Simple Summary
Despite the improvements in the survival rates and functional outcomes of childhood cancer survivors (CCS), most of them experience late effects with possible consequences to their occupational status. To date, a reliable estimate of the prevalence of employment among this population is still missing. This study aimed to assess, for the first time, the prevalence of employment among CCS and to examine the associations of socio-demographic and clinical factors with employment. Almost 100 cohorts worldwide have been included in this review, highlighting that two-thirds of childhood cancer survivors were employed. Different employment rates depending on socio-demographic and clinical factors were identified. The findings from this study could facilitate the design of targeted interventions aimed at promoting employment among CCS.
Abstract
To date, there are heterogeneous studies related to childhood cancer survivors’ (CCS) employment rates. Given the importance of this topic, we aimed to perform a systematic review and meta-analysis to investigate the prevalence of employment among CCS and to examine its association with socio-demographic and clinical factors. We followed the PRISMA guidelines to search for pertinent articles in relevant electronic databases. Eighty-nine articles comprising 93 cohorts were included. The overall prevalence of employment was 66% (CI: 95% 0.63–0.69). Subgroup meta-analyses showed that lower rates were found for central nervous system tumor survivors (51%, CI: 95% 0.43–0.59), and for CCS treated with cranial-radiotherapy (53%, CI: 95% 0.42–0.64) or haematopoietic stem-cell transplantation (56%, CI: 95% 0.46–0.65). The studies conducted in Asia highlighted employment rates of 47% (CI: 95%, 0.34–0.60). Univariate meta-regressions identified the following socio-demographic factors associated with higher rates of employment: a female gender (p = 0.046), a higher mean age at the time of investigation (p = 0.00), a longer time since diagnosis (p = 0.00), a higher educational level (p = 0.03), and a married status (p = 0.00). In conclusion, this systematic review and meta-analysis provides evidence that two-thirds of CCS are employed worldwide. Identifying vulnerable groups of CCS may allow for the design of multidisciplinary support strategies and interventions to promote employment in this population.
Keywords:
cancer survivors; neoplasm; paediatric; childhood; transition; employment; socio-economic status 1. Introduction
The survival rate for childhood cancer has considerably improved during the last few decades, and is now approaching 80% [1] due to substantial advances in diagnostics and treatment strategies [2]. Despite these improvements, two out of three Childhood Cancer Survivors (CCS) will experience at least one late effect (LE), while approximately 40% of them will experience severely disabling life-threatening or fatal clinical conditions over 30 years from diagnosis [3,4]. Nevertheless, CCS are a population at high risk for disrupted psychosocial development secondary to their primary disease, treatment, and physical LEs [5]. Previous studies highlighted that a childhood cancer diagnosis might negatively influence school performance, educational achievements, social life, and marital status [6]. Moreover, a recent study showed that one in six CCS are unemployed and that they are 1.5 times more likely to be unemployed than healthy controls [7]. Less is known about the CCS’s actual employment rate, which is currently considered a more reliable measure to assess trends in the occupational market. According to the definition of the International Labour Organisation (ILO), the term “employed” comprises all persons above a specified age (usually 15 years old) who, during a short reference period, were in one of the following categories: paid employment (at work or with a job but not at work) or self-employment generating an economic profit (at work or with an enterprise but not at work) [8]. The current scientific literature about CCS’s employment is heterogeneous along with the broad spectrum of childhood cancers in addition to country-specific educational systems.
Given the paucity of secondary literature on CCS’s employment status, yet, at the same time, its importance in terms of social impact, the purpose of this systematic review and meta-analysis is to provide comprehensive data on the prevalence of employment among CCS and to examine the associations of socio-demographic and clinical factors with employment rates.
2. Materials and Methods
This systematic review and meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The review protocol was registered on the international prospective register of systematic reviews (PROSPERO; CRD42022344410). This article presents aggregate data from primary studies; thus, no ethical approval was requested.
We conducted a systematic search for pertinent articles in five relevant electronic databases: Medline PubMed, Web of Science, Embase, Cochrane, and PsycINFO from their inception to May 2022. No limits were applied for language, and results were limited to studies conducted on humans. Search strings included terms related to the occupational field such as “employment, unemployment, absenteeism, presenteeism, work productivity, work capacity, work engagement, work ability, work performance, workload, workplace, job satisfaction, sickness absence”, and they were combined with the population of interest with terms such as “neoplasm, cancer, carcinoma […]” and “survivors, childhood survivors, paediatric […]”. The search strategy was firstly launched on PubMed and then adapted for all databases. (File S1). An expert librarian was involved in the database searches to ensure methodological rigor. The reference lists of included articles were also manually screened to identify further relevant articles. The literature search was conducted independently by three investigators and each abstract was evaluated in duplicate by two investigators. Full reports of potentially relevant articles were evaluated independently by two investigators. Disagreements were resolved between investigators and with the help of a third reviewer through consensus.
To be included in this systematic review, studies were required to be primary investigations based on a sample of CCS. Studies were included if they met the following criteria:
- -
- Included patients with a previous diagnosis of childhood cancer;
- -
- Mean age of 18 years or higher at the time of investigation;
- -
- Mean age of 16 years or lower at the time of the diagnosis;
- -
- Presented data on the employment status of included patients.
A proven diagnosis of childhood cancer, total number of CCS, and employment status of CCS were considered mandatory variables for inclusion. Only articles published in peer-reviewed journals were considered. Experimental studies, other systematic reviews or meta-analyses, and conference proceedings, theses, and letters to the editor were excluded. Articles for which the full text was not available either online or following request to the journal in which they were published were excluded.
For each cohort, the number of employed CCS was extracted as the primary outcome measure. An employed participant was intended as someone with paid employment who, during the article reference period, worked for at least one hour during a given week or had a job from which being absent was conditional on the reason of absence (e.g., holidays, maternity leave, etc.) or duration [8]. In the case of multiple reports from the same cohort, the most complete results (i.e., those based on the largest number of cases) were used. The following study characteristics were also extracted if reported in the article: publication year, country, study design, cohort size, number of males, cancer type, treatment type, mean age at the time of investigations, mean age at diagnosis, duration of the follow-up, ethnic groups, marital status, education level, number of students, and presence of a control group. Data were extracted by three independent reviewers, and any disagreement was solved by a fourth reviewer.
The CCS diagnoses were sorted into diagnostic groups: multiple cancers, central nervous system (CNS) cancers, haematological cancers, bone cancers/sarcomas, and thyroid cancers. Cohorts have been categorised in a treatment regimen if more than 50% of the CCS were treated with specific therapy, subdivided into radiotherapy (RT), cranial RT, stem-cell/bone marrow transplantation, and surgery. When data on mean age were not directly reported, they were calculated through quantile estimation [10].
Methodological quality was assessed using the Checklist for Prevalence Studies by the Joanna Brigs Institute [11], a nine-question tool with four standard answer options divided into four main domains (population and setting, condition measurement, statistics, and other), which allowed for the execution of a series of subgroup meta-analyses to assess the difference in prevalence reported by studies with different quality. As the assessment tool did not provide cut-off values, the average and median scores (M = 6.16; median = 6) were calculated to define the poor, fair, and good quality of articles. The quality assessment (QA) score of the articles was rated as poor (score = 4), fair (score = 5, 6, and 7), and good (score = 8). QA was performed by three independent reviewers, and results were discussed with a fourth reviewer until reaching consensus. The criteria were tested on a set of 10 articles to ensure agreement between assessors. Since the articles often considered employment status as a socio-demographic variable, studies were not excluded based on the QA scores.
2.1. Statistical Analysis
Overall Pooled Prevalence of Employment in CCS
Before conducting the overall pooled prevalence meta-analysis, the heterogeneity of prevalence estimates was assessed by calculating the I2 index and performing the Cochran Q test. An I2 > 50% and Cochran Q test p-values < 0.05 represented a high degree of significant heterogeneity. Due to the high heterogeneity that was both found and expected, we performed a random-effects meta-analysis of employment among CCS with 95% Confidence Intervals (CIs). As in highly heterogeneous meta-analyses, the random-effects model still has a high mean squared error; a meta-analysis using a quality effect estimator was also performed.
Sensitivity analyses included repetitions of the main meta-analysis; in each repetition, one article was removed to observe any individual effects. In addition, a subgroup meta-analysis by QA scores (poor, fair, and good) was performed to assess the variability between QA scores.
We assessed the presence of publication bias and small study effects by visual inspection of the funnel plots and applying the test proposed by Egger et al. [12].
2.2. Subgroup Meta-Analyses
We conducted subgroup meta-analyses to determine potential sources of heterogeneity. Four subgroup meta-analyses were performed to assess the prevalence of employed CCS according to different cancer diagnoses (grouped into five categories: multiple cancers, central nervous system, haematological cancers, bone cancers/sarcomas, and thyroid cancers treatment types), treatment types (multiple, mainly surgery, mainly RT, mainly cranial RT, and mainly stem-cell transplantation), and geographical areas (North America, Europe, and Asia). Data from at least three studies should be available to perform subgroup analyses.
2.3. Meta-Regressions
We performed a series of meta-regressions to examine the association between socio-demographic and clinical factors with respect to employment. The following parameters were investigated: mean age of the study group participants, mean age upon diagnosis, time since the diagnosis, percentages of males/females, percentage of participants that have graduated, and marital status. Firstly, we analysed the association of these variables in a univariate analysis. Variables statistically significantly associated with CCS’s employment were included in a multivariate analysis using a random-effects meta-regression model. Data on CCS characteristics from at least ten studies should be available to perform a univariate meta-regression and 20 for a multivariable meta-regression.
Data analyses were conducted using STATA SE/17 (StataCorp LLC, College Station, TX, USA).
