Single Cell RNA Sequencing: A New Frontier in Pancreatic Ductal Adenocarcinoma
Abstract
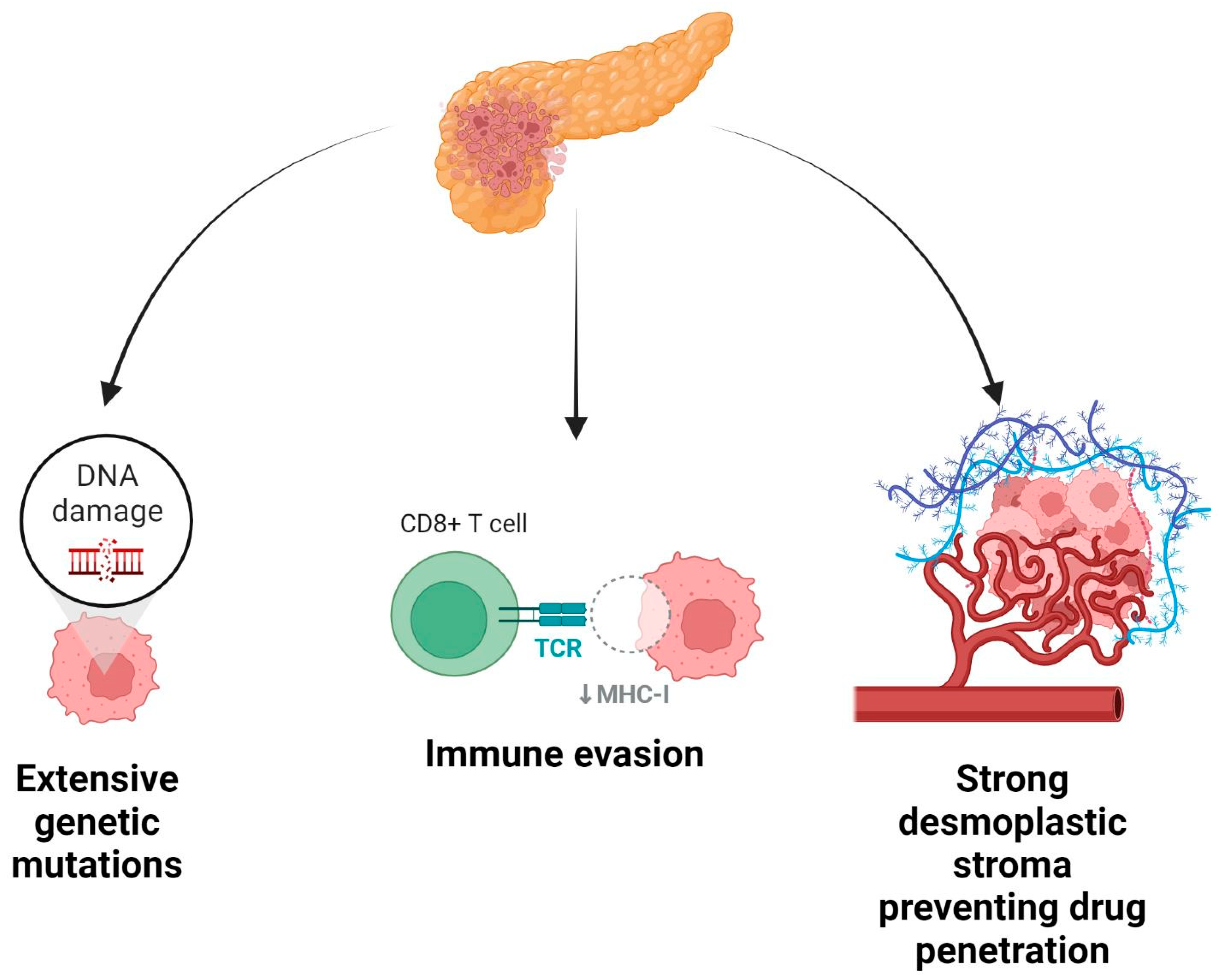
Simple Summary
Abstract
1. Introduction
2. Discussion
2.1. Genomic Approaches to Pancreatic Cancer Profiling
2.2. Transcriptomic Approaches to Pancreatic Cancer Profiling
2.3. scRNA-seq Provides New Insight into Pancreatic Cancer
2.4. Tumor Heterogeneity Analysis Using scRNA-seq
2.5. Predictive Modeling Using scRNA-seq
2.6. Stromal Biology and the Role of Cancer-Associated Fibroblasts
2.7. Current Classification of Pancreatic Cancer
2.8. Status of PDAC Chemotherapy
2.9. Mechanisms of Drug Resistance in Pancreatic Cancer
2.10. Utility of Molecular Profiling in Predicting Treatment Outcomes
2.11. Pharmaco-Typing Patient-Derived Organoids to Develop Treatment Modalities
2.12. Limitations of Molecular Profiling Techniques
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Races, A. SEER Cancer Statistics Review 1975–2014. 2022. Available online: https://seer.cancer.gov/archive/csr/1975_2011/results_single/sect_28_table.03.pdf (accessed on 29 May 2022).
