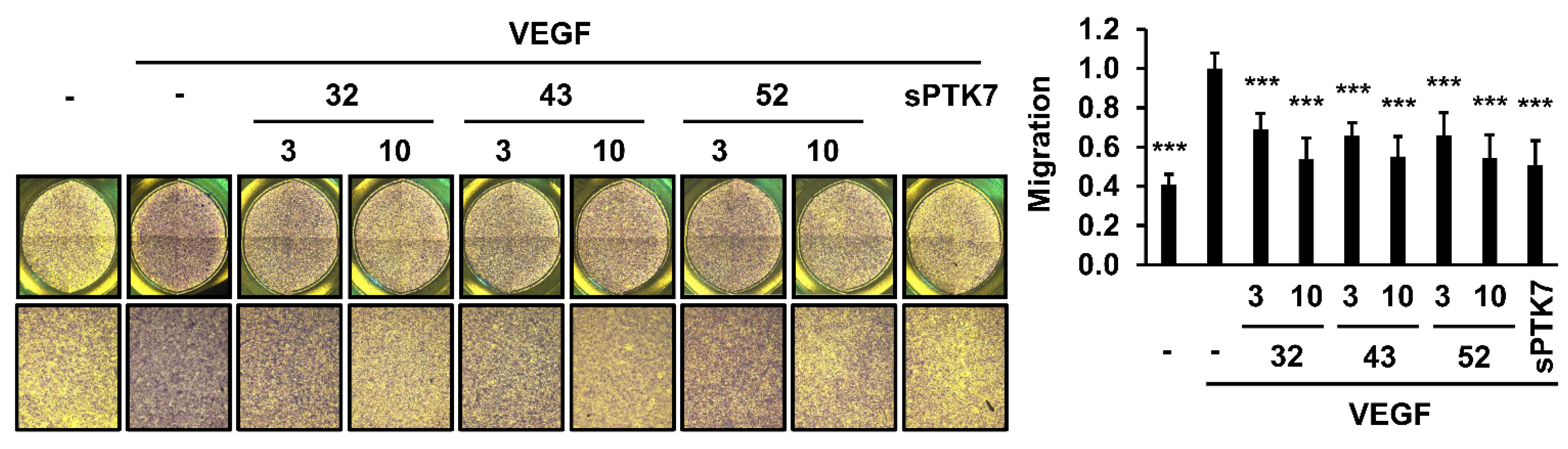
2.10. Chemotactic Migration and Invasion Assay
2.11. Capillary-like Tube Formation Assay
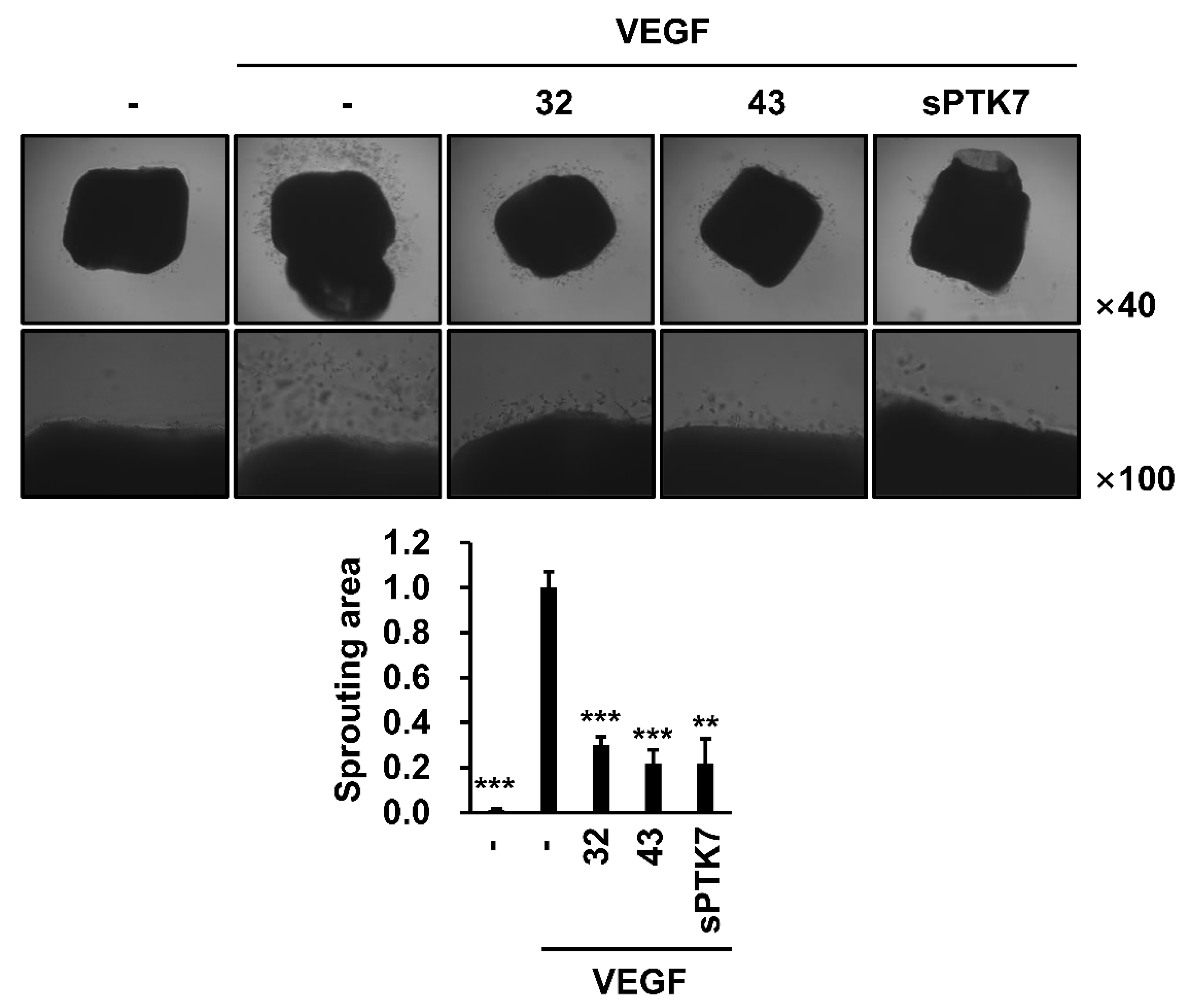
2.12. Mouse Aortic Ring Assay
2.13. Matrigel Plug Assay
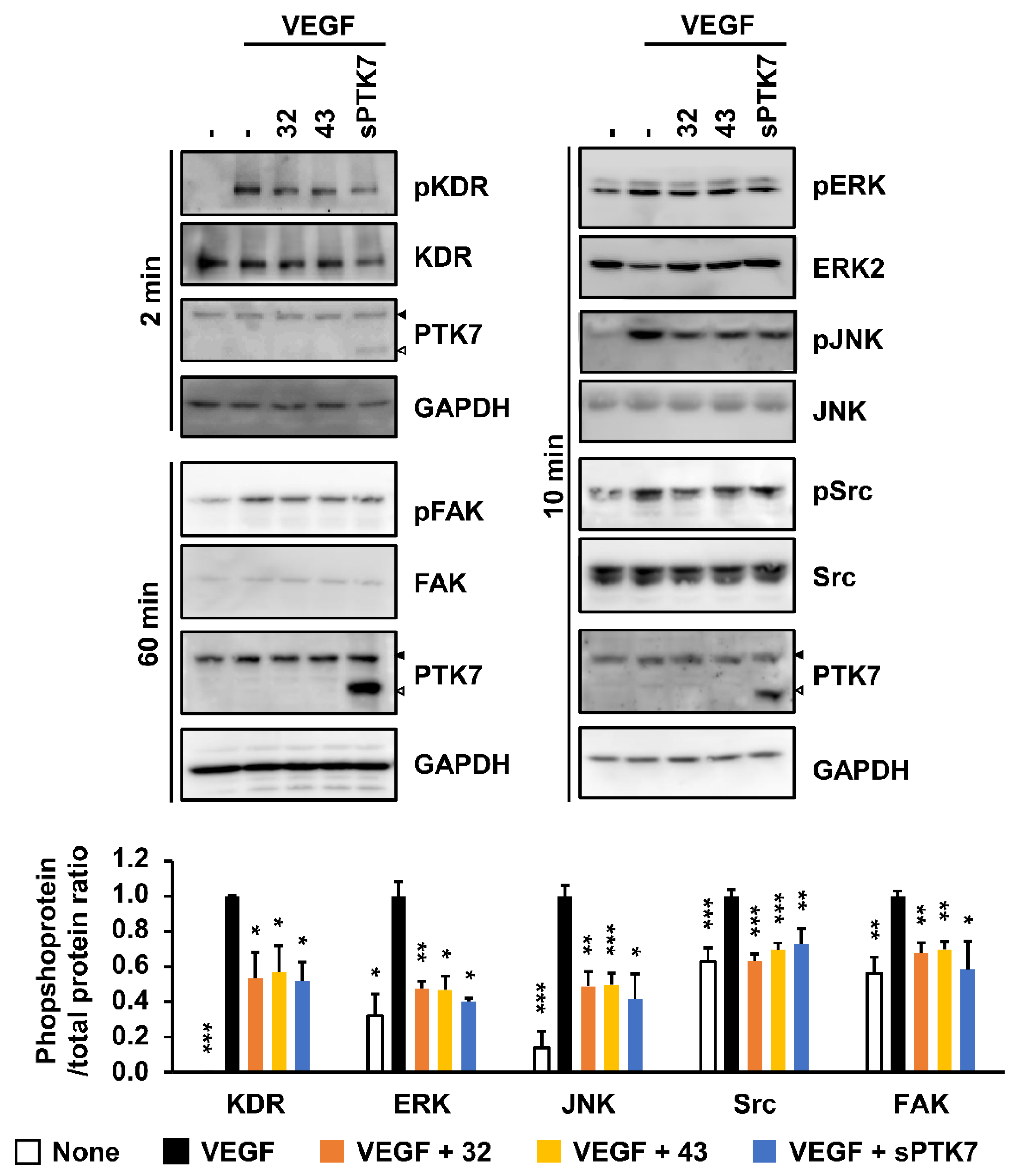
2.14. Analysis of Signaling Proteins in HUVECs
2.15. Co-Expression of PTK7 and KDR in HEK293 Cells
2.16. Pull-Down Assay
2.17. Statistical Analysis
3. Results
3.1. Analysis of the PTK7-Binding Domains of Anti-PTK7 mAbs
3.2. Effect of Anti-PTK7 mAbs on Angiogenic Phenotypes in HUVECs
3.3. Effect of Anti-PTK7 mAbs on Angiogenesis In Vitro and Ex Vivo
3.4. Effect of Anti-PTK7 mAbs on Angiogenesis In Vivo
3.5. Effect of Anti-PTK7 mAbs on VEGF-Induced KDR Signaling in HUVECs
3.6. Effect of Anti-PTK7 mAbs on PTK7–KDR Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, D.L.; Yarden, Y. Receptor Tyrosine Kinases: Family and Subfamilies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 539–558. [Google Scholar]
- Mendrola, J.M.; Shi, F.; Park, J.H.; Lemmon, M.A. Receptor tyrosine kinases with intracellular pseudokinase domains. Biochem. Soc. Trans. 2013, 41, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Ji, A.R.; Lee, J.; Kim, U.J.; Lee, S.-T. Organization of the human PTK7 gene encoding a receptor protein tyrosine kinase-like molecule and alternative splicing of its mRNA. Biochim. Biophys. Acta 2002, 1579, 153–163. [Google Scholar] [CrossRef]
- Wagner, G.; Peradziryi, H.; Wehner, P.; Borchers, A. PlexinA1 interacts with PTK7 and is required for neural crest migration. Biochem. Biophys. Res. Commun. 2010, 402, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Scerbo, P.; Giordano, M.; Daulat, A.M.; Lhoumeau, A.C.; Thomé, V.; Kodjabachian, L.; Borg, J.P. The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J. Biol. Chem. 2015, 290, 30562–30572. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Naito, M.; Daulat, A.; Angers, S.; Ciruna, B. Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development 2013, 140, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Bin-Nun, N.; Lichtig, H.; Malyarova, A.; Levy, M.; Elias, S.; Frank, D. PTK7 modulates Wnt signaling activity via LRP6. Development 2014, 141, 410–421. [Google Scholar] [CrossRef]