3. Results
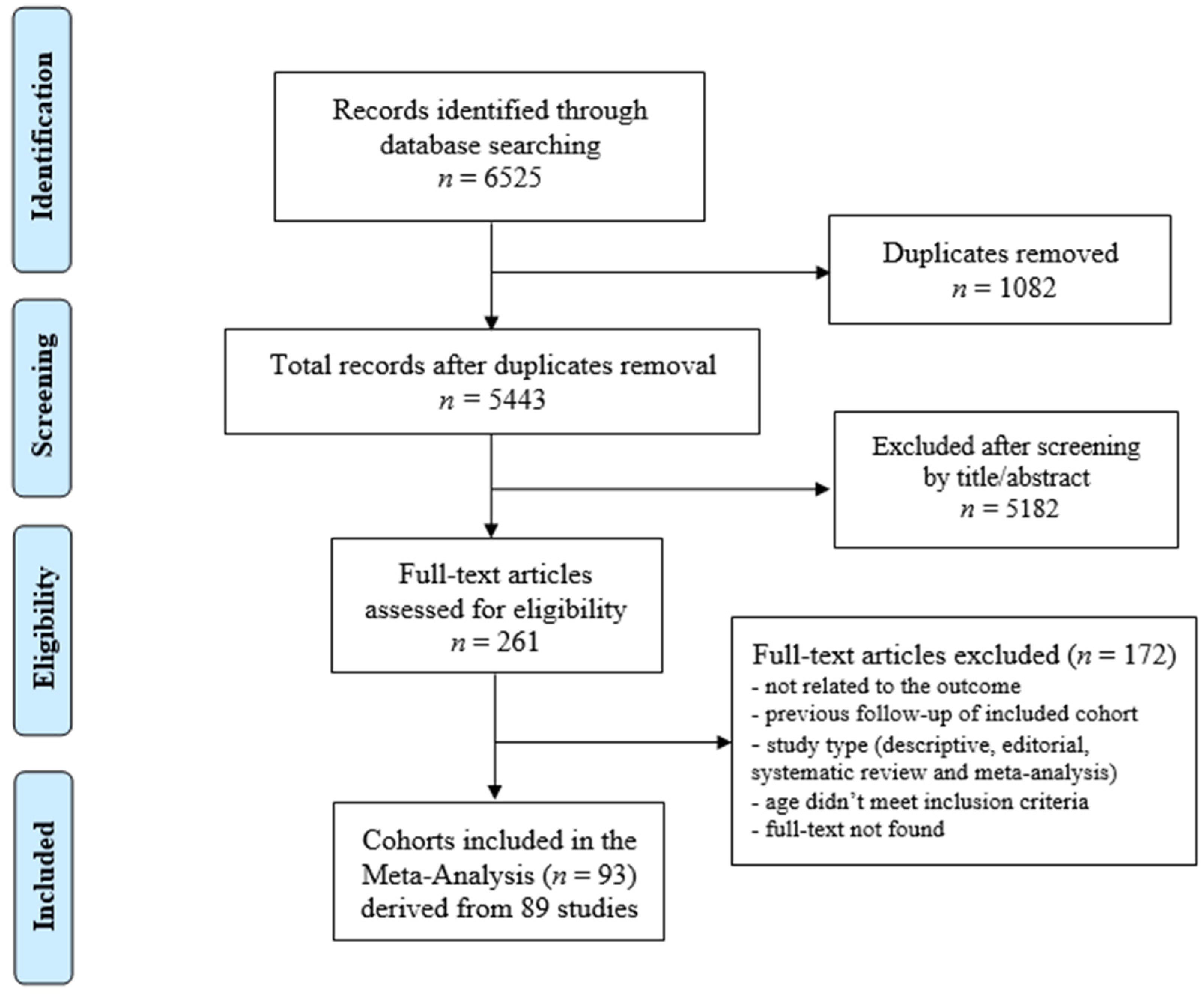
The database search yielded a total of 6525 articles. After the duplicates were removed (n = 1082), 5443 articles remained. After reviewing the articles by titles and abstracts, 261 articles were considered relevant for inclusion. The full texts of these articles were examined in detail and assessed against the inclusion and exclusion criteria. A manual search of the reference lists of the included articles did not reveal additional relevant studies.
Eighty-nine articles [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] met the inclusion criteria for the systematic review and meta-analysis. These articles reported on a total of 93 cohorts of CCS. The screening process is summarised in Figure 1.
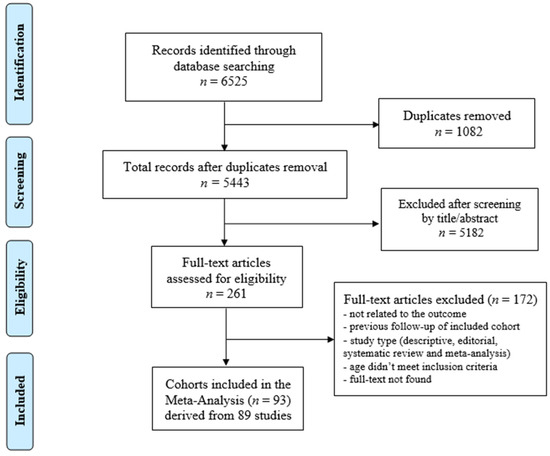
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses.
Forty-three studies were conducted in North America, forty in Europe, and six in Asia. The articles were published between 1989–2022.
The total number of CCS was 123,734. The mean age at the time of investigations was reported in 81.7% of the cohorts (27.99 years; SD ± 5.38), and the mean age at diagnosis was 9.12 years (SD ± 2.58), reported in 66.7% of the cohorts. The mean time since the diagnosis was 18.34 years (SD ± 5.27), reported in 66.7% of the cohorts. The gender was specified in 91.4% of the cohorts (50.93% males; SD ± 10.67), while marital status was specified in 63.4% of the cohorts (34.64% married; SD ± 15.96). The percentage of students was reported in 34.4% of the cohorts (mean 24.8%; range 2.5–51.3).
A total of 52 out of the 93 cohort studies analysed multiple cancer types, while 41 focused on a specific origin, namely CNS (n = 18), the haematopoietic system (n = 18), bone/soft tissue (n = 4), and thyroid gland (n = 1). In 40 of the 93 cohorts, CCS were mainly treated (>50% of the total population) with RT (n = 12), cranial-RT (n = 12), surgery (n = 11), and stem-cell/bone marrow transplantation (n = 5). Fifty-five studies (60%) reported data for CCS diagnosed and treated before 1990, whereas twenty studies (22%) reported data for after 1990. Seventeen articles (18%) did not report this information. Forty-seven studies enrolled a control population: 21 among siblings, 24 among the general population, and 2 from both. Table 1 summarises the characteristics of the included studies.

Table 1.
Characteristics of included studies.
The overall quality of the included studies was fair. Particularly, nine articles attained a high QA, whereas six were of poor quality, and the remaining seventy-four were of fair quality. The items that received a higher number of negative answers were related to the description of the setting and participants (question 4), the measurement of the condition in a standardised and reliable way for all participants (question 7), and the appropriateness of the applied statistical analysis (question 8). These were related to the scarce reporting and measurement of CCS’s employment rates, with their related clinical and socio-demographic characteristics, which were clearly stratified among those employed and unemployed. No QA questions were deemed unapplicable to the included articles. The complete quality assessment is reported in the Supplementary Material (Table S1).
3.1. Meta-Analyses
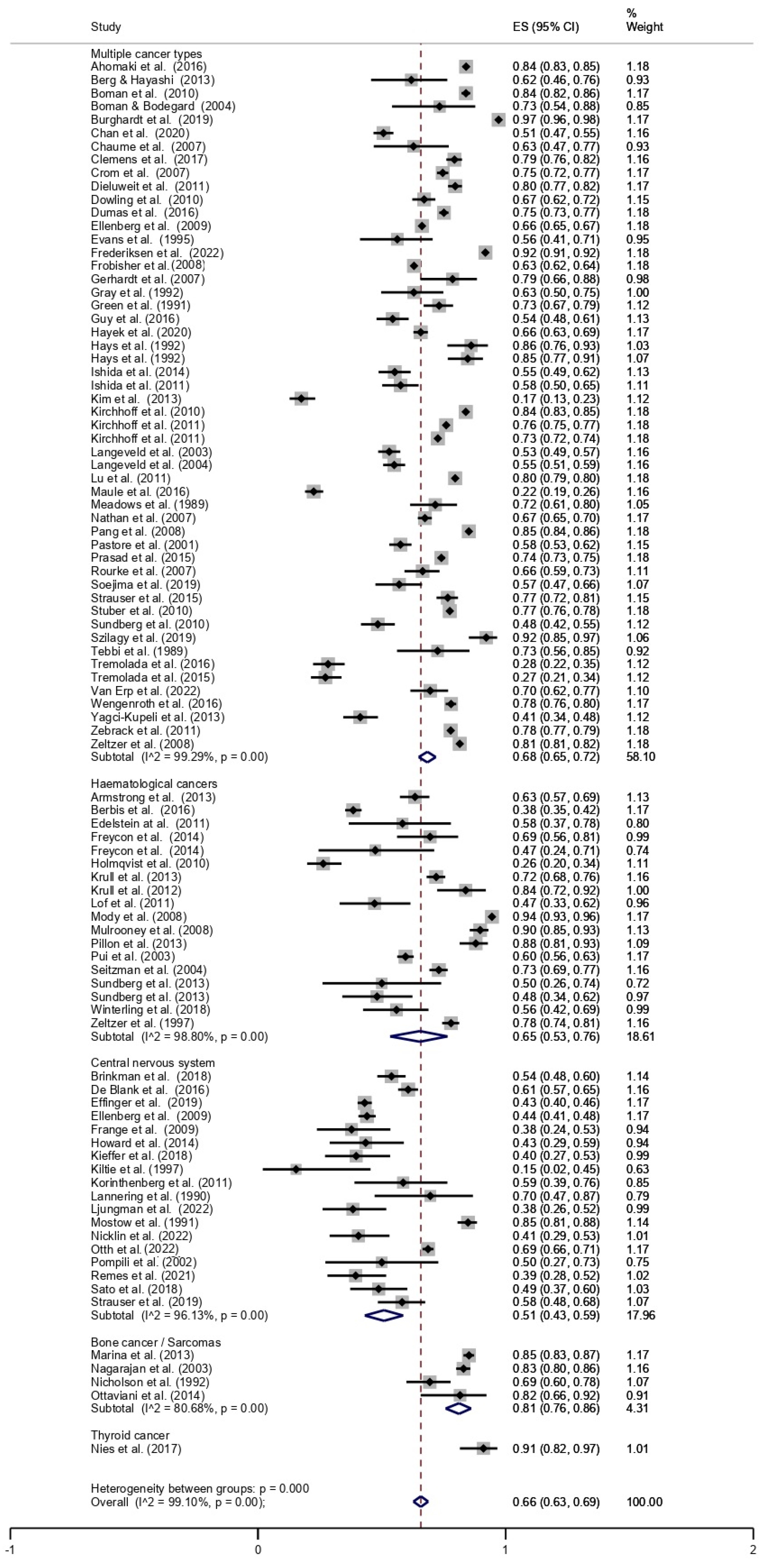
The overall prevalence of employment among CCS was 66% (95% CI, 0.63–0.69). The pooled prevalence of employment, stratified by cancer type, is shown in Figure 2. The lowest prevalence was found for CNS tumours (51%; 95% CI, 0.43–0.59), followed by haematologic malignancies (65%; 95% CI, 0.53–0.76), multiple cancers (68%; 95% CI, 0.65–0.72), bone cancer/sarcoma (81%; 95% CI, 0.76–0.86), and thyroid cancer (91%; 95% CI, 0.82–0.97).