- Siegal, R.; Miller, K.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory: Cancer today. Lyon Fr. Int. Agency Res. Cancer 2018, 3, 2019. [Google Scholar]
- Fesinmeyer, M.D.; Austin, M.A.; Li, C.I.; De Roos, A.J.; Bowen, D.J. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol. Prev. Biomark. 2005, 14, 1766–1773. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Löhr, J.-M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.-L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef]
- De La Cruz, M.S.D.; Young, A.P.; Ruffin, M.T. Diagnosis and management of pancreatic cancer. Am. Fam. Physician 2014, 89, 626–632. [Google Scholar]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332. [Google Scholar]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Pompella, L.; Tirino, G.; Pappalardo, A.; Caterino, M.; Ventriglia, A.; Nacca, V.; Orditura, M.; Ciardiello, F.; De Vita, F. Pancreatic cancer molecular classifications: From bulk genomics to single cell analysis. Int. J. Mol. Sci. 2020, 21, 2814. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Wu, Z.; Krautz, C.; Pilarsky, C.; Grützmann, R.; Weber, G.F. Current clinical strategies of pancreatic cancer treatment and open molecular questions. Int. J. Mol. Sci. 2019, 20, 4543. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Ciccarelli, F.D. The (r) evolution of cancer genetics. BMC Biol. 2010, 8, 74. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Moffitt, R.A.; Yeh, J.J.; Stewart, C.; Robertson, A.G.; Cherniack, A.D.; Gupta, M.; Getz, G. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lazarus, J.; Steele, N.G.; Yan, W.; Lee, H.-J.; Nwosu, Z.C.; Halbrook, C.J.; Menjivar, R.E.; Kemp, S.B.; Sirihorachai, V.R. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 2020, 10, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.A.; Simpson, F.; Thompson, E.W.; Hill, M.M.; Endo-Munoz, L.; Leggatt, G.; Minchin, R.F.; Guminski, A. Role of intratumoural heterogeneity in cancer drug resistance: Molecular and clinical perspectives. EMBO Mol. Med. 2012, 4, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Q.; Li, M.; Guo, H.; Liu, W.; Wang, F.; Tian, X.; Yang, Y. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine 2021, 66, 103315. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.-C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.-M.; Wu, J. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Makohon-Moore, A.P.; Zhang, M.; Reiter, J.G.; Bozic, I.; Allen, B.; Kundu, D.; Chatterjee, K.; Wong, F.; Jiao, Y.; Kohutek, Z.A. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 2017, 49, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.-C.; Mansour, J.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef]
- Moir, J.A.; Mann, J.; White, S.A. The role of pancreatic stellate cells in pancreatic cancer. Surg. Oncol. 2015, 24, 232–238. [Google Scholar] [CrossRef]
- Wood, L.D.; Hruban, R.H. Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 2012, 18, 492. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, M.; Zhang, M.; De Wilde, R.F.; Ottenhof, N.A.; Rezaee, N.; Wolfgang, C.L.; Blackford, A.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N. Very long-term survival following resection for pancreatic cancer is not explained by commonly mutated genes: Results of whole-exome sequencing analysis. Clin. Cancer Res. 2015, 21, 1944–1950. [Google Scholar] [CrossRef]
