Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article
Abstract
Simple Summary
Abstract
1. Introduction
2. Principles of DWI and Image Acquisition Technique
3. Clinical Applications in Gynecological Non-Malignant Conditions
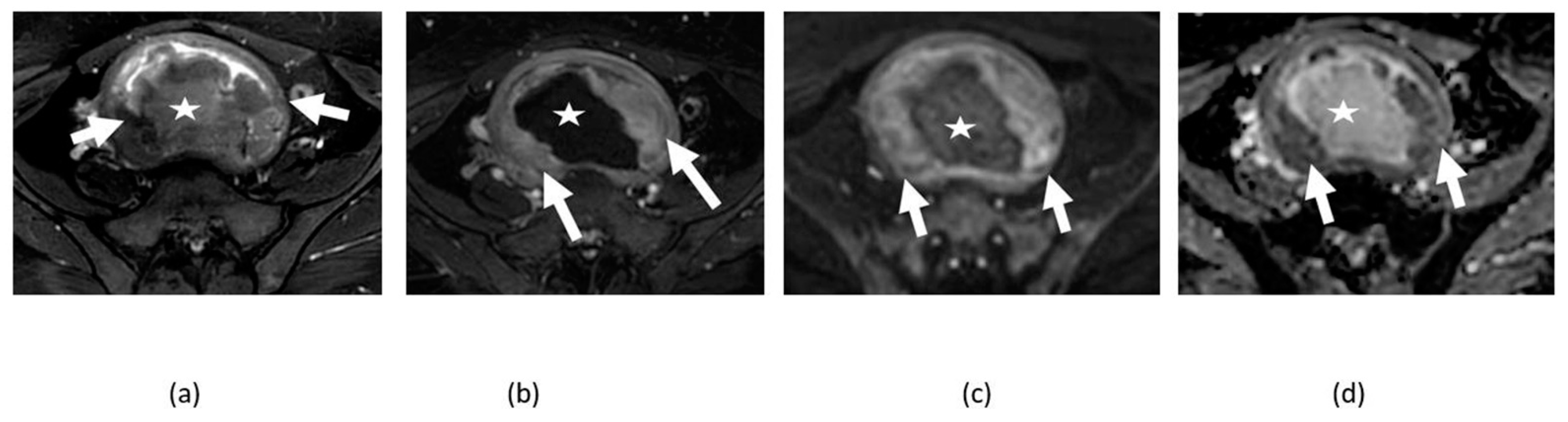
3.1. Uterine Fibroids
3.2. Adnexal Torsion
3.3. Endometriosis
3.4. Tubo-Ovarian Abscess
3.5. Mature Cystic Teratoma
3.6. Ovarian Fibroma, Fibrothecoma, and Thecoma
4. Clinical Applications in Gynecological Malignancies
4.1. Cervical Cancer
4.2. Endometrial Cancer
4.3. Ovarian Cancer
5. Pitfalls of DWI
5.1. T2 Shine-Through Effect
5.2. T2 Blackout Effect
5.3. Diagnostic Pitfalls
6. DWIBS and DTI
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coutinho, A.C., Jr.; Krishnaraj, A.; Pires, C.E.; Bittencourt, L.K.; Guimarães, A.R. Pelvic applications of diffusion magnetic resonance images. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A. Diffusion-weighted imaging in the abdomen and pelvis: Concepts and applications. Radiographics 2009, 29, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Morani, A.C.; Elsayes, K.M.; Liu, P.S.; Weadock, W.J.; Szklaruk, J.; Dillman, J.R.; Khan, A.; Chenevert, T.L.; Hussain, H.K. Abdominal applications of diffusion-weighted magnetic resonance imaging: Where do we stand. World J. Radiol. 2013, 5, 68–80. [Google Scholar] [CrossRef]
- Moore, W.A.; Khatri, G.; Madhuranthakam, A.J.; Sims, R.D.; Pedrosa, I. Added value of diffusion-weighted acquisitions in MRI of the abdomen and pelvis. AJR Am. J. Roentgenol. 2014, 202, 995–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujii, S.; Matsusue, E.; Kanasaki, Y.; Kanamori, Y.; Nakanishi, J.; Sugihara, S.; Kigawa, J.; Terakawa, N.; Ogawa, T. Detection of peritoneal dissemination in gynecological malignancy: Evaluation by diffusion-weighted MR imaging. Eur. Radiol. 2008, 18, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.S.; Coady, A.; Culver, L.; Rustin, G.; Padwick, M.; Padhani, A.R. Diffusion-weighted MR imaging of female pelvic tumors: A pictorial review. Radiographics 2009, 29, 759–774. [Google Scholar] [CrossRef]
- Manoharan, D.; Das, C.J.; Aggarwal, A.; Gupta, A.K. Diffusion weighted imaging in gynecological malignancies—Present and future. World J. Radiol. 2016, 8, 288–297. [Google Scholar] [CrossRef]
- Nougaret, S.; Tirumani, S.H.; Addley, H.; Pandey, H.; Sala, E.; Reinhold, C. Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR Am. J. Roentgenol. 2013, 200, 261–276. [Google Scholar] [CrossRef]
- Kubik-Huch, R.A.; Weston, M.; Nougaret, S.; Leonhardt, H.; Thomassin-Naggara, I.; Horta, M.; Cunha, T.M.; Maciel, C.; Rockall, A.; Forstner, R. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Leiomyomas. Eur. Radiol. 2018, 28, 3125–3137. [Google Scholar] [CrossRef]
- Gaetke-Udager, K.; McLean, K.; Sciallis, A.P.; Alves, T.; Maturen, K.E.; Mervak, B.M.; Moore, A.G.; Wasnik, A.P.; Erba, J.; Davenport, M.S. Diagnostic Accuracy of Ultrasound, Contrast-enhanced CT, and Conventional MRI for Differentiating Leiomyoma From Leiomyosarcoma. Acad. Radiol. 2016, 23, 1290–1297. [Google Scholar] [CrossRef]