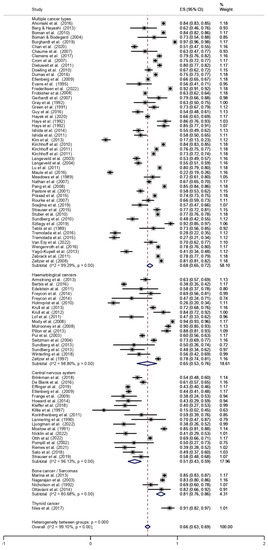
Figure 2.
Forest plot stratified by cancer type [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
Subgroup meta-analyses by type of treatment showed the lowest prevalence of employment in the cohorts of CCS mainly treated with cranial RT (53%; 95% CI, 0.42–0.64) or haematopoietic stem-cell/bone marrow transplantation (56%; 95% CI, 0.46–0.65), whereas they found the highest in the cohorts of CCS mainly treated with surgery (77%; 95% CI, 0.72–0.82). CCS diagnosed and treated before 1990 had higher employment rates (72%; 95% CI, 0.68–0.75) than those diagnosed and treated after 1990 (50%; 95% CI, 0.41–0.59). Finally, with regard to geographical differences, studies conducted in North America showed a prevalence of employment of 73% (95% CI, 0.70–075), in Europe of 60% (95% CI, 0.53–0.67), and in Asia of 47% (95% CI, 0.34–0.60).
There was evidence of significant heterogeneity (I2 > 50%; p = 0.00) in all the meta-analyses performed.
The funnel plot for the overall meta-analysis was scattered and asymmetrical, representing the possible presence of reporting bias. Similarly, the results of Egger’s tests were statistically significant for the presence of a small study effect.
3.2. Factors Associated with Employment
A univariate meta-regression (Table 2) identified the socio-demographic factors associated with a higher prevalence of employment: female gender (p = 0.046), higher mean age at the time of investigations (p = 0.00), longer time since diagnosis (p = 0.00), higher educational level (p = 0.03), and being married (p = 0.00). Moreover, the crude univariate meta-regression for Europe (p = 0.00) and Asia (p = 0.002) highlighted a lower employment prevalence than North America.

Table 2.
Univariate and multivariate random effects meta-regression model.
The clinical factors associated with lower employment prevalence were as follows: a diagnosis of a CNS tumor (p = 0.00), cranial RT (p = 0.021), CNS tumours treated with cranial RT (p = 0.00), haematologic malignancy treated with haematopoietic stem cell/bone marrow transplantation (p = 0.011), and a diagnosis and treatment after 1990 (p = 0.00).
In the multivariate analysis (Table 2), the prevalence of employment after adjustment for the age, gender, and geographical area was significantly lower for CNS tumours (p = 0.006) compared to the multiple cancer studies. The employment rates were lower for Asian studies compared with those conducted in Europe and North America after adjusting for the mean age, gender, and cancer type.
3.3. Sensitivity Analyses
The omission of any single article from the main meta-analysis did not influence the pooled prevalence of employment, with a maximum variation in the outcome of 1% (p < 0.01). The subgroup analyses by quality score revealed that the articles with a high methodological quality reported a prevalence of employment of 73% (95% CI, 0.64–0.81), while studies with a low-quality score had a 48% (95% CI, 0.35–0.62) prevalence. The majority of the articles had a fair quality score with a prevalence of 66% (95% CI, 0.63–0.69), consistent with the overall pooled prevalence found.
4. Discussion
This systematic review and meta-analysis determined that two-thirds of CCS are employed in their adulthood. However, the overall prevalence found is highly dependent on the cancer diagnosis and treatment. While almost all the survivors of bone cancer and sarcoma are employed, only half of those CCS who suffered from CNS tumours have a gainful occupation. Cranial RT and stem cell transplantation have worse outcomes, with approximately half of CCS treated with such therapies being employed. On the other hand, CCS subjected to surgery showed considerably higher employment rates, as well as those diagnosed and treated before 1990. A greater age, a longer time since diagnosis, a higher degree of education, a female gender, and being married were factors that were significantly associated with a higher rate of employment. Asian and European studies reported a significantly lower employment prevalence when compared to those conducted in North America.
To the authors’ knowledge, this is the first study assessing the overall employment prevalence estimate among CCS. Previous reviews on this population have considered their occupational status from an unemployment perspective. In particular, Mader et al. estimated that CCS were 1.5 times more likely to be unemployed than the healthy controls [7]. This value is even more favorable than those found by De Boer et al. in 2006, who highlighted that CCS were twice as likely to be unemployed as controls [102]. The quite elevated employment prevalence highlighted in this study, especially for some CCS groups, may represent a direct effect of the improvement in the safety of anticancer treatments and of recently implemented welfare policies aimed at improving social outcomes among CCS [103,104]. Indeed, the obtained findings for CCS are consistent with those found in 2009 among adult survivors, considering their unemployment rate of 33.8% [105], and recently among adolescent and young adult survivors, reporting an 84.4% lifetime prevalence of employment [106]. Nevertheless, the comparisons between employed and unemployed prevalence rates should be considered carefully, as these concepts are not completely complementary.
Consistently with previous reviews [7,102], a low prevalence of employment was found among CNS tumour survivors, while haematological malignancies, bone cancers, and sarcomas had better occupational outcomes [102]. These findings are not unexpected, as survivors of childhood-onset CNS tumours are almost five times more likely to be unemployed than healthy controls [7]. In particular, unemployment rates in these subjects ranged from 25 to 50% [102], and similar adverse employment outcomes have been reported for adult CNS cancer survivors [105]. Indeed, these patients are prone to suffer neurocognitive disorders, memory, and mobility limitations [107], which represent essential functions for workers. Moreover, they have limited chances of obtaining managerial or professional employment and high incomes [6]. Given their poor employment outcomes, childhood survivors of CNS tumours should receive specific training considering their impairments and benefit from sheltered employment opportunities.
Cranial RT plays a critical role in treating CNS tumours [108]. In addition to the development of numerous LEs such as fatigue and neurocognitive deficits, this therapeutic approach has already been identified as a predictor of unemployment among CCS [7]. Moreover, it has been shown that children who gained employment despite being treated with cranial RT reported reduced incomes [6]. Similarly, haematopoietic stem cell transplantation might result in more LEs and reduced growth among CCS, leading to higher unemployment rates among these individuals [109]. Furthermore, our findings showed no substantial differences in the prevalence of employment in CCS who underwent RT or chemotherapy, even if Ketterl et al. [106] reported increased levels of mental impairment in the work tasks among these groups.
The higher prevalence of employment among CCSs in North America compared with Europe and Asia would seem inconsistent with the data reported elsewhere in the literature. In particular, a recent review showed twofold odd unemployment rates for CCS from North America compared with healthy controls, which were higher than those observed in Europe [7]. The same trend was observed in adult cancer survivors [105]. However, as found in our study, the risk of unemployment in North American studies on adult survivors was no different from European studies after an adjustment for sociodemographic and clinical variables. The possible explanations regarding the higher values found in North America are related to the elevated occurrence of temporary or part-time jobs and the health insurance system [102]. Health insurance in North America is primarily provided and covered financially by employers [110] and could represent a crucial factor for CCS concerning their choice to work to ensure access to lifelong health care. On the other hand, employers in Europe ensure more flexible working conditions, helping survivors in maintaining their employment status [111,112]. Most European studies included were from Scandinavia, where social welfare systems are well-developed [6]. However, despite the positive aspects of social welfare systems, they can disincentivise CCS from seeking work, making unemployment financially attractive [113]. The significantly lower employment prevalence found in Asia compared with North America and Europe may reside in the Eastern culture of the preservation of vulnerable individuals or be related to the existence in these contexts of specific legislation to protect cancer survivors from employment discrimination [114].
