Simple Summary
Triple negative breast cancer (TNBC) has been a leading cause of cancer deaths among women and limited treatment options are currently available. Consequently, developing the promising therapy for TNBC remains an unmet medical need. The aim of this study was to assess the effect of Hsp70 inhibitors on breast cancer stem cells (BCSCs) in TNBC cells. The present study demonstrated the potent in vitro and in vivo anti-BCSCs activity of two Hsp70 inhibitors, compound 1 and 6, in TNBC cells. Our findings suggest that targeting Hsp70 could be an attractive strategy for eliminating BCSCs populations in TNBC.
Abstract
Triple negative breast cancer (TNBC) is considered the most aggressive breast cancer with high relapse rates and poor prognosis. Although great advances in the development of cancer therapy have been witnessed over the past decade, the treatment options for TNBC remain limited. In this study, we investigated the effect and potential underlying mechanism of the Hsp70 inhibitors, compound 1 and compound 6, on breast cancer stem cells (BCSCs) in TNBC cells. Our results showed that compound 1 and 6 exhibited potent tumor suppressive effects on cell viability and proliferation, and effectively inhibited BCSC expansion in TNBC cells. Reminiscent with the effect of Hsp70 inhibitors, Hsp70 knockdown effectively suppressed mammosphere formation and the expressions of BCSCs surface markers. Mechanistically, evidence showed that the Hsp70 inhibitors inhibited BCSCs by down-regulating β-catenin in TNBC cells. Moreover, we used the Hsp70 inhibitors treated TNBC cells and a stable Hsp70 knockdown clone of MDA-MB-231 cells to demonstrate the in vivo efficacy of Hsp70 inhibition in suppressing tumorigenesis and xenograft tumor growth. Together, these findings suggest the potential role of Hsp70 as a target for TNBC therapy and foster new therapeutic strategies to eliminate BCSCs by targeting Hsp70.
1. Introduction
Triple negative breast cancer (TNBC) is a subset of breast cancer, which is deficient in the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2); therefore, TNBC does not respond to hormonal and anti-HER2 antibody therapies [1]. TNBC accounts for 15–20% of all breast cancer cases. TNBC is usually more aggressive, with frequent metastasis, and has a relatively poor clinical outcome and higher grade than other types of breast cancer [2]. The massive heterogeneity of TNBC and the lack of defined molecular targets make it challenging to treat.
Cancer stem cells (CSCs), alternatively tumor-initiating cells, are the small subpopulation within cancer cells possessing the capacity to self-renew and to recapitulate the heterogeneity of the original tumor, which is one of the reasons contributing to cancer treatment failure and disease progression [3]. Moreover, accumulating evidence indicates that CSCs are responsible for tumor initiation, recurrence, metastasis, prognosis, and resistance to conventional therapy [4,5]. Breast cancer stem cells (BCSCs) are defined as a subset of breast cancer cells exhibiting the properties of self-renewal and differentiation capacity, which are thought to contribute to tumor relapse and metastasis [6]. The cell surface markers CD44+/CD24− and high aldehyde dehydrogenase (ALDH) activity have been established as biomarkers for BCSCs isolation and characterization in vitro and in vivo [7,8]. BCSCs are the leading cause of tumor progression and therapy resistance that results in poor patient outcomes [9]. Therefore, selective targeting of BCSCs is a promising therapeutic strategy to abolish the progression of breast cancer, reduce the risk of recurrence, and improve patient outcome [10]. Additionally, aberrant activation of the Wnt/β-catenin, Hedgehog, and Notch signaling pathways has been implicated in regulating BCSCs [11,12,13]. Accordingly, targeting these pathways to eliminate BCSCs might have therapeutic potential in breast cancer treatment.
Heat shock proteins (HSPs) are a large family of molecular chaperones that are crucial in diverse cellular processes by participating in DNA repair processes and acting as regulators of protein homeostasis, including folding, assembly/maturation, secretion, transportation, translocation, and stability [14,15]. The stress-inducible heat shock protein 70 (Hsp70) participates in the protection of proteins from aggregation and promotes refolding and degradation of damaged polypeptides [16,17]. Overexpression of Hsp70 has been observed in multiple forms of cancers, including breast, colon, liver, prostate, esophagus, and cervix [18,19,20,21,22,23], and correlates with tumor grade, metastasis, chemotherapy resistance and poor prognosis [19]. Moreover, elevated expression of Hsp70 has been shown to increase the tumorigenicity of cancer [24] and protect cancer cells from apoptosis in response to a variety conditions of survival stresses, for example, hypoxia, nutrient deprivation or anticancer treatment [25,26,27]. Hence, Hsp70 has emerged as a promising target for anticancer drug development. Indeed, various Hsp70 inhibitors have shown potent effectiveness and are currently in preclinical evaluation [28,29]. Since inactivation of the Hsp70 gene has been found to inhibit the tumorigenesis, invasion and metastasis of mammary tumors in vivo [30], targeting Hsp70 could be an attractive strategy for eliminating BCSCs populations in breast cancer.
