Pancreatic Cancer Cell-Derived Exosomes Promote Lymphangiogenesis by Downregulating ABHD11-AS1 Expression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Clinical Samples and Study Approval
2.2. Cell Lines and Cell Culture
2.3. Exosome Isolation, Characterization, and Treatment
2.4. Transmission Electron Microscopy
2.5. Wound Healing Assay
2.6. Transwell Migration Assay
2.7. Colony Formation Assay
2.8. Cell Counting Kit 8
2.9. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.10. Western Blotting
2.11. Immunofluorescent Staining
2.12. Immunohistochemical and Fluorescence In Situ Hybridization (FISH)
2.13. ABHD11-AS1 cDNA and siRNA Transfection
2.14. Statistical Analyses
3. Results
3.1. Pancreatic Cancer Exosome Isolation and Purification
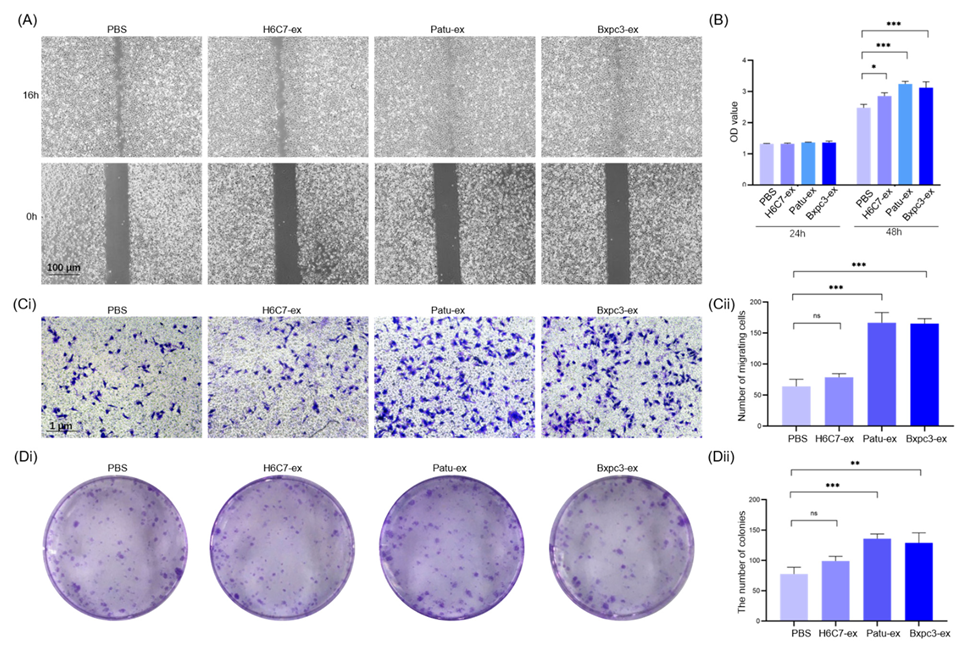
3.2. Exosomes Derived from Pancreatic Cancer Cells Promote the Proliferation and Migration of Lymphatic Endothelial Cells
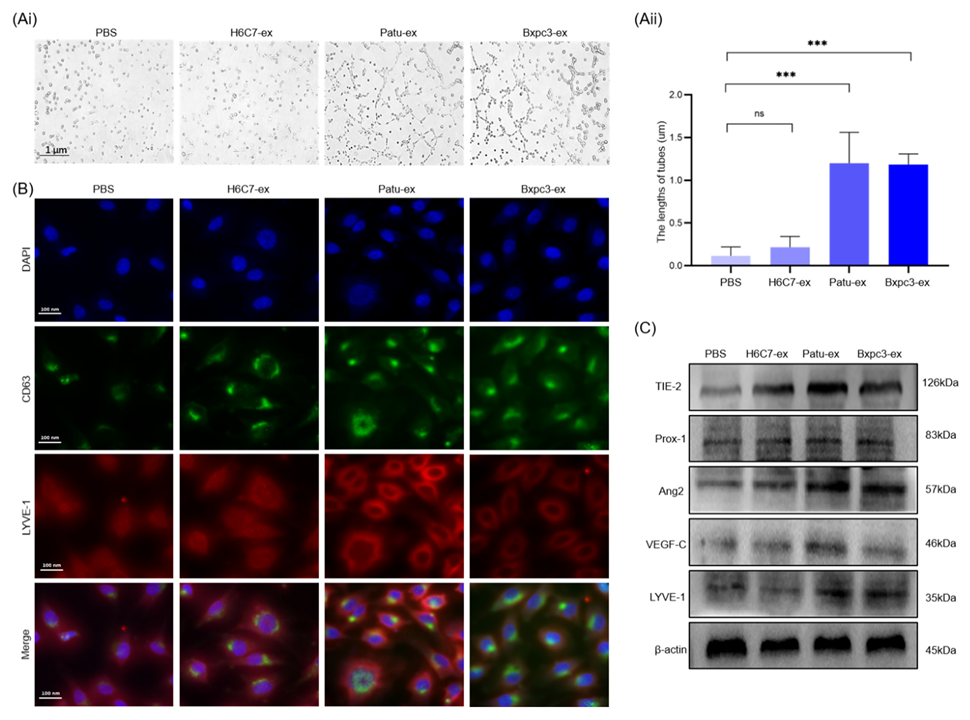
3.3. Pancreatic Cancer Cell-Derived Exosomes Promote the Tube Formation Surrounding Lymphatic Endothelial Cells
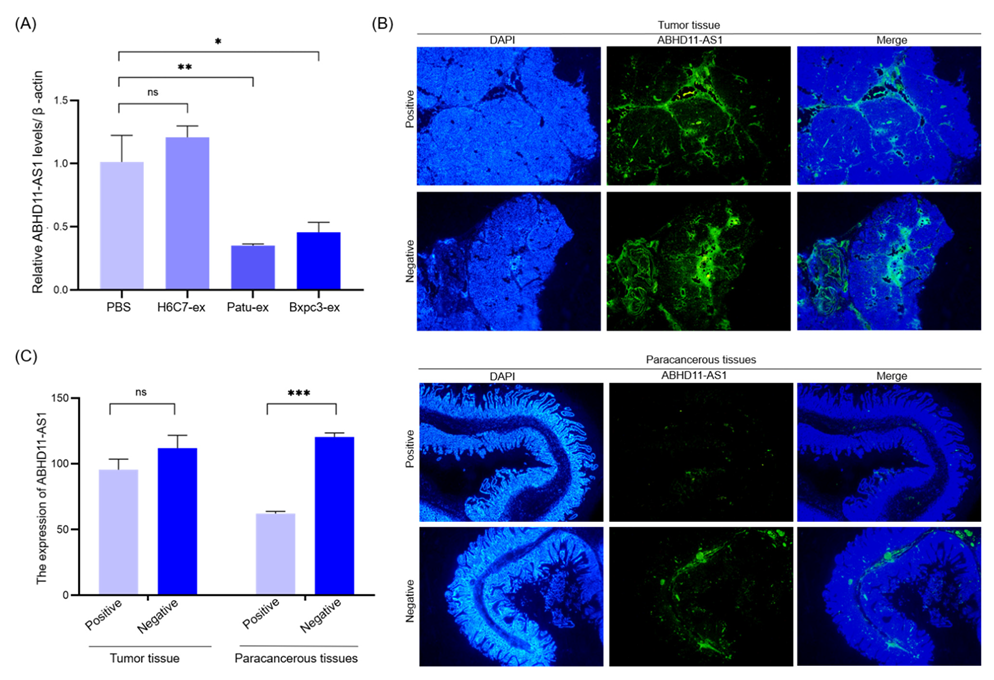
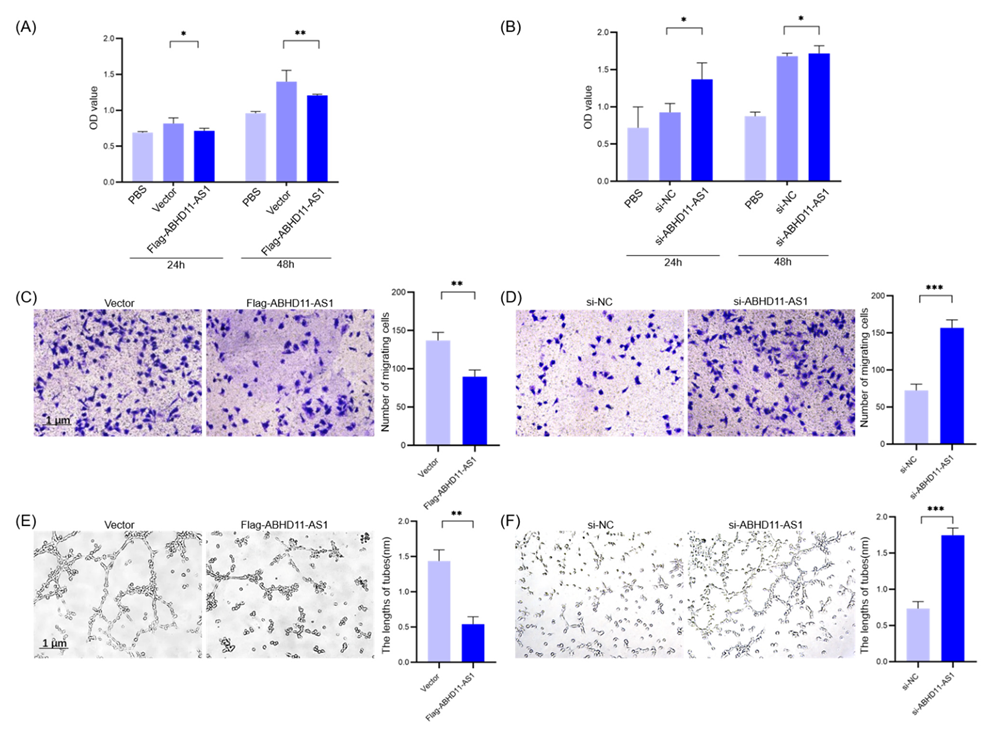
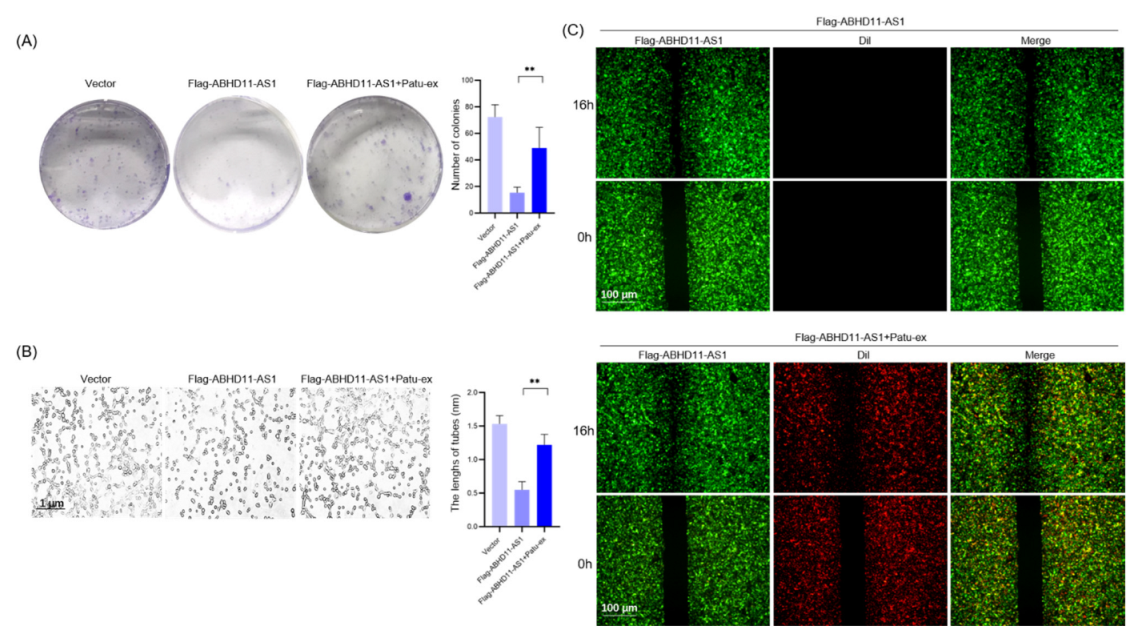
3.4. Pancreatic Cancer Cell-Derived Exosomes Promote Tube Formation by Downregulating ABHD11-AS1
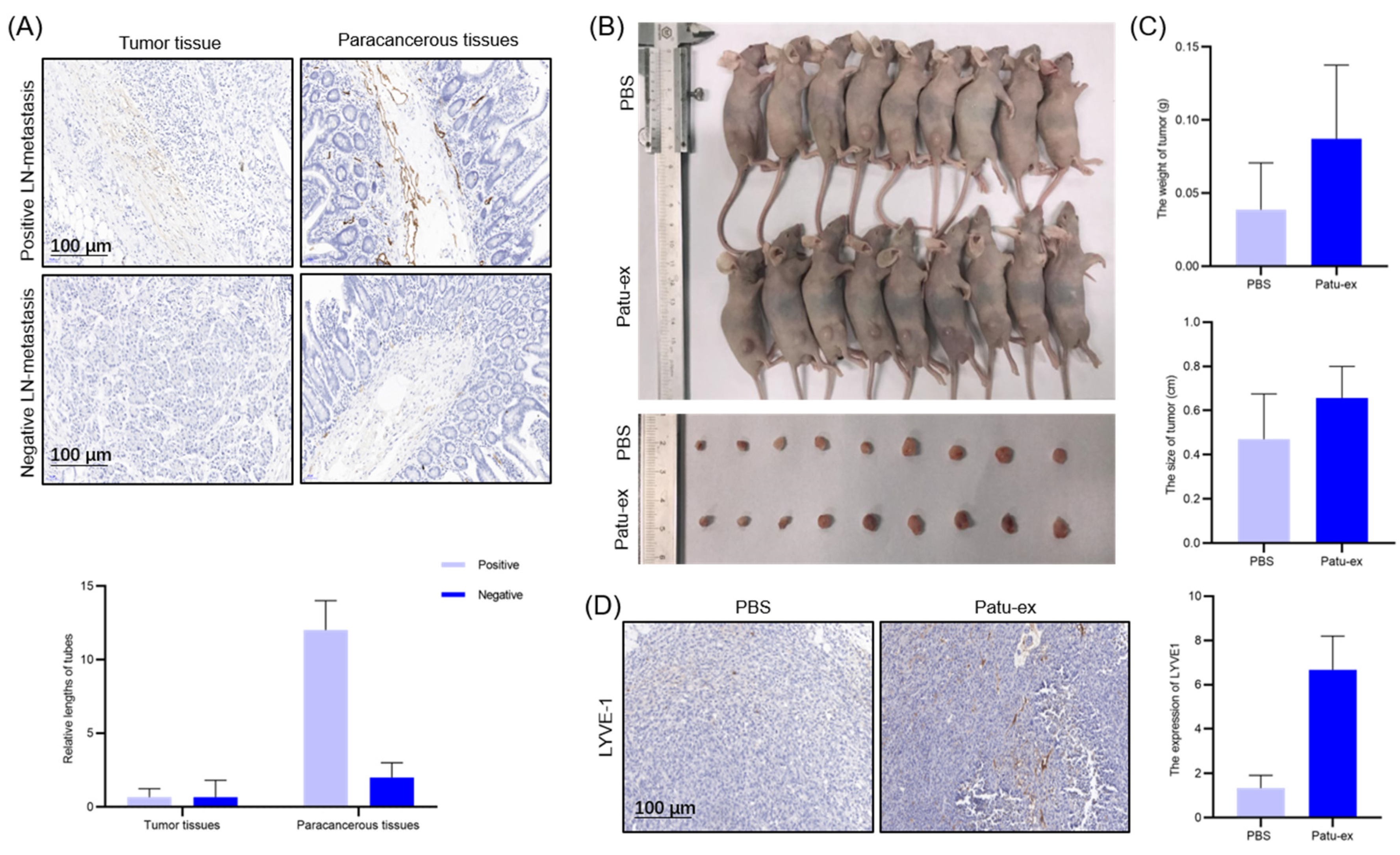
3.5. Pancreatic Cancer Cell-Derived Exosomes Can Promote Pancreatic Cancer Cell Proliferation and Lymphatic Metastasis In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.; Chang, D.; Kassahn, K.; Bailey, P.; Johns, A.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Shi, L.; Ye, Y.; Shi, H.; Zeng, L.; Tiwary, S.; Huse, J.; Huo, L.; Ma, L.; Ma, Y.; et al. YTHDF3 Induces the Translation of mA-Enriched Gene Transcripts to Promote Breast Cancer Brain Metastasis. Cancer Cell 2020, 38, 857–871.e857. [Google Scholar] [CrossRef]