- Pierce, S.E.; Kim, S.H.; Greenleaf, W.J. Finding needles in a haystack: Dissecting tumor heterogeneity with single-cell transcriptomic and chromatin accessibility profiling. Curr. Opin. Genet. Dev. 2021, 66, 36–40. [Google Scholar] [CrossRef]
- Shapiro, E.; Biezuner, T.; Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013, 14, 618–630. [Google Scholar] [CrossRef]
- Inda, M.C.; Joshi, S.; Wang, T.; Bolaender, A.; Gandu, S.; Koren III, J.; Che, A.Y.; Taldone, T.; Yan, P.; Sun, W. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat. Commun. 2020, 11, 319. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Stegle, O.; Teichmann, S.A.; Marioni, J.C. Computational and analytical challenges in single-cell transcriptomics. Nat. Rev. Genet. 2015, 16, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ning, B.; Shi, T. Single-cell RNA-seq technologies and related computational data analysis. Front. Genet. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Sun, B.-F.; Chen, C.-Y.; Zhou, J.-Y.; Chen, Y.-S.; Chen, H.; Liu, L.; Huang, D.; Jiang, J.; Cui, G.-S. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019, 29, 725–738. [Google Scholar] [CrossRef]
- Shirota, M.; Kinoshita, K. Discrepancies between human DNA, mRNA and protein reference sequences and their relation to single nucleotide variants in the human population. Database 2016, 2016, baw124. [Google Scholar] [CrossRef]
- Suvà, M.L.; Tirosh, I. Single-cell RNA sequencing in cancer: Lessons learned and emerging challenges. Mol. Cell 2019, 75, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tahkola, K.; Mecklin, J.-P.; Wirta, E.-V.; Ahtiainen, M.; Helminen, O.; Böhm, J.; Kellokumpu, I. High immune cell score predicts improved survival in pancreatic cancer. Virchows Arch. 2018, 472, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.; Froeling, F.E.; Burkhart, R.A.; Denroche, R.E.; Jang, G.-H. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef]
- Mehlen, P.; Delloye-Bourgeois, C.; Chédotal, A. Novel roles for Slits and netrins: Axon guidance cues as anticancer targets? Nat. Rev. Cancer 2011, 11, 188–197. [Google Scholar] [CrossRef]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.H.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H. Virtual microdissection identifies distinct tumor-and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Puleo, F.; Nicolle, R.; Blum, Y.; Cros, J.; Marisa, L.; Demetter, P.; Quertinmont, E.; Svrcek, M.; Elarouci, N.; Iovanna, J. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 2018, 155, 1999–2013.e3. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.; Nones, K.; Johns, A.; Patch, A.; Gingras, M.; Miller, D.; Christ, A.; Bruxner, T.; Quinn, M. Australian Pancreatic Cancer Genome I. Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar]
- Bernard, V.; Semaan, A.; Huang, J.; San Lucas, F.A.; Mulu, F.C.; Stephens, B.M.; Guerrero, P.A.; Huang, Y.; Zhao, J.; Kamyabi, N. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin. Cancer Res. 2019, 25, 2194–2205. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Hu, Y.; Huang, W. Single-cell RNA transcriptome reveals the intra-tumoral heterogeneity and regulators underlying tumor progression in metastatic pancreatic ductal adenocarcinoma. Cell Death Discov. 2021, 7, 331. [Google Scholar] [CrossRef]
- Lee, J.J.; Bernard, V.; Semaan, A.; Monberg, M.E.; Huang, J.; Stephens, B.M.; Lin, D.; Rajapakshe, K.I.; Weston, B.R.; Bhutani, M.S. Elucidation of Tumor-Stromal Heterogeneity and the Ligand-Receptor Interactome by Single-Cell Transcriptomics in Real-world Pancreatic Cancer BiopsiesscRNA-seq of Pancreatic Cancer Real-world Biopsies. Clin. Cancer Res. 2021, 27, 5912–5921. [Google Scholar] [CrossRef]