Previously, based on the screening of a novel series of rhodacyanine-based Hsp70 inhibitors, we identified that compound 1 and compound 6 exhibited high potency to inhibit Hsp70’s chaperone activity and antiproliferative activities against breast cancer cells [31]. Equally important, in contrast to the reported effect of Hsp90 inhibitors on Hsp70 upregulation, compounds 1 and 6 did not cause a compensatory elevation in Hsp90 expression [31]. Although the pre-clinical developments of Hsp70 inhibitors as a single agent or in combination with other drugs in cancer therapy are ongoing [28,32], the direct effect of Hsp70 inhibitors in targeting BCSCs remains unknown. The present study aimed to elucidate the effect of the Hsp70 inhibitors, compound 1 and compound 6, on BCSCs and the underlying mechanism of Hsp70 inhibition for maintaining breast cancer stemness in TNBC cells. Our results demonstrated that compounds 1 and 6 exhibited not only potent cytotoxicity and anti-proliferative activity against TNBC cells, but also high efficacy in inhibiting BCSCs characteristics, such as mammosphere-forming capacity and CSC-associated markers. Reminiscent with the effect of compounds 1 and 6 on BCSCs, genetic knockdown of Hsp70 also resulted in inhibition of BCSCs in TNBC MDA-MB-231 cells. Mechanistically, β-catenin is identified to be the potential target of Hsp70 inhibitors for maintaining the stemness properties of TNBC cells. Moreover, Hsp70 inhibition in suppressing tumorigenesis and xenograft tumor growth were further confirmed in vivo using Hsp70 inhibitors treated TNBC cells and a stable Hsp70 knockdown clone of MDA-MB-231 cells, respectively. Together, our findings provide and foster a novel anti-TNBC strategy to eliminate BCSCs by targeting Hsp70.
2. Materials and Methods
2.1. Cell Culture and Antibodies
Human breast cancer cell lines, MCF-7, T47D, MDA-MB-231, MDA-MB-468, HCC-1937, HCC-1806 and MCF-10A human mammary epithelial cells were obtained from the American Type Culture Collection (Manassas, VA, USA). These cell lines were maintained in recommended growth medium with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified incubator containing 5% CO2. The antibodies used in this study and their sources were as follows: Hsp70, Hsp90, β-Actin, β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); KLF4, NF-κB, Cyclin D1, Notch1 (Cell Signaling, Beverly, MA, USA); CD133, CD44 (GeneTex, Irvine, CA, USA).
2.2. Cell Viability Assay
The effects of the compound 1 and compound 6 on cell viability were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Cells were seeded in 96-well plates at a density of 5000 cells per well in the presence of 10% FBS. After overnight incubation, cells were exposed to test agents vis-à-vis vehicle in the presence of 5% FBS for 72 h. After treatment, cells were incubated with MTT (Biomatik, Wilmington, DE, USA) for an additional 1 h. The medium was then removed from each well and replaced with DMSO for subsequent colorimetric measurement of absorbance at 570 nm. Cell viabilities are expressed as percentages of viable cells relative to the corresponding vehicle-treated control group.
2.3. Colony Formation Assay
Cells were seeded in six-well plates at a density of 2 × 103 to 1 × 104 cells per well, left to attach overnight, and then incubated with media containing compound 1 or compound 6 for 7–14 days until colonies were visible. The colonies were fixed with 4% formaldehyde (Sigma-Aldrich, St. Louis, Mo, USA) and stained with crystal violet (5 mg/mL in 2% ethanol; Sigma-Aldrich). Colonies containing more than 50 cells were counted. Cell proliferation was determined from the numbers of colonies and expressed as a percentage relative to the corresponding vehicle-treated control group.
2.4. Mammosphere Formation Assay
A total of 2 × 103 MDA-MB-231 and 1 × 104 MDA-MB-468 cells/well were seeded in ultra-low attachment 24-well plates (Corning Inc., Union City, CA, USA) and maintained in MammoCult™ Human Medium (STEMCELL Technologies; Vancouver, BC, Canada) with test compounds. After 8–10 days, the formation of mammosphere was determined and expressed as percentage of CSCs relative to the corresponding vehicle-treated control group.
2.5. Stable Hsp70 Knockdown with shRNA Lentivirus
All of the lentiviral vectors and shRNAs against Hsp70 were obtained from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan). The lentivirus particles carrying control shRNA and Hsp70 shRNAs were generated by co-transfecting shRNA plasmids with lentiviral packaging plasmids pSPAX2 and pMD2.G into 293T cells for 72 h. Stable knockdown cell clones were established by infecting MDA-MB-231 cells with shRNA lentivirus followed by puromycin selection for 7–10 days.
2.6. Cell Migration Assay
The transwell cell migration assay using transwell chambers (8 µm pore-size, Millipore, Bedford, MA, USA) was performed to examine the migration ability of cells. Plate 3 × 104 cells in 300 μL of serum-free medium into the upper chambers of each transwell inserts in triplicate and then add 800 μL of medium with 10% FBS (Invitrogen) into the lower chambers. After 16–24 h incubation, the chambers were fixed in methanol and stained with Giemsa. The cells that migrated through the pores to the lower surface of the inserts were counted using a light microscope at 100× magnification in randomly selected fields of each independent experiment.