Age is a significant determinant of employment for the general population, with favourable outcomes reported in people aged 25–54 [115]. In CCS, a younger age at diagnosis and at the time of investigation has already been associated with unemployment [102]. Similarly, long-term CSS have a more successful recovery from LEs and showed no differences in absenteeism when compared to the healthy controls [83]. In contrast, a greater degree of presenteeism was reported among employed CCS with a longer time since diagnosis [83]. Therefore, it is not surprising that, in our study, a greater age and longer time since diagnosis have been associated with better employment outcomes. This finding could also explain the higher prevalence of employment found in CCS diagnosed and treated before 1990. Despite diagnostic and therapeutic advances in the last three decades, recently diagnosed CCS may still be young and already part of the working population. Regarding the high level of educational attainment by CCS, this could be hindered by treatments and their LEs, besides the delayed school progression [116]. This is of concern, as one’s educational level positively influences their possibility of employment and a higher income [117]. Compared with the general population, CCS showed lower academic success and marriage rates [7,118]. Moreover, among this population, a high frequency of divorce or separation has been reported, presumably influenced by financial stressors due to limited employment and a reduced income [119]. Thus, marital status or togetherness could represent protective factors for maintaining employment, since even in the case of reduced earnings partners could provide a motivation to continue working actively. Although several studies reported that female childhood survivors experience more health-related challenges towards employment [7], societal patterns suggest that they are more likely to achieve higher grades and educational success than males [6]. This could place them in a better position to acquire better employment and increased incomes, with male CCS more frequently employed at the manufacturing level and at a higher risk in terms of socioeconomic outcomes [6]. Indeed, CCS often require a physical component to perform their jobs, which is inevitably affected by the LEs of the treatment they were subjected to [106]. Nevertheless, although the obtained findings have shown better employment outcomes among females, the complexity of the labor market and the possible discrimination that CCS may face at present necessitate strategies that are above simple gender considerations.
Lastly, we found significant differences in the employment rates by conducting subgroup meta-analyses stratified by QA. The lower prevalence rates shown in the studies with poorer quality may be related to a lower sensitivity of the instruments used to assess employment among CCS. Moreover, among such studies, employment was not collected as a primary outcome. On the other hand, the studies with higher quality included large cohorts of participants, often stratifying the results between those CCS who were employed and those unemployed, thus generating a more reliable estimate of the prevalence.
4.1. Implications for Practice and Research
Despite the widespread emphasis on the need to develop interventions to promote social outcomes in CCS, to date, there is little evidence to guide healthcare providers in supporting these patients and their families. Multidisciplinary interventions involving physical, psycho-educational, and vocational components have shown efficacy in promoting adult cancer survivors’ return to work [105]. Similarly, interventions directed at CCS should be conducted with a multicomponent approach, focusing on clinical aspects and educational and social dimensions that may enable CCS to enter the labor market more confidently. These interventions should include a gradual approach to employment, the presence of referral figures over a lifetime to ensure work maintenance, and the opportunity to benefit from sheltered training periods in case of late effects’ occurrence or recurrence. Beyond the impact on employment, cancer has long-term effects on workability and work capacity, resulting in a potential reduction in income and the loss of life satisfaction for large groups of survivors [83,105]. There is a need to develop and prove the effectiveness of clinical and social support services focused on rehabilitation and workplace accommodation. The use of technology may represent a way to promote access to work and improve communication, by keeping young survivors and their families connected to healthcare and social providers [104]. Long-term follow-ups for CCS may lead to better health and educational outcomes [6]. Considering the results of our study, some CCS groups might benefit from a more focused follow-up and the implementation of appropriate support strategies [120]. These could increase the possibilities for CCS to be aware of the adverse socioeconomic situations that lead to unhealthy lifestyles and, consequently, more comorbidities [121]. From a social point of view, employment stability in CCS should be enhanced, as it is crucial for economic reasons and to prevent inequalities [122]. Better estimates of employment from high-quality studies involving large samples of CCS with matched control groups are needed to enable the reliable identification of vulnerable subgroups and the design of tailored interventions to promote employment among this population. Finally, future studies should apply reliable methodological standards to measure employment rates among CCS, ensuring the better comparability and statistical validity of the associated research. Particularly, they should report more accurately on participants’ clinical and socio-demographic characteristics, which are clearly differentiated among those employed and unemployed.
4.2. Strengths and limitations
This systematic review and meta-analysis has some limitations. The search of five databases could have excluded some relevant studies. Furthermore, all the studies included were conducted in high-income countries, thereby limiting the generalizability of their results. Nevertheless, the application of a systematic approach and the involvement of three independent researchers and an expert librarian in all the phases (the search, screening, and extraction) contributed to limiting the biases related to the selection of articles.
The quality of the included studies ranged widely, and strictly depended on their design and objectives. It is possible that, as reported by Mader and colleagues [7], there may be a tendency in uncontrolled studies to overestimate the phenomenon of interest. In this regard, all the studies with low quality have a small sample size (less than 100 CCS), and half of them were published before 2000, so it is arguable that the QA scores could be directly affected by an earlier period of a given study’s execution, beyond a limited recruited sample. Moreover, in some studies, employment was only a secondary outcome that was always reported in an unreliable form, whereas other studies examined CCS longitudinally with matched control groups, leading to heterogeneity of the quality of reported data. To address this limitation, we extracted all the information about employment status, excluding all students, homemakers, and retired CCS from our analyses, attempting to include only those effectively employed. As this study included a very large number of articles, we believe that a large proportion of the studies on employment have been selected by our inclusion criteria.
The issues about the estimates of the phenomenon of interest and the high heterogeneity found in the meta-analyses were reduced by stabilizing the prevalence variance using a double-arcsine transformation [123]. Moreover, all the performed meta-analyses used random-effect models. Using a quality effects estimator could have maintained a lower estimated variance while maintaining the correct confidence interval probability, regardless of the level of heterogeneity. We addressed this limitation by stratifying the studies by quality level, which allowed us to assess the differences that occurred in the prevalence rates obtained from the quality assessment scores. In this regard, higher quality studies reported a higher prevalence and might have used measures targeted to assess the employment rate. Conversely, lower quality studies could have assessed employment as a secondary outcome, limiting the reliability of the detection of employment prevalence. Lastly, the presence of a small study effect due to the heterogeneous number of CCS included in the cohorts could be partly explained by the variations in the cancers’ occurrence and contextual differences among different geographical areas.
Despite the above limitations, this systematic review focused specifically on employment among CCS, stratifying its findings and providing evidence for the association of employment with clinical and sociodemographic variables by using robust meta-analysis methods.