- Peradziryi, H.; Kaplan, N.A.; Podleschny, M.; Liu, X.; Wehner, P.; Borchers, A.; Tolwinski, N.S. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J. 2011, 30, 3729–3740. [Google Scholar] [CrossRef]
- Berger, H.; Wodarz, A.; Borchers, A. PTK7 Faces the Wnt in Development and Disease. Front. Cell Dev. Biol. 2017, 5, 31. [Google Scholar] [CrossRef]
- Chen, R.; Khatri, P.; Mazur, P.K.; Polin, M.; Zheng, Y.; Vaka, D.; Hoang, C.D.; Shrager, J.; Xu, Y.; Vicent, S.; et al. A meta-analysis of lung cancer gene expression identifies PTK7 as a survival gene in lung adenocarcinoma. Cancer Res. 2014, 74, 2892–2902. [Google Scholar] [CrossRef]
- Bie, J.; Hu, X.; Yang, M.; Shi, X.; Zhang, X.; Wang, Z. PTK7 promotes the malignant properties of cancer stem-like cells in esophageal squamous cell lines. Hum. Cell 2020, 33, 356–365. [Google Scholar] [CrossRef]
- Sun, J.J.; Li, H.L.; Guo, S.J.; Ma, H.; Liu, S.J.; Liu, D.; Xue, F.X. The Increased PTK7 Expression Is a Malignant Factor in Cervical Cancer. Dis. Markers 2019, 2019, 5380197. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Tang, J.; Kong, F.L.; Zou, H.W.; Ni, B.L.; Yu, J.C. Identification of PTK7 as a promising therapeutic target for thyroid cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6809–6817. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, L.H.; Wang, X.H.; Xing, X.F.; Cheng, X.J.; Dong, B.; Hu, Y.; Du, H.; Li, Y.A.; Zhu, Y.B.; et al. PTK7 as a novel marker for favorable gastric cancer patient survival. J. Surg. Oncol. 2012, 106, 880–886. [Google Scholar] [CrossRef]
- Lhoumeau, A.C.; Martinez, S.; Boher, J.M.; Monges, G.; Castellano, R.; Goubard, A.; Doremus, M.; Poizat, F.; Lelong, B.; de Chaisemartin, C.; et al. Overexpression of the Promigratory and Prometastatic PTK7 Receptor Is Associated with an Adverse Clinical Outcome in Colorectal Cancer. PLoS ONE 2015, 10, e0123768. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Angerer, R.; Kates, R.; Gunesch, A.; Knyazev, P.; Högel, B.; Becker, C.; Eiermann, W.; Harbeck, N. PTK7 expression in triple-negative breast cancer. Anticancer Res. 2013, 33, 3759–3763. [Google Scholar]