- Lin, G.; Yang, L.Y.; Huang, Y.T.; Ng, K.K.; Ng, S.H.; Ueng, S.H.; Chao, A.; Yen, T.C.; Chang, T.C.; Lai, C.H. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J. Magn. Reson. Imaging 2016, 43, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Aoki, M.; Miyagawa, C.; Murakami, K.; Takaya, H.; Kotani, Y.; Nakai, H.; Matsumura, N. Differential Diagnosis of Uterine Leiomyoma and Uterine Sarcoma using Magnetic Resonance Images: A Literature Review. Healthcare 2019, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, R.C.; Huang, Y.L.; Chen, K.; Tseng, S.C.; Wang, C.J.; Chao, A.; Lai, C.H.; Lin, G. Uterine fibroid-like tumors: Spectrum of MR imaging findings and their differential diagnosis. Abdom. Radiol. 2022, 47, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Abdel Wahab, C.; Jannot, A.-S.; Bonaffini, P.A.; Bourillon, C.; Cornou, C.; Lefrère-Belda, M.-A.; Bats, A.-S.; Thomassin-Naggara, I.; Bellucci, A.; Reinhold, C.; et al. Diagnostic Algorithm to Differentiate Benign Atypical Leiomyomas from Malignant Uterine Sarcomas with Diffusion-weighted MRI. Radiology 2020, 297, 361–371. [Google Scholar] [CrossRef]
- Agostinho, L.; Horta, M.; Salvador, J.C.; Cunha, T.M. Benign ovarian lesions with restricted diffusion. Radiol. Bras. 2019, 52, 106–111. [Google Scholar] [CrossRef]
- Lall, C.; Bura, V.; Lee, T.K.; Bhosale, P.; Faria, S.C.; Choi, J.I.; Wang, Z.J. Diffusion-weighted imaging in hemorrhagic abdominal and pelvic lesions: Restricted diffusion can mimic malignancy. Abdom. Radiol. 2018, 43, 1772–1784. [Google Scholar] [CrossRef]
- Balaban, M.; Idilman, I.S.; Toprak, H.; Unal, O.; Ipek, A.; Kocakoc, E. The utility of diffusion-weighted magnetic resonance imaging in differentiation of endometriomas from hemorrhagic ovarian cysts. Clin. Imaging 2015, 39, 830–833. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, S.; Kim, K.H.; Suh, D.S.; Kim, T.U.; Han, G.J.; Lee, J.W.; Kim, J.Y. Diffusion-weighted magnetic resonance imaging in the differentiation of endometriomas from hemorrhagic cysts in the ovary. Acta Radiol. 2016, 57, 998–1005. [Google Scholar] [CrossRef]
- Duarte, A.L.; Dias, J.L.; Cunha, T.M. Pitfalls of diffusion-weighted imaging of the female pelvis. Radiol. Bras. 2018, 51, 37–44. [Google Scholar] [CrossRef]
- Sakala, M.D.; Shampain, K.L.; Wasnik, A.P. Advances in MR Imaging of the Female Pelvis. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 415–431. [Google Scholar] [CrossRef]
- Chung, B.M.; Park, S.B.; Lee, J.B.; Park, H.J.; Kim, Y.S.; Oh, Y.J. Magnetic resonance imaging features of ovarian fibroma, fibrothecoma, and thecoma. Abdom. Imaging 2015, 40, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, G.-F.; Wang, T.-P.; Zhang, H. Value of 3.0 T diffusion-weighted imaging in discriminating thecoma and fibrothecoma from other adnexal solid masses. J. Ovarian Res. 2013, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Namimoto, T.; Awai, K.; Nakaura, T.; Yanaga, Y.; Hirai, T.; Yamashita, Y. Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur. Radiol. 2009, 19, 745–760. [Google Scholar] [CrossRef]