- Gopalan, V.; Singh, A.; Rashidi Mehrabadi, F.; Wang, L.; Ruppin, E.; Arda, H.E.; Hannenhalli, S. A Transcriptionally Distinct Subpopulation of Healthy Acinar Cells Exhibit Features of Pancreatic Progenitors and PDACAcinar Cells Show a Nongenetic Drift toward Malignancy. Cancer Res. 2021, 81, 3958–3970. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-D.; Wang, Y.; Xie, P.; Tan, S.-Y.; Li, H.-F.; Shen, H.; Zhang, Z.; Lei, Z.-Q.; Zhou, J.-H. The Crosstalk between Immune Infiltration, Circulating Tumor Cells, and Metastasis in Pancreatic Cancer: Identification of HMGB3 From a Multiple Omics Analysis. Front. Genet. 2022, 13, 892177. [Google Scholar] [CrossRef]
- Chen, K.; Liu, X.; Liu, W.; Wang, F.; Tian, X.; Yang, Y. Development and validation of prognostic and diagnostic model for pancreatic ductal adenocarcinoma based on scRNA-seq and bulk-seq datasets. Hum. Mol. Genet. 2022, 31, 1705–1719. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, C.; Lu, Y.; Ma, F.; Fan, Y.; Wang, C. Single-cell RNA-seq reveals invasive trajectory and determines cancer stem cell-related prognostic genes in pancreatic cancer. Bioengineered 2021, 12, 5056–5068. [Google Scholar] [CrossRef]
- Fang, Y.; Pei, S.; Huang, K.; Xu, F.; Xiang, G.; Lan, L.; Zheng, X. Single-Cell Transcriptome Reveals the Metabolic and Clinical Features of a Highly Malignant Cell Subpopulation in Pancreatic Ductal Adenocarcinoma. Front. Cell Dev. Biol. 2022, 10, 798165. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Dai, Y.; Tang, X.; Yin, T.; Wang, C.; Wang, T.; Dong, L.; Shi, M.; Qin, J. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut 2022. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Müller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Figueroa, E.F.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef]
- Guo, J.A.; Hoffman, H.I.; Weekes, C.D.; Zheng, L.; Ting, D.T.; Hwang, W.L. Refining the molecular framework for pancreatic cancer with single-cell and spatial technologies. Clin. Cancer Res. 2021, 27, 3825–3833. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, H.; Wang, J.; Liu, X.; Liu, H.; Wu, H.; Liang, Z. Identification and Validation of Immune Molecular Subtypes in Pancreatic Ductal Adenocarcinoma: Implications for Prognosis and Immunotherapy. Front. Immunol. 2021, 12, 690056. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Jamieson, N.B.; Chang, D.K.; Biankin, A.V. Molecular Subtyping of Pancreatic Cancer. In Textbook of Pancreatic Cancer; Springer: Berlin/Heidelberg, Germany, 2021; pp. 305–319. [Google Scholar]
- Mädler, S.C.; Julien-Laferriere, A.; Wyss, L.; Phan, M.; Sonrel, A.; Kang, A.S.; Ulrich, E.; Schmucki, R.; Zhang, J.D.; Ebeling, M. Besca, a single-cell transcriptomics analysis toolkit to accelerate translational research. NAR Genom. Bioinform. 2021, 3, lqab102. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Huang, Y.; Wang, Q.; Kaifi, J.T.; Kimchi, E.T.; Chabu, C.Y.; Liu, Z.; Joshi, T.; Li, G. Single-cell RNA sequencing to characterize the response of pancreatic cancer to anti-PD-1 immunotherapy. Transl. Oncol. 2022, 15, 101262. [Google Scholar] [CrossRef]
- Tempero, M.A. NCCN guidelines updates: Pancreatic cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 603–605. [Google Scholar]