- Shin, W.S.; Kwon, J.; Lee, H.W.; Kang, M.C.; Na, H.W.; Lee, S.-T.; Park, J.H. Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1120–1126. [Google Scholar] [CrossRef]
- Tian, X.; Yan, L.; Zhang, D.; Guan, X.; Dong, B.; Zhao, M.; Hao, C. PTK7 overexpression in colorectal tumors: Clinicopathological correlation and prognosis relevance. Oncol. Rep. 2016, 36, 1829–1836. [Google Scholar] [CrossRef]
- Jin, J.; Ryu, H.S.; Lee, K.B.; Jang, J.J. High expression of protein tyrosine kinase 7 significantly associates with invasiveness and poor prognosis in intrahepatic cholangiocarcinoma. PLoS ONE 2014, 9, e90247. [Google Scholar] [CrossRef]
- Shin, W.S.; Lee, H.W.; Lee, S.-T. Catalytically inactive receptor tyrosine kinase PTK7 activates FGFR1 independent of FGF. FASEB J. 2019, 33, 12960–12971. [Google Scholar] [CrossRef]
- Shin, W.S.; Maeng, Y.S.; Jung, J.W.; Min, J.K.; Kwon, Y.G.; Lee, S.-T. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem. Biophys. Res. Commun. 2008, 371, 793–798. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serratì, S.; Margheri, F.; Fibbi, G.; Di Cara, G.; Minafra, L.; Pucci-Minafra, I.; Liotta, F.; Annunziato, F.; Pucci, M.; Del Rosso, M. Endothelial cells and normal breast epithelial cells enhance invasion of breast carcinoma cells by CXCR-4-dependent up-regulation of urokinase-type plasminogen activator receptor (uPAR, CD87) expression. J. Pathol. 2008, 214, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Na, H.W.; Lee, S.-T. Biphasic effect of PTK7 on KDR activity in endothelial cells and angiogenesis. Biochim. Biophys. Acta 2015, 1853, 2251–2260. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Lee, H.K.; Lee, H.S.; Park, E.Y.; Jeong, E.; Dana, R. PTK7+ Mononuclear Cells Express VEGFR2 and Contribute to Vascular Stabilization by Upregulating Angiopoietin-1. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Shim, H.J.; Lee, Y.H.; Pyo, M.; Park, J.S.; Ahn, S.Y.; Lee, S.-T. PTK6 Localized at the Plasma Membrane Promotes Cell Proliferation and MigratiOn Through Phosphorylation of Eps8. J. Cell. Biochem. 