- Kim, O.; Hwangbo, C.; Tran, P.; Lee, J. Syntenin-1-mediated small extracellular vesicles promotes cell growth, migration, and angiogenesis by increasing onco-miRNAs secretion in lung cancer cells. Cell Death Dis. 2022, 13, 122. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.; Grogan, T.; Elashoff, D.; Ye, H.; Cohen, P.; Aronson, W. Effects of dietary omega-3 fatty acids on orthotopic prostate cancer progression, tumor associated macrophages, angiogenesis and T-cell activation-dependence on GPR120. Prostate Cancer Prostatic Dis. 2022, 25, 539–546. [Google Scholar] [CrossRef]
- Wang, S.; Nie, L.; Song, Y.; Zhang, F.; Chen, X.; Shi, W.; Yang, Z.; Sun, Y.; Dang, Q.; Gao, A. Neurturin promotes tumor cell motility and angiogenesis in colorectal cancer. Exp. Cell Res. 2022, 413, 113049. [Google Scholar] [CrossRef]
- Hunter, K.; Palermo, C.; Kester, J.; Simpson, K.; Li, J.; Tang, L.; Klimstra, D.; Vlodavsky, I.; Joyce, J. Heparanase promotes lymphangiogenesis and tumor invasion in pancreatic neuroendocrine tumors. Oncogene 2014, 33, 1799–1808. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Li, H.; Chen, J.; Ren, L.; Wu, C. KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 87–92. [Google Scholar] [CrossRef]
- Shi, K.; Queiroz, K.; Roelofs, J.; van Noesel, C.; Richel, D.; Spek, C. Protease-activated receptor 2 suppresses lymphangiogenesis and subsequent lymph node metastasis in a murine pancreatic cancer model. J. Pathol. 2014, 234, 398–409. [Google Scholar] [CrossRef]