2.7. Plasmid and Transient Transfection
The human β-catenin plasmid was obtained from Addgene (Addgene plasmid #16518). Transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
2.8. Western Blot
Total protein lysates were collected and solubilized in lysis buffer (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitors on ice for 30 min and then sonicated for 1 min. Protein concentration was determined by using BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Total proteins were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking in 5% non-fat milk for 1 h, the membranes were incubated overnight at 4 °C with primary antibodies. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse antibody was applied as a secondary antibody for 1 h at room temperature. Protein detection was performed by Chemiluminescence Reagent Plus (Perkin-Elmer, Waltham, MA, USA). The relative intensity of the protein band was measured densitometrically using the Image J software. Original western blot images are found in Supplementary File S1.
2.9. In Vivo Study
All experimental procedures using mice were performed in accordance with the relevant guidelines and regulations and approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University. For the tumorigenicity model, DMSO, compound 1, or compound 6 treated MDA-MB-231 cells (1 × 103 living cells in 0.1 mL; 50% Matrigel in PBS) were subcutaneously injected into both flanks of the 8-week-old athymic nude mice obtained from National Laboratory Animal Center (NLAC, Taipei, Taiwan) (n = 6). After 12 weeks post-injection, the tumor incidence in mice receiving compound 1 or compound 6 treated cells was compared to the control DMSO group. For the xenograft tumor growth model, stable clone of control (Scrb) or Hsp70 knockdown (shHsp70 #755) MDA-MB-231 cells (1 × 106 cells in 0.1 mL; 50% Matrigel in PBS) was subcutaneously injected in 8-week-old athymic nude mice (n = 4). Tumor volumes were calculated weekly from caliper measurements (with2 × length × 0.52).
2.10. Statistical Analysis
The experiments in this study were performed using 3–6 replicates in each group. Most data are expressed as the mean ± standard deviation (S.D.) from at least two experiments. Differences in the effects of various treatments were compared using Student’s t-test. Differences were considered significant at p < 0.05.
3. Results
3.1. Hsp70 Expression Is Elevated in Breast Tumor Tissue and Negatively Correlated with Breast Cancer Prognosis