2017, 118, 2887–2895. [Google Scholar] [CrossRef]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.-T. Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.H.; Cheon, D.H.; Kim, T.; Park, Y.E.; Oh, E.S.; Lee, J.E.; Lee, S.-T. Processing of syndecan-2 by matrix metalloproteinase-14 and effect of its cleavage on VEGF-induced tube formation of HUVECs. Biochem. J. 2017, 474, 3719–3732. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Bellacen, K.; Lewis, E.C. Aortic ring assay. J. Vis. Exp. 2009, e1564. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, R.; Cheng, K.; Li, Q.; Wang, Y.; Zhang, R.; Qin, X. Repulsive Guidance Molecule a Inhibits Angiogenesis by Downregulating VEGF and Phosphorylated Focal Adhesion Kinase In Vitro. Front. Neurol. 2017, 8, 504. [Google Scholar] [CrossRef]
- Sulpice, E.; Ding, S.; Muscatelli-Groux, B.; Bergé, M.; Han, Z.C.; Plouet, J.; Tobelem, G.; Merkulova-Rainon, T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol. Cell 2009, 101, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, H.D.; Song, M.J.; Lee, D.H.; Chung, J.H.; Lee, S.T. SOD3 Suppresses the Expression of MMP-1 and Increases the Integrity of Extracellular Matrix in Fibroblasts. Antioxidants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Park, M.K.; Kim, J.H.; Oh, S.W.; Jang, J.Y.; Lee, H.; Lee, S.-T. PTK7, a Catalytically Inactive Receptor Tyrosine Kinase, Increases Oncogenic Phenotypes in Xenograft Tumors of Esophageal Squamous Cell Carcinoma KYSE-30 Cells. Int. J. Mol. Sci. 2022, 23, 2391. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef]
- Haikala, H.M.; Jänne, P.A. Thirty Years of HER3: From Basic Biology to Therapeutic Interventions. Clin. Cancer Res. 2021, 27, 3528–3539. [Google Scholar] [CrossRef]
- Vafopoulou, P.; Kourti, M. Anti-angiogenic drugs in cancer therapeutics: A review of the latest preclinical and clinical studies of anti-angiogenic agents with anticancer potential. J. Cancer Metastasis Treat. 2022, 8, 18. [Google Scholar] [CrossRef]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef]