- Okusaka, T.; Furuse, J. Recent advances in chemotherapy for pancreatic cancer: Evidence from Japan and recommendations in guidelines. J. Gastroenterol. 2020, 55, 369–382. [Google Scholar] [CrossRef]
- O’Reilly, D.; Fou, L.; Hasler, E.; Hawkins, J.; O’Connell, S.; Pelone, F.; Callaway, M.; Campbell, F.; Capel, M.; Charnley, R. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.B.; Sorber, R.; Rozich, N.S.; Burkhart, R.A. A qualitative review of neoadjuvant chemotherapy in resectable pancreatic adenocarcinoma. Pancreas 2019, 48, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Saka, D.; Gökalp, M.; Piyade, B.; Cevik, N.C.; Arik Sever, E.; Unutmaz, D.; Ceyhan, G.O.; Demir, I.E.; Asimgil, H. Mechanisms of T-cell exhaustion in pancreatic cancer. Cancers 2020, 12, 2274. [Google Scholar] [CrossRef] [PubMed]
- Sankarasubramanian, S.; Pfohl, U.; Regenbrecht, C.R.; Reinhard, C.; Wedeken, L. Context Matters—Why We Need to Change From a One Size Fits all Approach to Made-to-Measure Therapies for Individual Patients with Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 760705. [Google Scholar] [CrossRef]
- Juiz, N.; Elkaoutari, A.; Bigonnet, M.; Gayet, O.; Roques, J.; Nicolle, R.; Iovanna, J.; Dusetti, N. Basal-like and classical cells coexist in pancreatic cancer revealed by single-cell analysis on biopsy-derived pancreatic cancer organoids from the classical subtype. FASEB J. 2020, 34, 12214–12228. [Google Scholar] [CrossRef]
- Froeling, F.E.; Casolino, R.; Pea, A.; Biankin, A.V.; Chang, D.K. Molecular subtyping and precision medicine for pancreatic cancer. J. Clin. Med. 2021, 10, 149. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, K.; Bailey, P.; Springfeld, C.; Roth, S.; Kurilov, R.; Brors, B.; Gress, T.; Buchholz, M.; An, J. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 743908. [Google Scholar] [CrossRef]
- Torres, C.; Grippo, P.J. Pancreatic cancer subtypes: A roadmap for precision medicine. Ann. Med. 2018, 50, 277–287. [Google Scholar] [CrossRef]
- Moreira, L.; Bakir, B.; Chatterji, P.; Dantes, Z.; Reichert, M.; Rustgi, A.K. Pancreas 3D organoids: Current and future aspects as a research platform for personalized medicine in pancreatic cancer. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 289–298. [Google Scholar] [CrossRef]
- Norberg, K.; Liu, X.; Fernández Moro, C.; Strell, C.; Nania, S.; Blümel, M.; Balboni, A.; Bozóky, B.; Heuchel, R.; Löhr, J. A novel pancreatic tumour and stellate cell 3D co-culture spheroid model. BMC Cancer 2020, 20, 475. [Google Scholar] [CrossRef]
- Tsai, S.; McOlash, L.; Palen, K.; Johnson, B.; Duris, C.; Yang, Q.; Dwinell, M.B.; Hunt, B.; Evans, D.B.; Gershan, J. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 2018, 18, 335. [Google Scholar] [CrossRef]
- Tiriac, H.; Bucobo, J.C.; Tzimas, D.; Grewel, S.; Lacomb, J.F.; Rowehl, L.M.; Nagula, S.; Wu, M.; Kim, J.; Sasson, A. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest. Endosc. 2018, 87, 1474–1480. [Google Scholar] [CrossRef]
- Chen, H.; Zhuo, Q.; Ye, Z.; Xu, X.; Ji, S. Organoid model: A new hope for pancreatic cancer treatment? Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188466. [Google Scholar] [CrossRef]
- Baker, L.A.; Tiriac, H.; Clevers, H.; Tuveson, D.A. Modeling pancreatic cancer with organoids. Trends Cancer 2016, 2, 176–190. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Zimmerman, J.W.; Sereni, E.; Plenker, D.; Suri, R.; Rozich, N.; Blair, A.; Thomas, D.L.; Teinor, J.; Javed, A. Patient-derived organoid pharmacotyping is a clinically tractable strategy for precision medicine in pancreatic cancer. Ann. Surg. 2020, 272, 427–435. [Google Scholar] [CrossRef]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar]