5. Conclusions
This is the first systematic review and meta-analysis assessing the prevalence of employment among CCS, which highlights difference rates depending on socio-demographic and clinical factors. A greater age at investigation resulted in a higher rate of employment among CCS, while CNS tumors were associated with worse occupational outcomes. The studies from Asia reported significantly lower employment rates. Identifying susceptible groups of CCS may facilitate the design of multidisciplinary support strategies and multicomponent interventions focused on clinical and social aspects to promote employment in this population. Future research should employ longitudinal, controlled, matched designs focusing on specific cancer diagnoses and treatments to ensure more reliable employment estimates among CCS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14194586/s1. Table S1. Quality Assessment of included studies, File S1. Search strategy on the Medline PubMed Database
Author Contributions
Conceptualization, A.G., A.C., M.C. and F.F. (Francesco Felicetti); methodology, M.C. and A.G.; investigation, T.P., F.G., M.D.-V., C.C., A.G. and A.C.; formal analysis, M.C. and A.G.; A.G., A.C. and F.F. (Francesco Felicetti) writing—original draft preparation; C.C., E.P., E.A. and F.F. (Francesco Felicetti), writing—review and editing; supervision E.P., E.A., E.B. and F.F. (Franca Fagioli) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood Cancer Survival in Europe 1999–2007: Results of EUROCARE-5—A Population-Based Study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood Cancer: Survival, Treatment Modalities, Late Effects and Improvements over Time. Cancer Epidemiol. 2021, 71, 101733. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, F.; Aimaretti, E.; Dal Bello, F.; Gatti, F.; Godono, A.; Saba, F.; Einaudi, G.; Collino, M.; Fagioli, F.; Aragno, M.; et al. Advanced Glycation End Products and Their Related Signaling Cascades in Adult Survivors of Childhood Hodgkin Lymphoma: A Possible Role in the Onset of Late Complications. Free Radic. Biol. Med. 2022, 178, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Rebholz, C.E.; von der Weid, N.X.; Bergstraesser, E.; Kuehni, C.E. Psychological Distress in Adult Survivors of Childhood Cancer: The Swiss Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E.; Mader, L.; Feychting, M.; Mogensen, H.; Madanat-Harjuoja, L.; Malila, N.; Tolkkinen, A.; Hasle, H.; Winther, J.F.; Erdmann, F. Surviving Childhood Cancer: A Systematic Review of Studies on Risk and Determinants of Adverse Socioeconomic Outcomes. Int. J. Cancer 2019, 144, 1796–1823. [Google Scholar] [CrossRef] [PubMed]
- Mader, L.; Michel, G.; Roser, K. Unemployment Following Childhood Cancer. Dtsch. Arztebl. Int. 2017, 114, 805–812. [Google Scholar] [CrossRef]
- Hussmanns, R. International Standards on the Measurement of Economic Activity, Employment, Unemployment and Underemployment. Labour Stat. A Mark. Econ. 2022, 1985, 77–106. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef]
- Munn, Z.; Tufanaru, C.; Aromataris, E. JBI’s Systematic Reviews: Data Extraction and Synthesis. Am. J. Nurs. 2014, 114, 49–54. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Ahomäki, R.; Harila-Saari, A.; Matomäki, J.; Lähteenmäki, P.M. Non-Graduation after Comprehensive School, and Early Retirement but Not Unemployment Are Prominent in Childhood Cancer Survivors—A Finnish Registry-Based Study. J. Cancer Surviv. 2017, 11, 284–294. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Reddick, W.E.; Petersen, R.C.; Santucci, A.; Zhang, N.; Srivastava, D.; Ogg, R.J.; Hillenbrand, C.M.; Sabin, N.; Krasin, M.J.; et al. Evaluation of Memory Impairment in Aging Adult Survivors of Childhood Acute Lymphoblastic Leukemia Treated with Cranial Radiotherapy. J. Natl. Cancer Inst. 2013, 105, 899–907. [Google Scholar] [CrossRef]
- Clemens, E.; Van Doorn, M.; Neggers, S.J.C.M.M.; de Vries, A.C.H.; van den Heuvel-Eibrink, M.M. Socio-Demographic Impact of Platinum-Induced Ototoxicity in Long-Term Survivors of Childhood Cancer. Curr. Pediatr. Res. 2017, 21, 470–479. [Google Scholar]
- Crom, D.B.; Lensing, S.Y.; Rai, S.N.; Snider, M.A.; Cash, D.K.; Hudson, M.M. Marriage, Employment, and Health Insurance in Adult Survivors of Childhood Cancer. J. Cancer Surviv. 2007, 1, 237–245. [Google Scholar] [CrossRef]
- De Blank, P.M.K.; Fisher, M.J.; Lu, L.; Leisenring, W.M.; Ness, K.K.; Sklar, C.A.; Stovall, M.; Vukadinovich, C.; Robison, L.L.; Armstrong, G.T.; et al. Impact of Vision Loss among Survivors of Childhood Central Nervous System Astroglial Tumors. Cancer 2016, 122, 730–739. [Google Scholar] [CrossRef]
- Dieluweit, U.; Debatin, K.-M.; Grabow, D.; Kaatsch, P.; Peter, R.; Seitz, D.C.M.; Goldbeck, L. Educational and Vocational Achievement among Long-Term Survivors of Adolescent Cancer in Germany. Pediatr. Blood Cancer 2011, 56, 432–438. [Google Scholar] [CrossRef]
- Dowling, E.; Yabroff, K.R.; Mariotto, A.; McNeel, T.; Zeruto, C.; Buckman, D. Burden of Illness in Adult Survivors of Childhood Cancers: Findings from a Population-Based National Sample. Cancer 2010, 116, 3712–3721. [Google Scholar] [CrossRef]
- Dumas, A.; Berger, C.; Auquier, P.; Michel, G.; Fresneau, B.; Sètchéou Allodji, R.; Haddy, N.; Rubino, C.; Vassal, G.; Valteau-Couanet, D.; et al. Educational and Occupational Outcomes of Childhood Cancer Survivors 30 Years after Diagnosis: A French Cohort Study. Br. J. Cancer 2016, 114, 1060–1068. [Google Scholar] [CrossRef]
- Edelstein, K.; D’Agostino, N.; Bernstein, L.J.; Nathan, P.C.; Greenberg, M.L.; Hodgson, D.C.; Millar, B.A.; Laperriere, N.; Spiegler, B.J. Long-Term Neurocognitive Outcomes in Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2011, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Effinger, K.E.; Stratton, K.L.; Fisher, P.G.; Ness, K.K.; Krull, K.R.; Oeffinger, K.C.; Armstrong, G.T.; Robison, L.L.; Hudson, M.M.; Leisenring, W.M.; et al. Long-Term Health and Social Function in Adult Survivors of Paediatric Astrocytoma: A Report from the Childhood Cancer Survivor Study. Eur. J. Cancer 2019, 106, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, L.; Liu, Q.; Gioia, G.; Yasui, Y.; Packer, R.J.; Mertens, A.; Donaldson, S.S.; Stovall, M.; Kadan-Lottick, N.; Armstrong, G.; et al. Neurocognitive Status in Long-Term Survivors of Childhood CNS Malignancies: A Report From the Childhood Cancer Survivor Study. Neuropsychology 2009, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Radford, M. Current Lifestyle of Young Adults Treated for Cancer in Childhood. Arch. Dis. Child. 1995, 72, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Berbis, J.; Reggio, C.; Michel, G.; Chastagner, P.; Bertrand, Y.; Kanold, J.; Sirvent, N.; Plantaz, D.; Baruchel, A.; Tabone, M.D.; et al. Employment in French Young Adult Survivors of Childhood Leukemia: An LEA Study (for Leucemies de l’Enfant et de l’Adolescent—Childhood and Adolescent Leukemia). J. Cancer Surviv. 2016, 10, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E.; Pedersen, C.; Mogensen, H.; Mader, L.; Bautz, A.; Talbäck, M.; Hirvonen, E.; Norsker, F.N.; Hasle, H.; Malila, N.; et al. Employment Status and Occupational Positions of Childhood Cancer Survivors from Denmark, Finland and Sweden: A Nordic Register-Based Cohort Study from the SALiCCS Research Programme. Lancet Reg. Health Eur. 2022, 12. [Google Scholar] [CrossRef]
- Frange, P.; Alapetite, C.; Gaboriaud, G.; Bours, D.; Zucker, J.M.; Zerah, M.; Brisse, H.; Chevignard, M.; Mosseri, V.; Bouffet, E.; et al. From Childhood to Adulthood: Long-Term Outcome of Medulloblastoma Patients. The Institut Curie Experience (1980–2000). J. Neurooncol. 2009, 95, 271–279. [Google Scholar] [CrossRef]
- Freycon, F.; Trombert-Paviot, B.; Casagranda, L.; Frappaz, D.; Mialou, V.; Armari-Alla, C.; Gomez, F.; Faure-Conter, C.; Plantaz, D.; Berger, C. Academic Difficulties and Occupational Outcomes of Adult Survivors of Childhood Leukemia Who Have Undergone Allogeneic Hematopoietic Stem Cell Transplantation and Fractionated Total Body Irradiation Conditioning. Pediatr. Hematol. Oncol. 2014, 31, 225–236. [Google Scholar] [CrossRef]
- Frobisher, C.; Winter, D.L.; Lancashire, E.R.; Reulen, R.C.; Taylor, A.J.; Eiser, C.; Stevens, M.C.G.; Hawkins, M.M. Extent of Smoking and Age at Initiation of Smoking among Adult Survivors of Childhood Cancer in Britain. J. Natl. Cancer Inst. 2008, 100, 1068–1081. [Google Scholar] [CrossRef]