- Simons, M.; Alitalo, K.; Annex, B.H.; Augustin, H.G.; Beam, C.; Berk, B.C.; Byzova, T.; Carmeliet, P.; Chilian, W.; Cooke, J.P.; et al. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: A scientific statement from the American heart association. Circ. Res. 2015, 116, e99–e132. [Google Scholar] [CrossRef]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Iqbal, F.; Gratch, Y.S.; Szaraz, P.; Librach, C.L. The Aortic Ring Co-culture Assay: A Convenient Tool to Assess the Angiogenic Potential of Mesenchymal Stromal Cells In Vitro. J. Vis. Exp. 2017, e56083. [Google Scholar] [CrossRef] [PubMed]
- Malinda, K.M. In vivo matrigel migration and angiogenesis assay. Methods Mol. Biol. 2009, 467, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Chauhan, S.K.; Kay, E.; Dana, R. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7–dependent pathway. Blood 2011, 117, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Muñoz-Chápuli, R.; Quesada, A.R.; Angel Medina, M. Angiogenesis and signal transduction in endothelial cells. Cell. Mol. Life Sci. 2004, 61, 2224–2243. [Google Scholar] [CrossRef]
- Shen, K.; Ji, L.; Lu, B.; Wang, Z. c-Jun N-terminal kinase mediated VEGFR2 sustained phosphorylation is critical for VEG-FA-induced angiogenesis in vitro and in vivo. Cell. Biochem. Biophys. 2012, 64, 17–27. [Google Scholar] [CrossRef]
- Vazgiourakis, V.M.; Zervou, M.I.; Eliopoulos, E.; Sharma, S.; Sidiropoulos, P.; Franek, B.S.; Myrthianou, E.; Melissourgaki, M.; Niewold, T.B.; Boumpas, D.T.; et al. Implication of VEGFR2 in systemic lupus erythematosus: A combined genetic and structural biological approach. Clin. Exp. Rheumatol. 2013, 31, 97–102. [Google Scholar]
- Endo, A.; Fukuhara, S.; Masuda, M.; Ohmori, T.; Mochizuki, N. Selective inhibition of vascular endothelial growth factor receptor-2 (VEGFR-2) identifies a central role for VEGFR-2 in human aortic endothelial cell responses to VEGF. J. Recept. Signal Transduct. Res. 2003, 23, 239–254. [Google Scholar] [CrossRef]
- O’Farrell, A.M.; Abrams, T.J.; Yuen, H.A.; Ngai, T.J.; Louie, S.G.; Yee, K.W.; Wong, L.M.; Hong, W.; Lee, L.B.; Town, A.; et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003, 101, 3597–3605. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar] [CrossRef]