- Silva, L.M.; Caro, T.M.; Gerstberger, R.; Vila-Viçosa, M.J.; Cortes, H.C.; Hermosilla, C.; Taubert, A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar]
- Chantrill, L.A.; Nagrial, A.M.; Watson, C.; Johns, A.L.; Martyn-Smith, M.; Simpson, S.; Mead, S.; Jones, M.D.; Samra, J.S.; Gill, A.J. Precision medicine for advanced pancreas cancer: The individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin. Cancer Res. 2015, 21, 2029–2037. [Google Scholar]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar]
- Baek, H.W.; Park, M.J.; Rhee, Y.-Y.; Lee, K.B.; Kim, M.A.; Park, I.A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic lesions. J. Pathol. Transl. Med. 2015, 49, 52. [Google Scholar] [CrossRef]
- Cros, J.; Raffenne, J.; Couvelard, A.; Poté, N. Tumor heterogeneity in pancreatic adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar]
- Vietsch, E.E.; Graham, G.T.; McCutcheon, J.N.; Javaid, A.; Giaccone, G.; Marshall, J.L.; Wellstein, A. Circulating cell-free DNA mutation patterns in early and late stage colon and pancreatic cancer. Cancer Genet. 2017, 218, 39–50. [Google Scholar] [CrossRef]
- Dreyer, S.; Jamieson, N.; Evers, L.; Duthie, F.; Cooke, S.; Marshall, J.; Beraldi, D.; Knight, S.; Upstill-Goddard, R.; Dickson, E. A large study reporting the feasibility of NGS sequencing on endoscopic ultrasound-acquired specimens. Chin. Clin. Oncol 2019, 8, 16. [Google Scholar]
- Aguirre, A.J.; Nowak, J.A.; Camarda, N.D.; Moffitt, R.A.; Ghazani, A.A.; Hazar-Rethinam, M.; Raghavan, S.; Kim, J.; Brais, L.K.; Ragon, D. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 2018, 8, 1096–1111. [Google Scholar] [CrossRef]
- Aung, K.L.; Fischer, S.E.; Denroche, R.E.; Jang, G.-H.; Dodd, A.; Creighton, S.; Southwood, B.; Liang, S.-B.; Chadwick, D.; Zhang, A. Genomics-driven precision medicine for advanced pancreatic cancer: Early results from the COMPASS trial. Clin. Cancer Res. 2018, 24, 1344–1354. [Google Scholar]
- González-Silva, L.; Quevedo, L.; Varela, I. Tumor Functional Heterogeneity Unraveled by scRNA-seq Technologies. Trends Cancer 2020, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Zerdan, M.; Shatila, M.; Sarwal, D.; Bouferraa, Y.; Bou Zerdan, M.; Allam, S.; Ramovic, M.; Graziano, S. Single Cell RNA Sequencing: A New Frontier in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 4589. https://doi.org/10.3390/cancers14194589
Bou Zerdan M, Shatila M, Sarwal D, Bouferraa Y, Bou Zerdan M, Allam S, Ramovic M, Graziano S. Single Cell RNA Sequencing: A New Frontier in Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(19):4589. https://doi.org/10.3390/cancers14194589
Chicago/Turabian StyleBou Zerdan, Maroun, Malek Shatila, Dhruv Sarwal, Youssef Bouferraa, Morgan Bou Zerdan, Sabine Allam, Merima Ramovic, and Stephen Graziano. 2022. "Single Cell RNA Sequencing: A New Frontier in Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 19: 4589. https://doi.org/10.3390/cancers14194589
APA StyleBou Zerdan, M., Shatila, M., Sarwal, D., Bouferraa, Y., Bou Zerdan, M., Allam, S., Ramovic, M., & Graziano, S. (2022). Single Cell RNA Sequencing: A New Frontier in Pancreatic Ductal Adenocarcinoma. Cancers, 14(19), 4589. https://doi.org/10.3390/cancers14194589