- Gerhardt, C.A.; Dixon, M.; Miller, K.; Vannatta, K.; Valerius, K.S.; Correll, J.; Noll, R.B. Educational and Occupational Outcomes among Survivors of Childhood Cancer during the Transition to Emerging Adulthood. J. Dev. Behav. Pediatr. 2007, 28, 448–455. [Google Scholar] [CrossRef]
- Gray, R.E.; Doan, B.D.; Shermer, P.; Fitzgerald, A.V.; Bery, M.P.; Jenkin, D.; Doherty, M.A. Psychologic Adaptation of Survivors of Childhood Cancer. Cancer 1992, 70, 2713–2721. [Google Scholar] [CrossRef]
- Green, D.M.; Zevon, M.A.; Hall, B. Life Goals by Adult Survivors. Distribution 1990, 67, 206–213. [Google Scholar]
- Guy, G.P.; Berkowitz, Z.; Ekwueme, D.U.; Rim, S.H.; Yabroff, K.R. Annual Economic Burden of Productivity Losses Among Adult Survivors of Childhood Cancers. Pediatrics 2016, 138, S15–S21. [Google Scholar] [CrossRef]
- Hayek, S.; Brinkman, T.M.; Plana, J.C.; Joshi, V.M.; Leupker, R.V.; Durand, J.B.; Green, D.M.; Partin, R.E.; Santucci, A.K.; Howell, R.M.; et al. Association of Exercise Intolerance with Emotional Distress, Attainment of Social Roles, and Health-Related Quality of Life among Adult Survivors of Childhood Cancer. JAMA Oncol. 2020, 6, 1194–1202. [Google Scholar] [CrossRef]
- Hays, D.M.; Landsverk, J.; Sallan, S.E.; Hewett, K.D.; Patenaude, A.F.; Schoonover, D.; Zilber, S.L.; Ruccione, K.; Siegel, S.E. Educational, Occupational, and Insurance Status of Childhood Cancer Survivors in Their Fourth and Fifth Decades of Life. J. Clin. Oncol. 1992, 10, 1397–1406. [Google Scholar] [CrossRef]
- Berg, C.; Hayashi, R.J. Participation and Self-Management Strategies of Young Adult Childhood Cancer Survivors. OTJR Occup. Particip. Health 2013, 33, 21–30. [Google Scholar] [CrossRef]
- Holmqvist, A.S.; Wiebe, T.; Hjorth, L.; Lindgren, A.; Øra, I.; Moëll, C. Young Age at Diagnosis Is a Risk Factor for Negative Late Socio-Economic Effects after Acute Lymphoblastic Leukemia in Childhood. Pediatr. Blood Cancer 2010, 55, 698–707. [Google Scholar] [CrossRef]
- Howard, A.F.; Hasan, H.; Bobinski, M.A.; Nurcombe, W.; Olson, R.; Parkinson, M.; Goddard, K. Parents’ Perspectives of Life Challenges Experienced by Long-Term Paediatric Brain Tumour Survivors: Work and Finances, Daily and Social Functioning, and Legal Difficulties. J. Cancer Surviv. 2014, 8, 372–383. [Google Scholar] [CrossRef]
- Ishida, Y.; Hayashi, M.; Inoue, F.; Ozawa, M. Recent Employment Trend of Childhood Cancer Survivors in Japan: A Cross-Sectional Survey. Int. J. Clin. Oncol. 2014, 19, 973–981. [Google Scholar] [CrossRef]
- Ishida, Y.; Honda, M.; Kamibeppu, K.; Ozono, S.; Okamura, J.; Asami, K.; Maeda, N.; Sakamoto, N.; Inada, H.; Iwai, T.; et al. Social Outcomes and Quality of Life of Childhood Cancer Survivors in Japan: A Cross-Sectional Study on Marriage, Education, Employment and Health-Related QOL (SF-36). Int. J. Hematol. 2011, 93, 633–644. [Google Scholar] [CrossRef]
- Kieffer, V.; Chevignard, M.P.; Dellatolas, G.; Puget, S.; Dhermain, F.; Grill, J.; Valteau-Couanet, D.; Dufour, C. Intellectual, Educational, and Situation-Based Social Outcome in Adult Survivors of Childhood Medulloblastoma. Dev. Neurorehabilit. 2019, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kiltie, A.E.; Lashford, L.S.; Gattamaneni, H.R. Survival and Late Effects in Medulloblastoma Patients Treated with Craniospinal Irradiation under Three Years Old. Med. Pediatr. Oncol. 1997, 28, 348–354. [Google Scholar] [CrossRef]
- Kim, M.A.; Yi, J. Psychological Distress in Adolescent and Young Adult Survivors of Childhood Cancer in Korea. J. Pediatr. Oncol. Nurs. 2013, 30, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Leisenring, W.; Krull, K.R.; Ness, K.K.; Friedman, D.L.; Armstrong, G.T.; Stovall, M.; Park, E.R.; Oeffinger, K.C.; Hudson, M.M.; et al. Unemployment among Adult Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Med. Care 2010, 48, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Krull, K.R.; Ness, K.K.; Armstrong, G.T.; Park, E.R.; Stovall, M.; Robison, L.L.; Leisenring, W. Physical, Mental, and Neurocognitive Status and Employment Outcomes in the Childhood Cancer Survivor Study Cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Krull, K.R.; Ness, K.K.; Park, E.R.; Oeffinger, K.C.; Hudson, M.M.; Stovall, M.; Robison, L.L.; Wickizer, T.; Leisenring, W. Occupational Outcomes of Adult Childhood Cancer Survivors. Cancer 2011, 117, 3033–3044. [Google Scholar] [CrossRef]
- Boman, K.K.; Lindblad, F.; Hjern, A. Long-Term Outcomes of Childhood Cancer Survivors in Sweden: A Population-Based Study of Education, Employment, and Income. Cancer 2010, 116, 1385–1391. [Google Scholar] [CrossRef]
- Korinthenberg, R.; Neuburger, D.; Nikkhah, G.; Teske, C.; Schnabel, K.; Calaminus, G. Assessing Quality of Life in Long-Term Survivors after 125I Brachytherapy for Low-Grade Glioma in Childhood. Neuropediatrics 2011, 42, 110–115. [Google Scholar] [CrossRef]
- Krull, K.R.; Brinkman, T.M.; Li, C.; Armstrong, G.T.; Ness, K.K.; Kumar Srivastava, D.; Gurney, J.G.; Kimberg, C.; Krasin, M.J.; Pui, C.H.; et al. Neurocognitive Outcomes Decades after Treatment for Childhood Acute Lymphoblastic Leukemia: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2013, 31, 4407–4415. [Google Scholar] [CrossRef]
- Krull, K.R.; Sabin, N.D.; Reddick, W.E.; Zhu, L.; Armstrong, G.T.; Green, D.M.; Arevalo, A.R.; Krasin, M.J.; Srivastava, D.K.; Robison, L.L.; et al. Neurocognitive Function and CNS Integrity in Adult Survivors of Childhood Hodgkin Lymphoma. J. Clin. Oncol. 2012, 30, 3618–3624. [Google Scholar] [CrossRef]
- Langeveld, N.E.; Ubbink, M.C.; Last, B.F.; Grootenhuis, M.A.; Voûte, P.A.; De Haan, R.J. Educational Achievement, Employment and Living Situation in Long-Term Young Adult Survivors of Childhood Cancer in The Netherlands. Psychooncology 2003, 12, 213–225. [Google Scholar] [CrossRef]
- Langeveld, N.E.; Grootenhuis, M.A.; Voûte, P.A.; De Haan, R.J. Posttraumatic Stress Symptoms in Adult Survivors of Childhood Cancer. Pediatr. Blood Cancer 2004, 42, 604–610. [Google Scholar] [CrossRef]
- Lannering, B.; Marky, I.; Lundberg, A.; Olsson, E. Long-term Sequelae after Pediatric Brain Tumors: Their Effect on Disability and Quality of Life. Med. Pediatr. Oncol. 1990, 18, 304–310. [Google Scholar] [CrossRef]
- Ljungman, L.; Remes, T.; Westin, E.; Huittinen, A.; Lönnqvist, T.; Sirkiä, K.; Rantala, H.; Ojaniemi, M.; Harila, M.; Lähteenmäki, P.; et al. Health-Related Quality of Life in Long-Term Survivors of Childhood Brain Tumors: A Population-Based Cohort Study. Support. Care Cancer 2022, 30, 5157–5166. [Google Scholar] [CrossRef]
- Löf, C.M.; Winiarski, J.; Ljungman, P.; Forinder, U. The Socioeconomic and Psychosocial Circumstances of Adult Long-Term Survivors of Hematopoietic Stem Cell Transplantation in Childhood. Pediatr. Transplant. 2011, 15, 691–698. [Google Scholar] [CrossRef]
- Lu, Q.; Krull, K.R.; Leisenring, W.; Owen, J.E.; Kawashima, T.; Tsao, J.C.I.; Zebrack, B.; Mertens, A.; Armstrong, G.T.; Stovall, M.; et al. Pain in Long-Term Adult Survivors of Childhood Cancers and Their Siblings: A Report from the Childhood Cancer Survivor Study. Pain 2011, 152, 2616–2624. [Google Scholar] [CrossRef]
- Marina, N.; Hudson, M.M.; Jones, K.E.; Mulrooney, D.A.; Avedian, R.; Donaldson, S.S.; Popat, R.; West, D.W.; Fisher, P.; Leisenring, W.; et al. Changes in Health Status Among Aging Survivors of Pediatric Upper and Lower Extremity Sarcoma: A Report From the Childhood Cancer Survivor Study. Arch. Phys. Med. Rehabil. 2013, 94, 1062–1073. [Google Scholar] [CrossRef]
- Boman, K.K.; Bodegård, G. Life after Cancer in Childhood: Social Adjustment and Educational and Vocational Status of Young-Adult Survivors. J. Pediatr. Hematol. Oncol. 2004, 26, 354–362. [Google Scholar] [CrossRef]
- Maule, M.; Zugna, D.; Migliore, E.; Alessi, D.; Merletti, F.; Onorati, R.; Zengarini, N.; Costa, G.; Spadea, T. Surviving a Childhood Cancer: Impact on Education and Employment. Eur. J. Cancer Prev. 2017, 26, 351–356. [Google Scholar] [CrossRef]
- Meadows, A.T.; McKee, L.; Kazak, A.E. Psychosocial Status of Young Adult Survivors of Childhood Cancer: A Survey. Med. Pediatr. Oncol. 1989, 17, 466–470. [Google Scholar] [CrossRef]
- Mody, R.; Li, S.; Dover, D.C.; Sallan, S.; Leisenring, W.; Oeffinger, K.C.; Yasui, Y.; Robison, L.L.; Neglia, J.P. Twenty-Five-Year Follow-up among Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. Blood 2008, 111, 5515–5523. [Google Scholar] [CrossRef]