- Ali, R.; Nassef, H.; Ibrahim, A.; Chalabi, N.; Mohamed, A. The Role of Diffusion Weighted Imaging in suspected cases of ovarian cancer. Egypt. J. Radiol. Nucl. Med. 2020, 51, 97. [Google Scholar] [CrossRef]
- Punwani, S. Diffusion weighted imaging of female pelvic cancers: Concepts and clinical applications. Eur. J. Radiol. 2011, 78, 21–29. [Google Scholar] [CrossRef]
- Addley, H.; Moyle, P.; Freeman, S. Diffusion-weighted imaging in gynaecological malignancy. Clin. Radiol. 2017, 72, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Haldorsen, I.S.; Lura, N.; Blaakær, J.; Fischerova, D.; Werner, H.M.J. What Is the Role of Imaging at Primary Diagnostic Work-Up in Uterine Cervical Cancer? Curr. Oncol. Rep. 2019, 21, 77. [Google Scholar] [CrossRef]
- Dappa, E.; Elger, T.; Hasenburg, A.; Düber, C.; Battista, M.J.; Hötker, A.M. The value of advanced MRI techniques in the assessment of cervical cancer: A review. Insights Imaging 2017, 8, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Dashottar, S.; Preeth Pany, T.; Lohia, N. Role of apparent diffusion coefficient as a biomarker in the evaluation of cervical cancer. Indian J. Radiol. Imaging 2019, 29, 25–32. [Google Scholar] [CrossRef]
- Meyer, H.-J.; Wienke, A.; Surov, A. Pre-treatment Apparent Diffusion Coefficient Does Not Predict Therapy Response to Radiochemotherapy in Cervical Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2021, 41, 1163. [Google Scholar] [CrossRef]
- Nakamura, K.; Joja, I.; Nagasaka, T.; Fukushima, C.; Kusumoto, T.; Seki, N.; Hongo, A.; Kodama, J.; Hiramatsu, Y. The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol. Oncol. 2012, 127, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Mahawar, V.; Chaturvedi, A.K.; Rao, A.; Singla, R.; Mitra, S.; Goyal, S.; Kesan, S.; Pasricha, S.; Maheshwari, U.; et al. Role of ADC values in assessing clinical response and identifying residual disease post-chemo radiation in uterine cervix cancer. Indian J. Radiol. Imaging 2019, 29, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Oshima, N.; Miyasaka, N.; Wakana, K.; Wakabayashi, A.; Sakamoto, J.; Saida, Y.; Tateishi, U.; Kobayashi, D. Texture Analysis of Apparent Diffusion Coefficient Maps in Cervical Carcinoma: Correlation with Histopathologic Findings and Prognosis. Radiol. Imaging Cancer 2020, 2, e190085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, K.; Li, X.-T.; Zhu, H.-T.; Sun, Y.-S. Whole lesion histogram analysis of apparent diffusion coefficients on MRI predicts disease-free survival in locally advanced squamous cell cervical cancer after radical chemo-radiotherapy. BMC Cancer 2019, 19, 1115. [Google Scholar] [CrossRef]