- Kupsch, P.; Henning, B.F.; Passarge, K.; Richly, H.; Wiesemann, K.; Hilger, R.A.; Scheulen, M.E.; Christensen, O.; Brendel, E.; Schwartz, B.; et al. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin. Colorectal Cancer 2005, 5, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, M.I.; Sabbaga, J.; Hoff, P.M. Bevacizumab: Overview of the literature. Expert Rev. Anticancer Ther. 2012, 12, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Hida, Y.; Amin, D.N.; Flint, A.F.; Panigrahy, D.; Morton, C.C.; Klagsbrun, M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004, 64, 8249–8255. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Shojaei, F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett. 2012, 320, 130–137. [Google Scholar] [CrossRef]
- Butz, H.; Ding, Q.; Nofech-Mozes, R.; Lichner, Z.; Ni, H.; Yousef, G.M. Elucidating mechanisms of sunitinib resistance in renal cancer: An integrated pathological-molecular analysis. Oncotarget 2018, 9, 4661–4674. [Google Scholar] [CrossRef]
- Selvakumaran, M.; Yao, K.S.; Feldman, M.D.; O’Dwyer, P.J. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem. Pharmacol. 2008, 75, 627–638. [Google Scholar] [CrossRef]
- Mésange, P.; Poindessous, V.; Sabbah, M.; Escargueil, A.E.; de Gramont, A.; Larsen, A.K. Intrinsic bevacizumab resistance is associated with prolonged activation of autocrine VEGF signaling and hypoxia tolerance in colorectal cancer cells and can be overcome by nintedanib, a small molecule angiokinase inhibitor. Oncotarget 2014, 5, 4709–4721. [Google Scholar] [CrossRef]
- Sun, H.J.; Cai, W.W.; Gong, L.L.; Wang, X.; Zhu, X.X.; Wan, M.Y.; Wang, P.Y.; Qiu, L.Y. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed. Pharmacother. 2017, 95, 144–152. [Google Scholar] [CrossRef]