- Mostow, E.N.; Byrne, J.; Connelly, R.R.; Mulvihill, J.J. Quality of Life in Long-Term Survivors of CNS Tumors of Childhood and Adolescence. J. Clin. Oncol. 1991, 9, 592–599. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Dover, D.C.; Li, S.; Yasui, Y.; Ness, K.K.; Mertens, A.C.; Neglia, J.P.; Sklar, C.; Robison, L.L.; Davies, S.M.; et al. Twenty Years of Follow-up among Survivors of Childhood and Young Adult Acute Myeloid Leukemia: A Report from the Childhood Cancer Survivor Study. Cancer 2008, 112, 2071–2079. [Google Scholar] [CrossRef]
- Nagarajan, R.; Neglia, J.P.; Clohisy, D.R.; Yasui, Y.; Greenberg, M.; Hudson, M.; Zevon, M.A.; Tersak, J.M.; Ablin, A.; Robison, L.L. Education, Employment, Insurance, and Marital Status among 694 Survivors of Pediatric Lower Extremity Bone Tumors: A Report from the Childhood Cancer Survivor Study. Cancer 2003, 97, 2554–2564. [Google Scholar] [CrossRef]
- Nathan, P.C.; Ness, K.K.; Greenberg, M.L.; Hudson, M.; Wolden, S.; Davidoff, A.; Laverdiere, C.; Mertens, A.; Whitton, J.; Robison, L.L.; et al. Health-Related Quality of Life in Adult Survivors of Childhood Wilms Tumor or Neuroblastoma: A Report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2007, 49, 704–715. [Google Scholar] [CrossRef]
- Nicholson, H.S.; Mulvihill, J.J.; Byrne, J. Late Effects of Therapy in Adult Survivors of Osteosarcoma and Ewing’s Sarcoma. Med. Pediatr. Oncol. 1992, 20, 6–12. [Google Scholar] [CrossRef]
- Nicklin, E.; Velikova, G.; Glaser, A.; Kwok-Williams, M.; Debono, M.; Sarwar, N.; Boele, F. Long-Term Unmet Supportive Care Needs of Teenage and Young Adult (TYA) Childhood Brain Tumour Survivors and Their Caregivers: A Cross-Sectional Survey. Support. Care Cancer 2022, 30, 1981–1992. [Google Scholar] [CrossRef]
- Nies, M.; Hesselink, M.S.K.; Huizinga, G.A.; Sulkers, E.; Brouwers, A.H.; Burgerhof, J.G.M.; Van Dam, E.W.C.M.; Havekes, B.; Van Den Heuvel-Eibrink, M.M.; Corssmit, E.P.M.; et al. Long-Term Quality of Life in Adult Survivors of Pediatric Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, T.M.; Ness, K.K.; Li, Z.; Huang, I.C.; Krull, K.R.; Gajjar, A.; Merchant, T.E.; Klosky, J.L.; Partin, R.E.; Olsson, I.T.; et al. Attainment of Functional and Social Independence in Adult Survivors of Pediatric CNS Tumors: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2018, 36, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Robert, R.S.; Huh, W.W.; Palla, S.; Jaffe, N. Sociooccupational and Physical Outcomes More than 20 Years after the Diagnosis of Osteosarcoma in Children and Adolescents: Limb Salvage versus Amputation. Cancer 2013, 119, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Otth, M.; Michel, G.; Gerber, N.U.; Guerreiro Stücklin, A.S.; von Bueren, A.O.; Scheinemann, K. Educational Attainment and Employment Outcome of Survivors of Pediatric CNS Tumors in Switzerland—A Report from the Swiss Childhood Cancer Survivor Study. Children 2022, 9, 411. [Google Scholar] [CrossRef]
- Pang, J.W.Y.; Friedman, D.L.; Whitton, J.A.; Stovall, M.; Mertens, A.C.; Robison, L.L.; Weiss, N.S. Employment Status among Adult Survivors in the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2008, 50, 104–110. [Google Scholar] [CrossRef]
- Pastore, G.; Mosso, M.L.; Magnani, C.; Luzzatto, L.; Bianchi, M.; Terracini, B. Physical Impairment and Social Life Goals among Adult Long-Term Survivors of Childhood Cancer: A Population-Based Study from the Childhood Cancer Registry of Piedmont, Italy. Tumori 2001, 87, 372–378. [Google Scholar] [CrossRef]
- Pillon, M.; Tridello, G.; Boaro, M.P.; Messina, C.; Putti, M.C.; Varotto, S.; Petris, M.G.; Scrimin, S.; Zanesco, L.; Rosolen, A.; et al. Psychosocial Life Achievements in Adults Even If They Received Prophylactic Cranial Irradiation for Acute Lymphoblastic Leukemia during Childhood. Leuk. Lymphoma 2013, 54, 315–320. [Google Scholar] [CrossRef]
- Pompili, A.; Caperle, M.; Pace, A.; Ramazzotti, V.; Raus, L.; Jandolo, B.; Occhipinti, E. Quality-of-Life Assessment in Patients Who Had Been Surgically Treated for Cerebellar Pilocytic Astrocytoma in Childhood. J. Neurosurg. 2002, 96, 229–234. [Google Scholar] [CrossRef]
- Prasad, P.K.; Hardy, K.K.; Zhang, N.; Edelstein, K.; Srivastava, D.; Zeltzer, L.; Stovall, M.; Seibel, N.L.; Leisenring, W.; Armstrong, G.T.; et al. Psychosocial and Neurocognitive Outcomes in Adult Survivors of Adolescent and Early Young Adult Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2015, 33, 2545–2552. [Google Scholar] [CrossRef]
- Pui, C.-H.; Cheng, C.; Leung, W.; Rai, S.N.; Rivera, G.K.; Sandlund, J.T.; Ribeiro, R.C.; Relling, M.V.; Kun, L.E.; Evans, W.E.; et al. Extended Follow-up of Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2003, 349, 640–649. [Google Scholar] [CrossRef]
- Remes, T.M.; Hovén, E.; Ritari, N.; Pohjasniemi, H.; Puosi, R.; Arikoski, P.M.; Arola, M.O.; Lähteenmäki, P.M.; Lönnqvist, T.R.I.; Ojaniemi, M.K.; et al. Neurocognitive Impairment, Employment, and Social Status in Radiotherapy-Treated Adult Survivors of Childhood Brain Tumors. Neuro-Oncol. Pract. 2021, 8, 266–277. [Google Scholar] [CrossRef]
- Rourke, M.T.; Hobbie, W.L.; Schwartz, L.; Kazak, A.E. Posttraumatic Stress Disorder (PTSD) in Young Adult Survivors of Childhood Cancer. Pediatr. Blood Cancer 2007, 49, 177–182. [Google Scholar] [CrossRef]
- Burghardt, J.; Klein, E.; Brähler, E.; Ernst, M.; Schneider, A.; Eckerle, S.; Neu, M.A.; Wingerter, A.; Henninger, N.; Panova-Noeva, M.; et al. Prevalence of Mental Distress among Adult Survivors of Childhood Cancer in Germany—Compared to the General Population. Cancer Med. 2019, 8, 1865–1874. [Google Scholar] [CrossRef]
- Sato, I.; Higuchi, A.; Yanagisawa, T.; Murayama, S.; Kumabe, T.; Sugiyama, K.; Mukasa, A.; Saito, N.; Sawamura, Y.; Terasaki, M.; et al. Employment Status and Termination among Survivors of Pediatric Brain Tumors: A Cross-Sectional Survey. Int. J. Clin. Oncol. 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Seitzman, R.L.; Glover, D.A.; Meadows, A.T.; Mills, J.L.; Nicholson, H.S.; Robison, L.L.; Byrne, J.; Zeltzer, L.K. Self-Concept in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Cooperative Children’s Cancer Group and National Institutes of Health Study. Pediatr. Blood Cancer 2004, 42, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Soejima, T.; Sato, I.; Takita, J.; Koh, K.; Kaneko, T.; Inada, H.; Ozono, S.; Kamibeppu, K. Do Childhood Cancer and Physical Late Effects Increase Worries about Future Employment in Adulthood? Cancer Rep. 2019, 2, e1175. [Google Scholar] [CrossRef]
- Strauser, D.R.; Chan, F.; Fine, E.; Iwanaga, K.; Greco, C.; Liptak, C. Development of the Perceived Barriers Scale: A New Instrument Identifying Barriers to Career Development and Employment for Young Adult Survivors of Pediatric CNS Tumors. J. Cancer Surviv. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Strauser, D.; Klosky, J.L.; Brinkman, T.M.; Wong, A.W.K.; Chan, F.; Lanctot, J.; Ojha, R.P.; Robison, L.L.; Hudson, M.M.; Ness, K.K. Career Readiness in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. J. Cancer Surviv. 2015, 9, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Stuber, M.L.; Meeske, K.A.; Krull, K.R.; Leisenring, W.; Stratton, K.; Kazak, A.E.; Huber, M.; Zebrack, B.; Uijtdehaage, S.H.; Mertens, A.C.; et al. Prevalence and Predictors of Posttraumatic Stress Disorder in Adult Survivors of Childhood Cancer. Pediatrics 2010, 125, e1124–e1134. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, K.K.; Doukkali, E.; Lampic, C.; Eriksson, L.E.; Arvidson, J.; Wettergren, L. Long-Term Survivors of Childhood Cancer Report Quality of Life and Health Status in Parity with a Comparison Group. Pediatr. Blood Cancer 2010, 55, 337–343. [Google Scholar] [CrossRef]
- Sundberg, K.K.; Wettergren, L.; Frisk, P.; Arvidson, J. Self-Reported Quality of Life in Long-Term Survivors of Childhood Lymphoblastic Malignancy Treated with Hematopoietic Stem Cell Transplantation versus Conventional Therapy. Pediatr. Blood Cancer 2013, 60, 1382–1387. [Google Scholar] [CrossRef]
- Szilagy, I.S.; Nagele, E.; Fürschuß, C.; Mohapp, A.; Wiegele, K.; Lackner, H.; Urban, C. Influencing Factors on Career Choice and Current Occupation Analysis of Adult Survivors of Childhood Cancer: A Special Focus on Health-Related Occupations. Memo Mag. Eur. Med. Oncol. 2019, 12, 83–90. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Bromberg, C.; Piedmonte, M. Long-term Vocational Adjustment of Cancer Patients Diagnosed during Adolescence. Cancer 1989, 63, 213–218. [Google Scholar] [CrossRef]