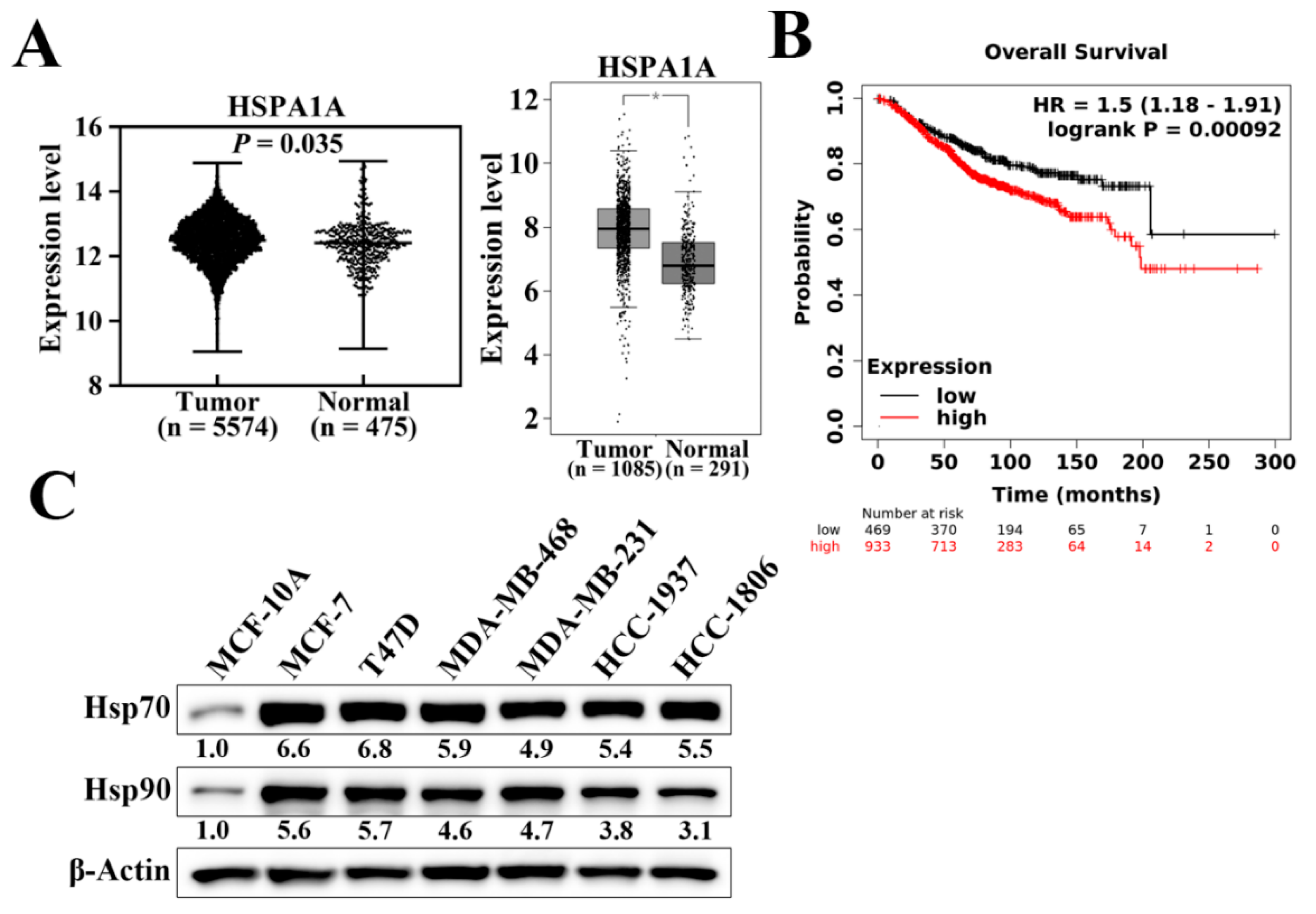
Hsp70 has been reported to be overexpressed and associated with enhanced resistance to chemotherapy and poor prognosis in various cancer types [33]. We analyzed the mRNA expression levels of Hsp70 (HSPA1A) in normal and tumoral breast tissues using two published databases, the Gene Expression database of Normal and Tumor tissues (GENT2) (Figure 1A, left panel) and Gene Expression Profiling Interactive Analysis (GEPIA) (Figure 1A, right panel). As shown, breast tumor samples exhibited significantly higher Hsp70 mRNA expression levels compared to corresponding normal tissues (Figure 1A). We then examined and analyzed the correlation between Hsp70 expression and clinical progression in breast cancer patients using the Kaplan–Meier plotter database [34]. Kaplan–Meier analysis showed that Hsp70 expression was negatively associated with overall survival (p = 9.2 × 10−4). Patients with higher Hsp70 mRNA expression had shorter overall survival time than those with lower Hsp70 expression (Figure 1B). In addition, the protein expression level of Hsp70 was higher in human breast cancer cell lines, including TNBC cell lines, than in MCF-10A normal mammary epithelial cells (Figure 1C) [31]. Taken together, these results suggested that Hsp70 expression is overexpressed in breast cancer and negatively associated with clinical breast cancer prognosis. Therefore, it shows that Hsp70 acts as a clinically relevant target for breast cancer treatment.
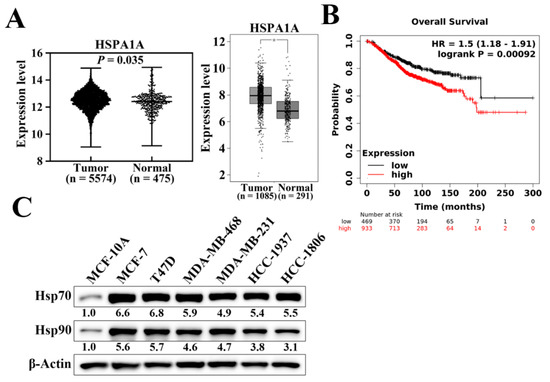
Figure 1.
Hsp70 gene and protein expression in clinical breast tissues and breast cancer cells. (A) Expression level of Hsp70 in breast cancer (left panel, GENT2 database, n = 5574 in cancerous tissue and n = 481 in normal tissue, p = 0.035; right panel, GEPIA expression database, n = 1085 in cancerous tissue and n = 291 in normal tissue, * p < 0.01). (B) Kaplan–Meier analysis of the prognostic value of Hsp70 mRNA expression in the dataset [Affymetrix ID, 202581_at (HSPA1A)]. Survival curves were plotted for all breast cancer patients (n = 1402). Cut-off value, median expression level. HR, hazard ratio (with 95% confidence interval). (C) Differential expression of Hsp70 and Hsp90 proteins in human breast cancer cell lines versus MCF10A normal human mammary cells. Values under Hsp70 and Hsp90 blots represent the relative expression compared to MCF-10A. The band intensity was measured densitometrically using Image J software and normalized to the β-Actin band.