- Keklikoglou, I.; Hosaka, K.; Bender, C.; Bott, A.; Koerner, C.; Mitra, D.; Will, R.; Woerner, A.; Muenstermann, E.; Wilhelm, H.; et al. MicroRNA-206 functions as a pleiotropic modulator of cell proliferation, invasion and lymphangiogenesis in pancreatic adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene 2015, 34, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, Y.; Luo, Y.; Zhu, J.; Zheng, H.; Gao, B.; Guo, X.; Li, Z.; Chen, R.; Chen, C. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol. Cancer 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.; Imasuen-Williams, I.; Conteh, A.; Craven, K.; Cheng, M.; Korc, M. Combined targeting of TGF-β, EGFR and HER2 suppresses lymphangiogenesis and metastasis in a pancreatic cancer model. Cancer Lett. 2016, 379, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.; Simpson, R. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut 2022, 71, 568–579. [Google Scholar] [CrossRef]
- Yao, X.; Mao, Y.; Wu, D.; Zhu, Y.; Lu, J.; Huang, Y.; Guo, Y.; Wang, Z.; Zhu, S.; Li, X.; et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 2021, 512, 38–50. [Google Scholar] [CrossRef]
- Ruivo, C.F.; Bastos, N.; Adem, B.; Batista, I.; Duraes, C.; Melo, C.A.; Castaldo, S.A.; Campos-Laborie, F.; Moutinho-Ribeiro, P.; Morão, B.; et al. Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression. Gut 2022, 71, 2043–2068. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, Z.; Lin, Q.; Yang, B.; Yan, F.; Liu, B.; Chen, H.; Kong, J. Surface Plasmon Coupling Electrochemiluminescence Immunosensor Based on Polymer Dots and AuNPs for Ultrasensitive Detection of Pancreatic Cancer Exosomes. Anal. Chem. 2022, 94, 837–846. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Sun, S.; Ding, H.; Lan, L.; Li, X.; Han, S. Knockdown of lncRNA ABHD11-AS1 Suppresses the Tumorigenesis of Pancreatic Cancer via Sponging miR-1231. OncoTargets Ther. 2020, 13, 11347–11358. [Google Scholar] [CrossRef]
- Zhuang, X.; Tong, H.; Ding, Y.; Wu, L.; Cai, J.; Si, Y.; Zhang, H.; Shen, M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Jiang, X.; Yong, J.; Xie, H.; Yuan, J.; Zeng, D.; Dou, Y.; Xiao, S. lncRNA ABHD11-AS1, regulated by the EGFR pathway, contributes to the ovarian cancer tumorigenesis by epigenetically suppressing TIMP2. Cancer Med. 2019, 8, 7074–7085. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, J.; Lin, Y.; Liu, D.; Yang, Q.; Jian, J.; Peng, J. m6A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J. Cell. Physiol. 2021, 236, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Yan, Z.; Cao, J. Long non-coding RNA ABHD11-AS1 boosts gastric cancer development by regulating miR-361-3p/PDPK1 signalling. J. Biochem. 2020, 168, 465–476. [Google Scholar] [CrossRef]
- He, D.; Yue, Z.; Liu, L.; Fang, X.; Chen, L.; Han, H. Long noncoding RNA ABHD11-AS1 promote cells proliferation and invasion of colorectal cancer via regulating the miR-1254-WNT11 pathway. J. Cell. Physiol. 2019, 234, 12070–12079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, W.; Liu, W.; Kong, X.; Li, L.; He, J.; Wang, D.; Zhang, M.; Zhou, G.; Xu, W.; et al. Circulating lncRNA ABHD11-AS1 serves as a biomarker for early pancreatic cancer diagnosis. J. Cancer 2019, 10, 3746–3756. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Dore, M.P.; Sepulveda, A.R.; Pedroni, A.; Realdi, G.; Delitala, G. Reversal of elevated pancreatic enzymes after Helicobacter pylori eradication. Intern. Emerg. Med. 2008, 3, 269–270. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Bai, Y.; Zhi, F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 2019, 509, 767–772. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Clos-Garcia, M.; Telleria, O.; Nafría, B.; Alonso, C.; Iruarrizaga-Lejarreta, M.; Franke, A.; Crespo, A.; Iglesias, A.; Cubiella, J.; et al. Interplay between Genome, Metabolome and Microbiome in Colorectal Cancer. Cancers 2021, 13, 6216. [Google Scholar] [CrossRef]
- Jiang, J.W.; Chen, X.H.; Ren, Z.; Zheng, S.S. Gut microbial dysbiosis associates hepatocellular carcinoma via the gut-liver axis. Hepatobiliary Pancreat Dis. Int. 2019, 18, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 2020, 41, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Zhang, B.; Huang, S.; Li, P.; He, X.; Cao, G.; Kang, M.; Dong, X.; Wu, Y. Pancreatic cancer-derived exosomes promoted pancreatic stellate cells recruitment by pancreatic cancer. J. Cancer 2019, 10, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.; Roy, A.; Rajpoot, S.; Liu, D.; Savai, R.; Banerjee, S.; Kawada, M.; Faisal, S.; Saluja, R.; Saqib, U.; et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020, 69, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, P.; Li, J.; Qi, Q.; Sun, Z.; Shi, S.; Xie, Y.; Liu, S.; Wang, Y.; Du, L.; et al. Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Mol. Ther. Oncolytics 2021, 23, 163–180. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, J.; Huang, L.; Yi, H.; Zhang, Y.; Wu, X.; Yan, R.; Liang, L.; Zhong, M.; Yu, Y.; et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 2019, 38, 1256–1268. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Xue, X.; Sun, D.; Cai, P.; Song, Q.; Zhang, B.; Qin, L. HANR promotes lymphangiogenesis of hepatocellular carcinoma via secreting miR-296 exosome and regulating EAG1/VEGFA signaling in HDLEC cells. J. Cell. Biochem. 2019, 120, 17699–17708. [Google Scholar] [CrossRef]
- Meller, V.H.; Joshi, S.S.; Deshpande, N. Modulation of Chromatin by Noncoding RNA. Annu. Rev. Genet. 2015, 49, 673–695. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, C.; Chen, Y.; Ruan, Y.; Fan, L.; Chen, Q.; Wei, Q. LncRNA ABHD11-AS1 promotes tumor progression in papillary thyroid carcinoma by regulating EPS15L1/EGFR signaling pathway. Clin. Transl. Oncol. 2022, 24, 1124–1133. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, X.; Yang, J. Long non-coding RNA ABHD11-AS1 facilitates the progression of cervical cancer by competitively binding to miR-330-5p and upregulating MARK2. Exp. Cell Res. 2022, 410, 112929. [Google Scholar] [CrossRef]
- Mehrpour Layeghi, S.; Arabpour, M.; Shakoori, A.; Naghizadeh, M.; Mansoori, Y.; Tavakkoly Bazzaz, J.; Esmaeili, R. Expression profiles and functional prediction of long non-coding RNAs LINC01133, ZEB1-AS1 and ABHD11-AS1 in the luminal subtype of breast cancer. J. Transl. Med. 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, Y.; Wu, L.; Zhuo, D.; Zhang, S.; Jiang, X.; Sun, Y.; Huang, Y. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer progression and invasion through targeting the integrin subunit alpha 5/focal adhesion kinase/phosphoinositide 3 kinase/Akt signaling pathway. Aging 2021, 13, 20179–20191. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Q.; Liu, X.; Wang, F.; Yang, Y.; Tian, X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int. J. Biol. Sci. 2022, 18, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Sulidankazha, Q.; Nie, X.; Yilidan, R.; Len, K. Pancreatic cancer cells-derived exosomal long non-coding RNA CCAT1/microRNA-138-5p/HMGA1 axis promotes tumor angiogenesis. Life Sci. 2021, 278, 119495. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhong, F.; Yan, Y.; Wu, S.; Wang, H.; Liu, J.; Li, F.; Cui, D.; Xu, M. Pancreatic Cancer Cell-Derived Exosomes Promote Lymphangiogenesis by Downregulating ABHD11-AS1 Expression. Cancers 2022, 14, 4612. https://doi.org/10.3390/cancers14194612
Zhou X, Zhong F, Yan Y, Wu S, Wang H, Liu J, Li F, Cui D, Xu M. Pancreatic Cancer Cell-Derived Exosomes Promote Lymphangiogenesis by Downregulating ABHD11-AS1 Expression. Cancers. 2022; 14(19):4612. https://doi.org/10.3390/cancers14194612
Chicago/Turabian StyleZhou, Xulin, Fengyun Zhong, Yongmin Yan, Sihui Wu, Huizhi Wang, Junqiang Liu, Feifan Li, Dawei Cui, and Min Xu. 2022. "Pancreatic Cancer Cell-Derived Exosomes Promote Lymphangiogenesis by Downregulating ABHD11-AS1 Expression" Cancers 14, no. 19: 4612. https://doi.org/10.3390/cancers14194612
APA StyleZhou, X., Zhong, F., Yan, Y., Wu, S., Wang, H., Liu, J., Li, F., Cui, D., & Xu, M. (2022). Pancreatic Cancer Cell-Derived Exosomes Promote Lymphangiogenesis by Downregulating ABHD11-AS1 Expression. Cancers, 14(19), 4612. https://doi.org/10.3390/cancers14194612