- Chan, C.W.H.; Choi, K.C.; Chien, W.T.; Sit, J.W.H.; Wong, R.; Cheng, K.K.F.; Li, C.K.; Yuen, H.L.; Li, C.K. Health Behaviors of Chinese Childhood Cancer Survivors: A Comparison Study with Their Siblings. Int. J. Environ. Res. Public Health 2020, 17, 6136. [Google Scholar] [CrossRef]
- Tremolada, M.; Schiavo, S.; Varotto, S.; Basso, G.; Pillon, M. Patient Satisfaction in Italian Childhood Cancer Survivors: Human Aspects of Treatment as a Key Factor in Patients’ Quality of Life. Health Soc. Work 2015, 40, e148–e155. [Google Scholar] [CrossRef]
- Tremolada, M.; Bonichini, S.; Basso, G.; Pillon, M. Perceived Social Support and Health-Related Quality of Life in AYA Cancer Survivors and Controls. Psychooncology 2016, 25, 1408–1417. [Google Scholar] [CrossRef]
- Van Erp, L.M.E.; Maurice-Stam, H.; Kremer, L.C.M.; Tissing, W.J.E.; van der Pal, H.J.H.; Beek, L.; de Vries, A.C.H.; van den Heuvel-Eibrink, M.M.; Versluys, B.A.B.; van der Heiden-van der Loo, M.; et al. Support Needs of Dutch Young Adult Childhood Cancer Survivors. Support. Care Cancer 2022, 30, 3291–3302. [Google Scholar] [CrossRef]
- Wengenroth, L.; Sommer, G.; Schindler, M.; Spycher, B.D.; Von Der Weid, N.X.; Stutz-Grunder, E.; Michel, G.; Kuehni, C.E.; Angst, R.; Ansari, M.; et al. Income in Adult Survivors of Childhood Cancer. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Winterling, J.; Johansson, E.; Wettergren, L.; Ljungman, P.; Alexanderson, K. Occupational Status among Adult Survivors Following Allogeneic Stem Cell Transplantation in Childhood. Eur. J. Cancer Care 2018, 27, e12808. [Google Scholar] [CrossRef]
- Yağci-Küpeli, B.; Yalçin, B.; Küpeli, S.; Varan, A.; Akyüz, C.; Kutluk, T.; Büyükpamukçu, M. Educational Achievement, Employment, Smoking, Marital, and Insurance Statuses in Long-Term Survivors of Childhood Malignant Solid Tumors. J. Pediatr. Hematol. Oncol. 2013, 35, 129–133. [Google Scholar] [CrossRef]
- Zebrack, B.J.; Stuber, M.L.; Meeske, K.A.; Phipps, S.; Krull, K.R.; Liu, Q.; Yasui, Y.; Parry, C.; Hamilton, R.; Robison, L.L.; et al. Perceived Positive Impact of Cancer among Long-Term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Psychooncology 2012, 21, 630–639. [Google Scholar] [CrossRef]
- Zeltzer, L.K.; Lu, Q.; Leisenring, W.; Tsao, J.C.I.; Recklitis, C.; Armstrong, G.; Mertens, A.C.; Robison, L.L.; Ness, K.K. Psychosocial Outcomes and Health-Related Quality of Life in Adult Childhood Cancer Survivors: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 435–446. [Google Scholar] [CrossRef]
- Zeltzer, L.K.; Chen, E.; Weiss, R.; Guo, M.D.; Robison, L.L.; Meadows, A.T.; Mills, J.L.; Nicholson, H.S.; Byrne, J. Comparison of Psychologic Outcome in Adult Survivors of Childhood Acute Lymphoblastic Leukemia versus Sibling Controls: A Cooperative Children’s Cancer Group and National Institutes of Health Study. J. Clin. Oncol. 1997, 15, 547–556. [Google Scholar] [CrossRef]
- Chaume, A.G.; Berger, C.; Cathébras, P. Séquelles et Qualité de Vie Chez de Jeunes Adultes Survivants de Cancers Pédiatriques. Rev. Med. Interne 2007, 28, 450–457. [Google Scholar] [CrossRef]
- De Boer, A.G.E.M.; Verbeek, J.H.A.M.; van Dijk, F.J.H. Adult Survivors of Childhood Cancer and Unemployment. Cancer 2006, 107, 1–11. [Google Scholar] [CrossRef]
- Michel, G.; Mulder, R.L.; van der Pal, H.J.H.; Skinner, R.; Bárdi, E.; Brown, M.C.; Vetsch, J.; Frey, E.; Windsor, R.; Kremer, L.C.M.; et al. Evidence-Based Recommendations for the Organization of Long-Term Follow-up Care for Childhood and Adolescent Cancer Survivors: A Report from the PanCareSurFup Guidelines Working Group. J. Cancer Surviv. 2019, 13, 759–772. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Mann, K.; Kaul, S.; Zamora, E.R.; Smits-Seemann, R.R.; Kirchhoff, A.C. Follow-Up Care Provider Preferences of Adolescent and Young Adult Cancer Survivors. J. Adolesc. Young Adult Oncol. 2018, 7, 204–209. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.G.E.M.; Taskila, T.; Ojajärvi, A.; van Dijk, F.J.H.; Verbeek, J.H.A.M. Cancer Survivors and Unemployment: A Meta-Analysis and Meta-Regression. JAMA 2009, 301, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ketterl, T.G.; Syrjala, K.L.; Casillas, J.; Jacobs, L.A.; Palmer, S.C.; McCabe, M.S.; Ganz, P.A.; Overholser, L.; Partridge, A.; Rajotte, E.J.; et al. Lasting Effects of Cancer and Its Treatment on Employment and Finances in Adolescent and Young Adult Cancer Survivors. Cancer 2019, 125, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Barlow-Krelina, E.; Chen, Y.; Yasui, Y.; Till, C.; Gibson, T.M.; Ness, K.K.; Leisenring, W.M.; Howell, R.M.; Nathan, P.C.; Oeffinger, K.C.; et al. Consistent Physical Activity and Future Neurocognitive Problems in Adult Survivors of Childhood Cancers: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2020, 38, 2041–2052. [Google Scholar] [CrossRef]
- DeNunzio, N.J.; Yock, T.I. Modern Radiotherapy for Pediatric Brain Tumors. Cancers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Ishida, Y.; Honda, M.; Ozono, S.; Okamura, J.; Asami, K.; Maeda, N.; Sakamoto, N.; Inada, H.; Iwai, T.; Kamibeppu, K.; et al. Late Effects and Quality of Life of Childhood Cancer Survivors: Part 1. Impact of Stem Cell Transplantation. Int. J. Hematol. 2010, 91, 865–876. [Google Scholar] [CrossRef]
- Currie, J.; Madrian, B. Health, Health Insurance and the Labor Market Amsterdam. In Handbook of Labor Economics; Elsevier: Amsterdam, The Netherlands, 1999; Volume 3, pp. 3309–3416. [Google Scholar]
- Ghaderi, S.; Engeland, A.; Moster, D.; Ruud, E.; Syse, A.; Wesenberg, F.; Bjørge, T. Increased Uptake of Social Security Benefits among Long-Term Survivors of Cancer in Childhood, Adolescence and Young Adulthood: A Norwegian Population-Based Cohort Study. Br. J. Cancer 2013, 108, 1525–1533. [Google Scholar] [CrossRef]
- Sieswerda, E.; Font-Gonzalez, A.; Reitsma, J.B.; Dijkgraaf, M.G.W.; Heinen, R.C.; Jaspers, M.W.; van der Pal, H.J.; van Leeuwen, F.E.; Caron, H.N.; Geskus, R.B.; et al. High Hospitalization Rates in Survivors of Childhood Cancer: A Longitudinal Follow-Up Study Using Medical Record Linkage. PLoS ONE 2016, 11, e0159518. [Google Scholar] [CrossRef]
- Hjern, A.; Lindblad, F.; Boman, K.K. Disability in Adult Survivors of Childhood Cancer: A Swedish National Cohort Study. J. Clin. Oncol. 2007, 25, 5262–5266. [Google Scholar] [CrossRef]
- Feuerstein, M.; Gehrke, A.K.; McMahon, B.T.; McMahon, M.C. Challenges Persist Under Americans With Disabilities Act Amendments Act: How Can Oncology Providers Help? J. Oncol. Pract. 2017, 13, e543–e551. [Google Scholar] [CrossRef]
- OECD Employment Outlook 2021; OECD Employment Outlook; OECD: Paris, France, 2021; ISBN 9789264708723.
- Molcho, M.; D’Eath, M.; Alforque Thomas, A.; Sharp, L. Educational Attainment of Childhood Cancer Survivors: A Systematic Review. Cancer Med. 2019, 8, 3182–3195. [Google Scholar] [CrossRef]
- Card, D. The Causal Effect of Education on Earnings. In Handbook of Labor Economics; Ashenfelter, O., Ed.; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Lown, E.A.; Phillips, F.; Schwartz, L.A.; Rosenberg, A.R.; Jones, B. Psychosocial Follow-Up in Survivorship as a Standard of Care in Pediatric Oncology. Pediatr. Blood Cancer 2015, 62, S514–S584. [Google Scholar] [CrossRef]
- Stone, D.S.; Ganz, P.A.; Pavlish, C.; Robbins, W.A. Young Adult Cancer Survivors and Work: A Systematic Review. J. Cancer Surviv. 2017, 11, 765–781. [Google Scholar] [CrossRef]
- Wiener, L.; Kazak, A.E.; Noll, R.B.; Patenaude, A.F.; Kupst, M.J. Standards for the Psychosocial Care of Children With Cancer and Their Families: An Introduction to the Special Issue. Pediatr. Blood Cancer 2015, 62, S419–S424. [Google Scholar] [CrossRef]
- Nathan, P.C.; Ford, J.S.; Henderson, T.O.; Hudson, M.M.; Emmons, K.M.; Casillas, J.N.; Lown, E.A.; Ness, K.K.; Oeffinger, K.C. Health Behaviors, Medical Care, and Interventions to Promote Healthy Living in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2009, 27, 2363–2373. [Google Scholar] [CrossRef]
- Torp, S.; Nielsen, R.A.; Gudbergsson, S.B.; Fosså, S.D.; Dahl, A.A. Sick Leave Patterns among 5-Year Cancer Survivors: A Registry-Based Retrospective Cohort Study. J. Cancer Surviv. 2012, 6, 315–323. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-Analysis of Prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).