3.2. Cytotoxic and Antiproliferative Activities of Compound 1 and Compound 6 in TNBC Cells
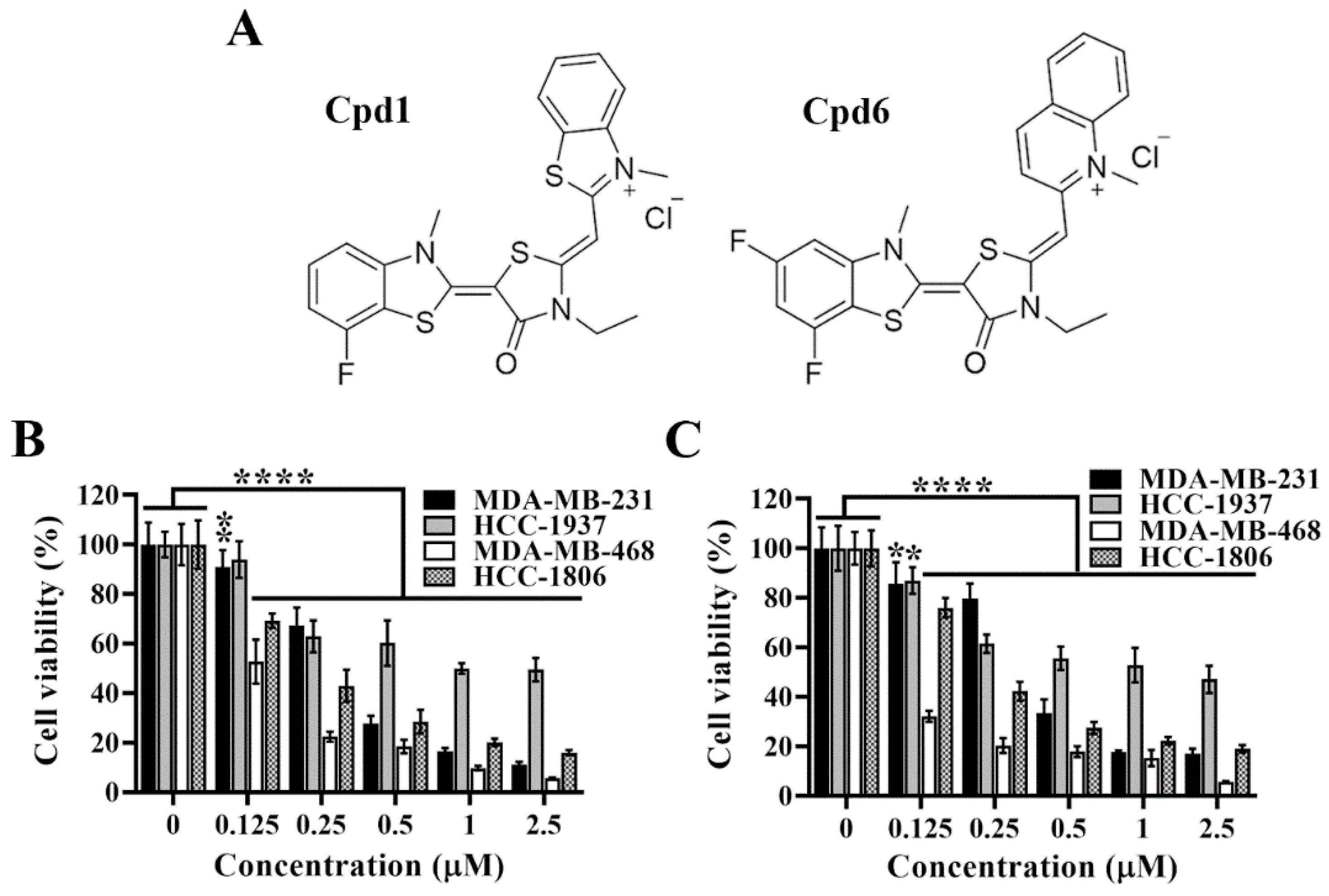
Pursuant to the previous study, we have developed two novel rhodacyanine-based Hsp70 inhibitors, represented by compound 1 and compound 6 (Figure 2A), that effectively inhibited the chaperone activity of Hsp70 and suppressed the expression of Hsp70’s client proteins [31]. We first investigated the cytotoxic activities of compounds 1 and 6 in four TNBC cell lines, and cell viability was determined by MTT assays. As shown, compound 1 (Figure 2B) and compound 6 (Figure 2C) significantly suppressed cell viability in a dose dependent manner in MDA-MB-231, HCC-1937, MDA-MB-468, and HCC-1806 cells.
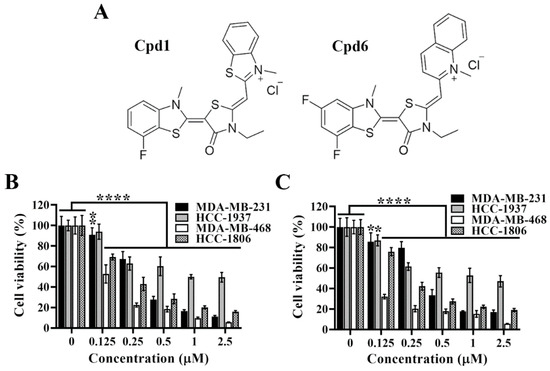
Figure 2.
Evidence that compound 1 and compound 6 exhibit high potency in suppressing triple negative breast cancer cells viability. (A) Chemical structures of compound 1 and compound 6. (B) Dose-dependent suppressive effects of compound 1 and (C) compound 6 on the viability of MDA-MB-231, HCC-1937, MDA-MB-468 and HCC-1806 cells after 72 h treatment. Cell viability was determined by MTT assays. Columns, means; bars, S.D., * p < 0.05, ** p < 0.01, **** p < 0.0001.