- Cui, N.P.; Qiao, S.; Jiang, S.; Hu, J.L.; Wang, T.T.; Liu, W.W.; Qin, Y.; Wang, Y.N.; Zheng, L.S.; Zhang, J.C.; et al. Protein Tyrosine Kinase 7 Regulates EGFR/Akt Signaling Pathway and Correlates with Malignant Progression in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 699889. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, H.P.; Senter, P.D.; Grewal, I.S. Antibody drug-conjugates targeting the tumor vasculature: Current and future developments. MAbs 2009, 1, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Maitland, M.L.; Sachdev, J.C.; Sharma, M.R.; Moreno, V.; Boni, V.; Kummar, S.; Stringer-Reasor, E.; Lakhani, N.; Moreau, A.R.; Xuan, D.; et al. First-in-Human Study of PF-06647020 (Cofetuzumab Pelidotin), an Antibody-Drug Conjugate Targeting Protein Tyrosine Kinase 7, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Bankovich, A.; Bernstein, J.; Lucas, J.; Chen, L.; Williams, S.; Park, A.; Aguilar, J.; Ernstoff, E.; Charati, M.; et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci. Transl. Med. 2017, 9, eaag2611. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Antibody-drug conjugate targeting protein tyrosine kinase 7, a receptor tyrosine kinase-like molecule involved in WNT and vascular endothelial growth factor signaling: Effects on cancer stem cells, tumor microenvironment and whole-body homeostasis. Ann. Transl. Med. 2017, 5, 462. [Google Scholar] [CrossRef] [Green Version]
- Nejadmoghaddam, M.R.; Minai-Tehrani, A.; Ghahremanzadeh, R.; Mahmoudi, M.; Dinarvand, R.; Zarnani, A.H. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J. Med. Biotechnol. 2019, 11, 3–23. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.W.; Shin, W.-S.; Lee, S.-T. Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function. Cancers 2022, 14, 4463. https://doi.org/10.3390/cancers14184463
Oh SW, Shin W-S, Lee S-T. Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function. Cancers. 2022; 14(18):4463. https://doi.org/10.3390/cancers14184463
Chicago/Turabian StyleOh, Si Won, Won-Sik Shin, and Seung-Taek Lee. 2022. "Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function" Cancers 14, no. 18: 4463. https://doi.org/10.3390/cancers14184463
APA StyleOh, S. W., Shin, W. -S., & Lee, S. -T. (2022). Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function. Cancers, 14(18), 4463. https://doi.org/10.3390/cancers14184463