- Dolciami, M.; Capuani, S.; Celli, V.; Maiuro, A.; Pernazza, A.; Palaia, I.; Di Donato, V.; Santangelo, G.; Rizzo, S.M.R.; Ricci, P.; et al. Intravoxel Incoherent Motion (IVIM) MR Quantification in Locally Advanced Cervical Cancer (LACC): Preliminary Study on Assessment of Tumor Aggressiveness and Response to Neoadjuvant Chemotherapy. J. Pers. Med. 2022, 12, 638. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Feng, M.; Ren, J.; Mi, K.; Cheng, J.; Song, B.; Lang, J. Early changes in the apparent diffusion coefficient and MMP-9 expression of a cervical carcinoma U14 allograft model following irradiation. Oncol. Lett. 2017, 14, 6769–6775. [Google Scholar] [CrossRef]
- Wormald, B.W.; Doran, S.J.; Ind, T.E.J.; D’Arcy, J.; Petts, J.; deSouza, N.M. Radiomic features of cervical cancer on T2-and diffusion-weighted MRI: Prognostic value in low-volume tumors suitable for trachelectomy. Gynecol. Oncol. 2020, 156, 107–114. [Google Scholar] [CrossRef]
- Neves, T.R.; Correia, M.T.; Serrado, M.A.; Horta, M.; Caetano, A.P.; Cunha, T.M. Staging of Endometrial Cancer Using Fusion T2-Weighted Images with Diffusion-Weighted Images: A Way to Avoid Gadolinium? Cancers 2022, 14, 384. [Google Scholar] [CrossRef]
- Lin, M.Y.; Dobrotwir, A.; McNally, O.; Abu-Rustum, N.R.; Narayan, K. Role of imaging in the routine management of endometrial cancer. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 109–117. [Google Scholar] [CrossRef]
- Lee, M.S.; Moon, M.H.; Kim, S.Y.; Jang, S.; Oh, S.; Cho, J.Y. Preoperative risk stratification in women with endometrial cancer: A comparison of contrast-enhanced MR imaging and diffusion-weighted MR imaging. Eur. J. Radiol. 2022, 150, 10276. [Google Scholar] [CrossRef]
- Xie, M.; Ren, Z.; Bian, D.; Li, D.; Yu, L.; Zhu, F.; Huang, R.; Zhang, Z.; Suye, S.; Fu, C. High resolution diffusion-weighted imaging with readout segmentation of long variable echo-trains for determining myometrial invasion in endometrial carcinoma. Cancer Imaging 2020, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.T.; Cunha, T.M.; Horta, M.; Alves, I. The added value of diffusion-weighted imaging in the preoperative assessment of endometrial cancer. Radiol. Bras. 2019, 52, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.H.; Chiou, Y.Y.; Wang, J.H.; Yen, M.S.; Lee, R.C.; Lai, C.R.; Chang, C.Y. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR Am. J. Roentgenol. 2008, 190, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Sanli, S.; Bakir, V.L.; Ayas, S.; Yildiz, S.O.; Iyibozkurt, A.C.; Kartal, M.G.; Yavuz, E. Role of diffusion weighted MRI in the differential diagnosis of endometrial cancer, polyp, hyperplasia, and physiological thickening. Clin. Imaging 2017, 41, 86–94. [Google Scholar] [CrossRef]
- Lefebvre, T.L.; Ueno, Y.; Dohan, A.; Chatterjee, A.; Vallières, M.; Winter-Reinhold, E.; Saif, S.; Levesque, I.R.; Zeng, X.Z.; Forghani, R.; et al. Development and Validation of Multiparametric MRI-based Radiomics Models for Preoperative Risk Stratification of Endometrial Cancer. Radiology 2022, 212873. [Google Scholar] [CrossRef]