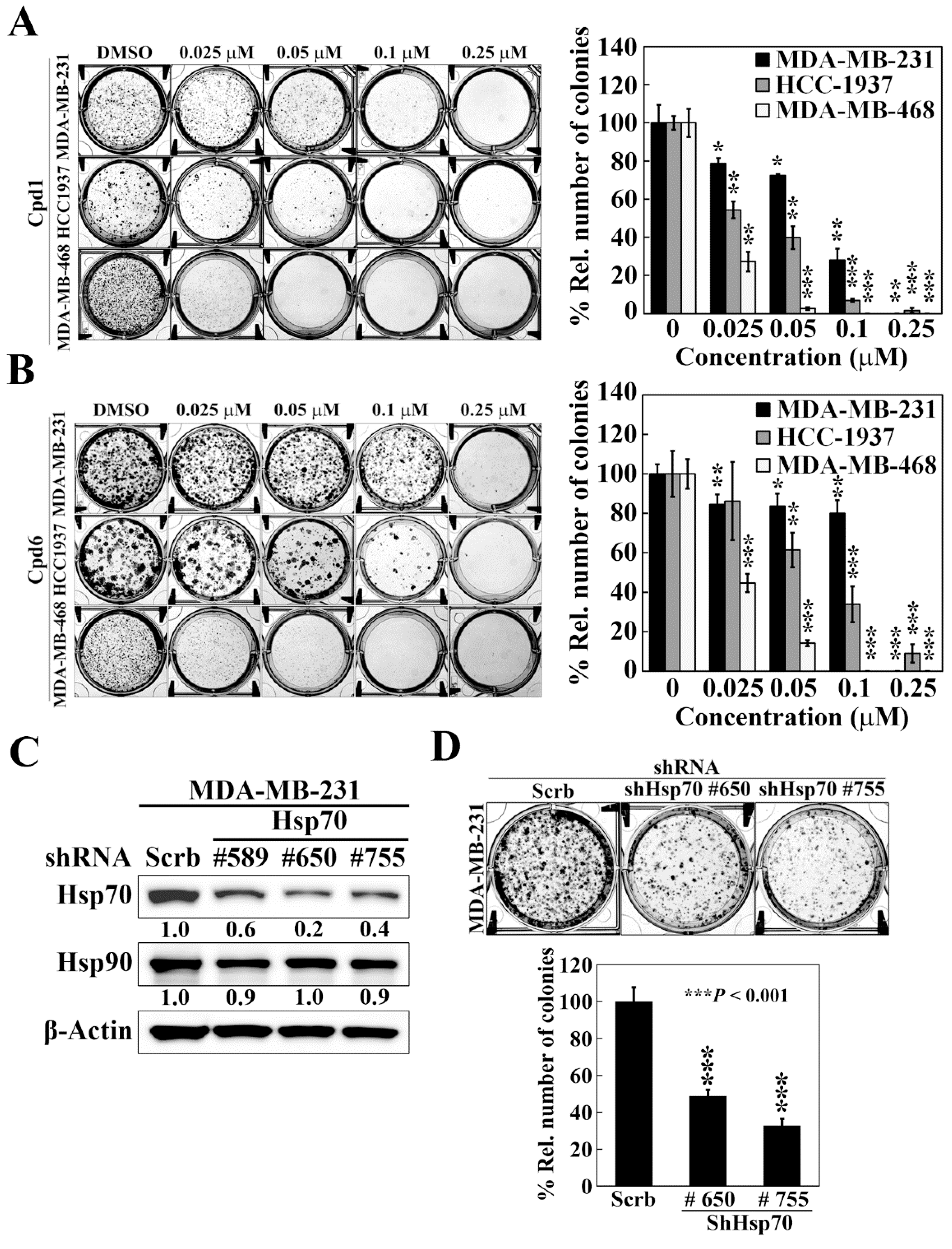
To shed light on the efficacy of Hsp70 inhibitors against TNBC cells growth, we further assessed the effect of compounds 1 and 6 on cell proliferation by colony formation assays. Compared to the control treatment of DMSO, both compound 1 and compound 6 significantly reduced colony formations in a dose dependent manner in MDA-MB-231, HCC-1937, and MDA-MB-468 cells (Figure 3A,B). Among the three TNBC cell lines, MDA-MB-468 cells showed the most sensitivity to Hsp70 inhibitors with 73% and 55% inhibition of colony formation at 0.025 μM by compound 1 and compound 6, respectively. These results suggested that inhibition of Hsp70 leads to suppression of TNBC cell growth. The role of Hsp70 in TNBC cell growth was further confirmed by genetic knockdown of Hsp70. We used three different shRNAs (#589, #650, and #755) to reduce Hsp70 expression, and two stable Hsp70 knockdown clones (shHsp70 #650 and shHsp70 #755) were selected to examine the consequent effects on colony formation in MDA-MB-231 cells (Figure 3C). Consistent with the effect of compounds 1 and 6 treatment, knockdown of Hsp70 could significantly suppress the colony forming ability of MDA-MB-231 cells (Figure 3D), suggesting that Hsp70 is involved in the proliferation of TNBC cells and targeting Hsp70 could be a promising option in the treatment of TNBC.
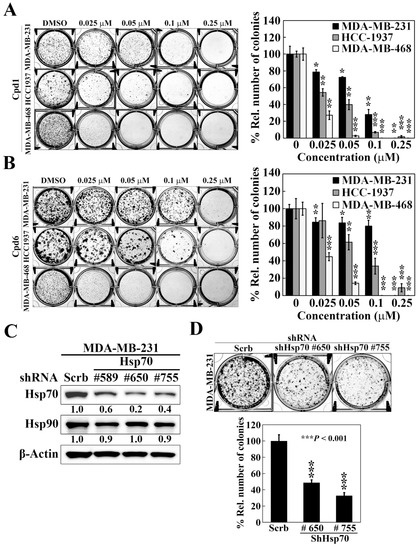
Figure 3.
Effects of compound 1, compound 6, and Hsp70 knockdown on cell proliferation in triple negative breast cancer cells. (A) Left, representative images showing the effect of compound 1 on colony formation in MDA-MB-231, HCC-1937, and MDA-MB-468 cells. Right, quantitative results of colony formation expressed as the percentage compared with the DMSO control. Data, means ± S.D., * p < 0.05, ** p < 0.01, *** p < 0.001. (B) Left, representative images showing the effect of compound 6 on colony formation in MDA-MB-231, HCC-1937, and MDA-MB-468 cells. Right, quantitative results of colony formation expressed as the percentage compared with the DMSO control. Data, means ± S.D., * p < 0.05, ** p < 0.01, *** p < 0.001. (C) Western blot analyses of the effect of stable knockdown of Hsp70 on the expression of Hsp70 and Hsp90 in MDA-MB-231 cells. Values under Hsp70 and Hsp90 blots represent the relative expression compared to control shRNA. The band intensity was measured densitometrically using Image J software and normalized to the β-Actin band. (D) Upper, representative images showing the effect of stable knockdown of Hsp70 on colony formation in MDA-MB-231 cells. Lower, quantitative results of colony formation from stable knockdown of Hsp70 in MDA-MB-231 cells. Data, means ± S.D., *** p < 0.001.
3.3. Suppressive Effect of Hsp70 Inhibitors on BCSCs in TNBC
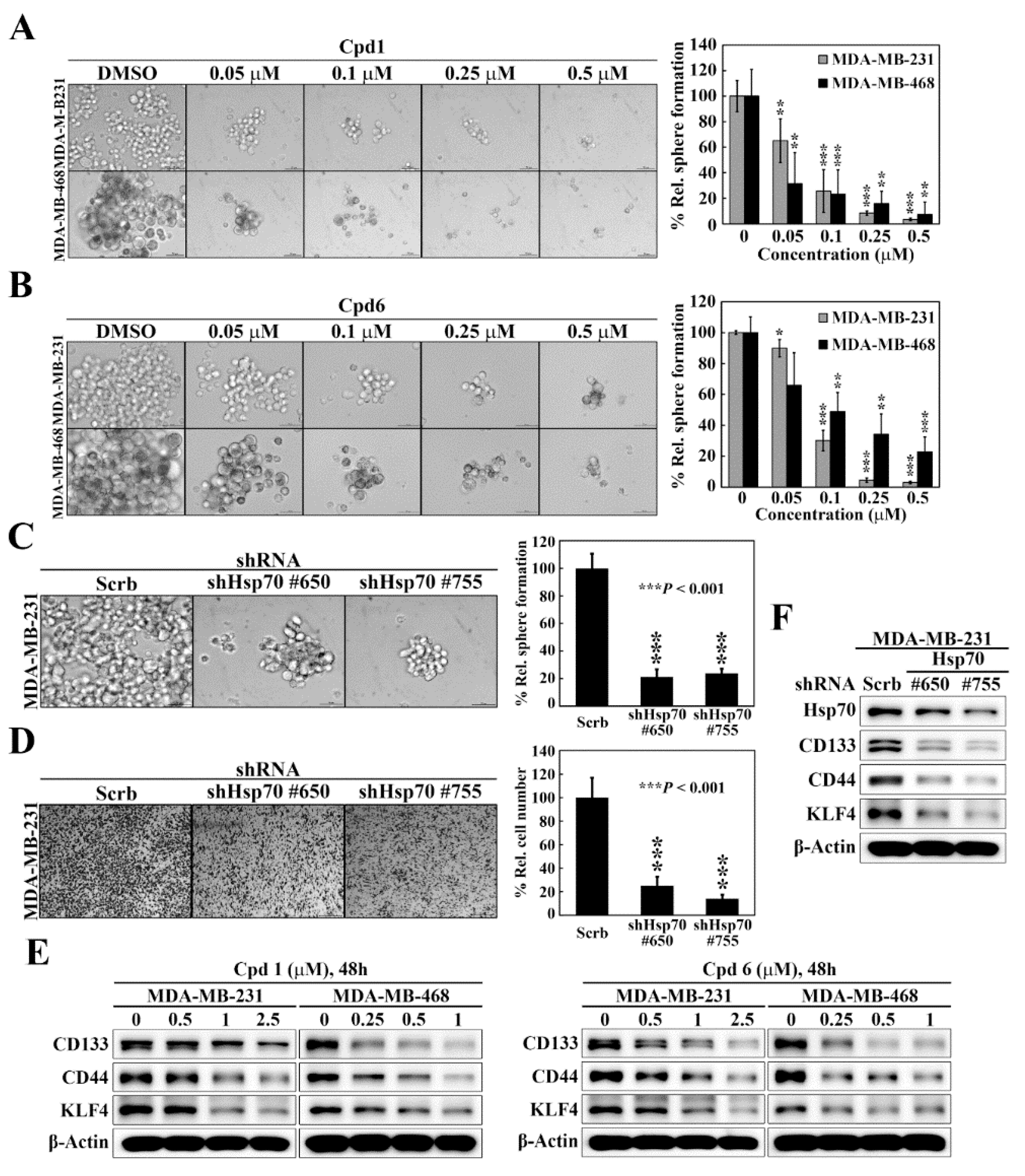
As selective targeting BCSCs is a promising therapeutic strategy to abolish the progression, reduce the risk of recurrence, and improve patient outcome of breast cancer [35], we assessed the effects of compound 1 and compound 6 on mammosphere formation, a surrogate measure of CSC expansion [36], in two TNBC cell lines, MDA-MB-231 and MDA-MB-468. The mammosphere formation assay showed that compound 1 (Figure 4A) and compound 6 (Figure 4B) exhibited potent dose-dependent suppressive effects on mammosphere formation in both cell lines. Moreover, knockdown of Hsp70 in MDA-MB-231 cells, using two different shRNAs (#650 and #755), decreased the ability of MDA-MB-231 to form mammospheres as compared to control cells (Figure 4C), which was reminiscent of the effects observed with compound 1 and compound 6 treatment. Since CSCs have been found to contribute to tumor metastasis, we also examined the effect of Hsp70 inhibition by genetic knockdown on cancer cell motility. The