- Bereby-Kahane, M.; Dautry, R.; Matzner-Lober, E.; Cornelis, F.; Sebbag-Sfez, D.; Place, V.; Mezzadri, M.; Soyer, P.; Dohan, A. Prediction of tumor grade and lymphovascular space invasion in endometrial adenocarcinoma with MR imaging-based radiomic analysis. Diagn. Interv. Imaging 2020, 101, 401–411. [Google Scholar] [CrossRef]
- Satta, S.; Dolciami, M.; Celli, V.; Di Stadio, F.; Perniola, G.; Palaia, I.; Pernazza, A.; Della Rocca, C.; Rizzo, S.; Catalano, C.; et al. Quantitative diffusion and perfusion MRI in the evaluation of endometrial cancer: Validation with histopathological parameters. Br. J. Radiol. 2021, 94, 20210054. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Du, D.; Zheng, T.; Liu, L.; Wang, Z.; Du, J.; Dong, Y.; Yi, H.; Cui, Y. Multi-Parameter MR Radiomics Based Model to Predict 5-Year Progression-Free Survival in Endometrial Cancer. Front. Oncol. 2022, 12, 813069. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Daraï, E.; Cuenod, C.A.; Fournier, L.; Toussaint, I.; Marsault, C.; Bazot, M. Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur. Radiol. 2009, 19, 1544–1552. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Thomassin-Naggara, I.; Rockall, A.; Maturen, K.E.; Forstner, R.; Jha, P.; Nougaret, S.; Siegelman, E.S.; Reinhold, C. O-RADS MRI Risk Stratification System: Guide for Assessing Adnexal Lesions from the ACR O-RADS Committee. Radiology 2022, 303, 35–47. [Google Scholar] [CrossRef]
- Türkoğlu, S.; Kayan, M. Differentiation between benign and malignant ovarian masses using multiparametric MRI. Diagn. Interv. Imaging 2020, 101, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Derlatka, P.; Grabowska-Derlatka, L.; Halaburda-Rola, M.; Szeszkowski, W.; Czajkowski, K. The Value of Magnetic Resonance Diffusion-Weighted Imaging and Dynamic Contrast Enhancement in the Diagnosis and Prognosis of Treatment Response in Patients with Epithelial Serous Ovarian Cancer. Cancers 2022, 14, 2464. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Song, Y.; Li, H.; Lu, J.; Li, Y.; Duan, S.; Qiang, J. Histogram Analysis Comparison of Monoexponential, Advanced Diffusion-Weighted Imaging, and Dynamic Contrast-Enhanced MRI for Differentiating Borderline From Malignant Epithelial Ovarian Tumors. J. Magn. Reson. Imaging 2020, 52, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Gauquelin, L.; Nougaret, S.; Chicart, M.; Pulido, M.; Floquet, A.; Guyon, F.; Croce, S.; Kind, M.; Cazeau, A.L. Diffusion-weighted MRI and PET/CT reproducibility in epithelial ovarian cancers during neoadjuvant chemotherapy. Diagn. Interv. Imaging 2021, 102, 629–639. [Google Scholar] [CrossRef]
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F.; et al. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, P.; Sun, Z.; Ma, X.; Wang, H.; Wu, W.; Wen, Z.; Kisrieva-Ware, Z.; Woodard, P.K.; Wang, Q.; et al. Magnetic resonance diffusion tensor imaging of cervical microstructure in normal early and late pregnancy in vivo. Am. J. Obstet. Gynecol. 2021, 224, 101.e101–101.e111. [Google Scholar] [CrossRef]
- Tian, S.; Niu, M.; Xie, L.; Song, Q.; Liu, A. Diffusion-tensor imaging for differentiating uterine sarcoma from degenerative uterine fibroids. Clin. Radiol. 2021, 76, 313.e327–313.e332. [Google Scholar] [CrossRef]
- Drake-Pérez, M.; Boto, J.; Fitsiori, A.; Lovblad, K.; Vargas, M.I. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018, 9, 535–547. [Google Scholar] [CrossRef]
- van der Jagt, P.K.; Dik, P.; Froeling, M.; Kwee, T.C.; Nievelstein, R.A.; ten Haken, B.; Leemans, A. Architectural configuration and microstructural properties of the sacral plexus: A diffusion tensor MRI and fiber tractography study. Neuroimage 2012, 62, 1792–1799. [Google Scholar] [CrossRef]
- Manganaro, L.; Porpora, M.G.; Vinci, V.; Bernardo, S.; Lodise, P.; Sollazzo, P.; Sergi, M.E.; Saldari, M.; Pace, G.; Vittori, G.; et al. Diffusion tensor imaging and tractography to evaluate sacral nerve root abnormalities in endometriosis-related pain: A pilot study. Eur. Radiol. 2014, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Vinci, V.; De Vito, C.; Migliara, G.; Anastasi, E.; Ticino, A.; Resta, S.; Catalano, C.; Benedetti Panici, P.; Manganaro, L. The Role of Magnetic Resonance Imaging-Diffusion Tensor Imaging in Predicting Pain Related to Endometriosis: A Preliminary Study. J. Minim. Invasive Gynecol. 2018, 25, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Oshima, N.; Wakabayashi, A.; Miyasaka, N.; Wakana, K.; Saida, Y.; Tateishi, U.; Kobayashi, D. Diffusion-Tensor Imaging of Uterine Cervical Carcinoma: Correlation With Histopathologic Findings. J. Comput. Assist. Tomogr. 2020, 44, 426–435. [Google Scholar] [CrossRef]
- Di Paola, V.; Perillo, F.; Gui, B.; Russo, L.; Pierconti, F.; Fiorentino, V.; Autorino, R.; Ferrandina, G.; Valentini, V.; Scambia, G.; et al. Detection of parametrial invasion in women with uterine cervical cancer using diffusion tensor imaging at 1.5T MRI. Diagn. Interv. Imaging, 2022; in press. [Google Scholar] [CrossRef] [PubMed]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonde, A.; Andreazza Dal Lago, E.; Foster, B.; Javadi, S.; Palmquist, S.; Bhosale, P. Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article. Cancers 2022, 14, 4468. https://doi.org/10.3390/cancers14184468
Bonde A, Andreazza Dal Lago E, Foster B, Javadi S, Palmquist S, Bhosale P. Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article. Cancers. 2022; 14(18):4468. https://doi.org/10.3390/cancers14184468
Chicago/Turabian StyleBonde, Apurva, Eduardo Andreazza Dal Lago, Bryan Foster, Sanaz Javadi, Sarah Palmquist, and Priya Bhosale. 2022. "Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article" Cancers 14, no. 18: 4468. https://doi.org/10.3390/cancers14184468
APA StyleBonde, A., Andreazza Dal Lago, E., Foster, B., Javadi, S., Palmquist, S., & Bhosale, P. (2022). Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article. Cancers, 14(18), 4468. https://doi.org/10.3390/cancers14184468