transwell migration assays revealed that the migration abilities were also greatly decreased by Hsp70 knockdown (shHsp70 #650 and shHsp70 #755) compared with control shRNA cells (Figure 4D). The effect of Hsp70 inhibition by compound 1 and compound 6 on BCSCs in TNBC cells was also verified by reduced expressions of the BCSCs-related markers CD133, CD44, and Kruppel-like factor 4 (KLF4) in MDA-MB-231 and MDA-MB-468 cells (Figure 4E). In addition, knockdown of Hsp70 in MDA-MB-231 cells led to concomitant decreases in the expression of CD133, CD44, and KLF4 (Figure 4F). These results indicated that Hsp70 plays a vital role in the regulation of the BCSC populations in TNBC cells.
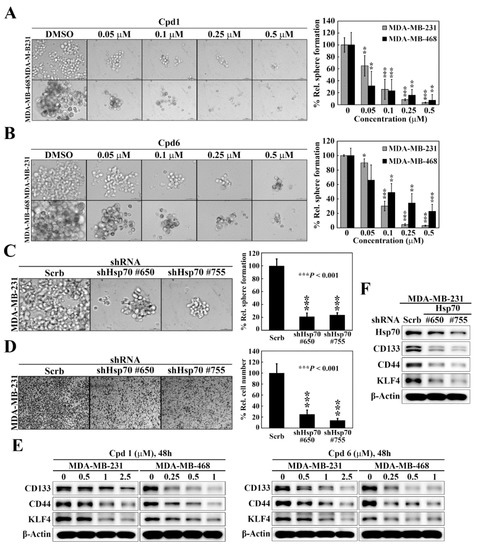
Figure 4.
Hsp70 is involved in maintaining cancer stem cells in triple negative breast cancer cells. (A) Left, representative images showing the effect of compound 1 on mammosphere formation in MDA-MB-231 and MDA-MB-468 cells (100× magnification). Right, quantitative results of mammosphere formation expressed as the percentage compared with the DMSO control. Data, means ± S.D., ** p < 0.01, *** p < 0.001. (B) Left, representative images showing the effect of compound 6 on mammosphere formation in MDA-MB-231 and MDA-MB-468 cells (100× magnification). Right, quantitative results of mammosphere formation expressed as the percentage compared with the DMSO control. Data, means ± S.D., * p < 0.05, ** p < 0.01, *** p < 0.001. (C) Left, effects of stable knockdown of Hsp70 on mammosphere formation in MDA-MB-231 cells (100× magnification). Right, quantitative results of mammosphere formation expressed as the percentage compared with the control shRNA. Data, means ± S.D., *** p < 0.001. (D) Left, representative images showing the effect of stable knockdown of Hsp70 on cell motility in MDA-MB-231 cells, determined by transwell migration assay. Right, quantitative analyses of the number of migrated cells on the lower side of chambers expressed as the percentage compared with the control shRNA. Data, means; bars, S.D., *** p < 0.001. (E) Western blot analyses of the effect of compound 1 (left) and compound 6 (right) on the expressions of various cancer stem cell markers, including CD133, CD44, and KLF4 in MDA-MB-231 and MDA-MB-468 cells. (F) Western blot analyses of the effect of stable knockdown of Hsp70 on the expressions of cancer stem cell markers, including CD133, CD44, and KLF4 in MDA-MB-231 cells.
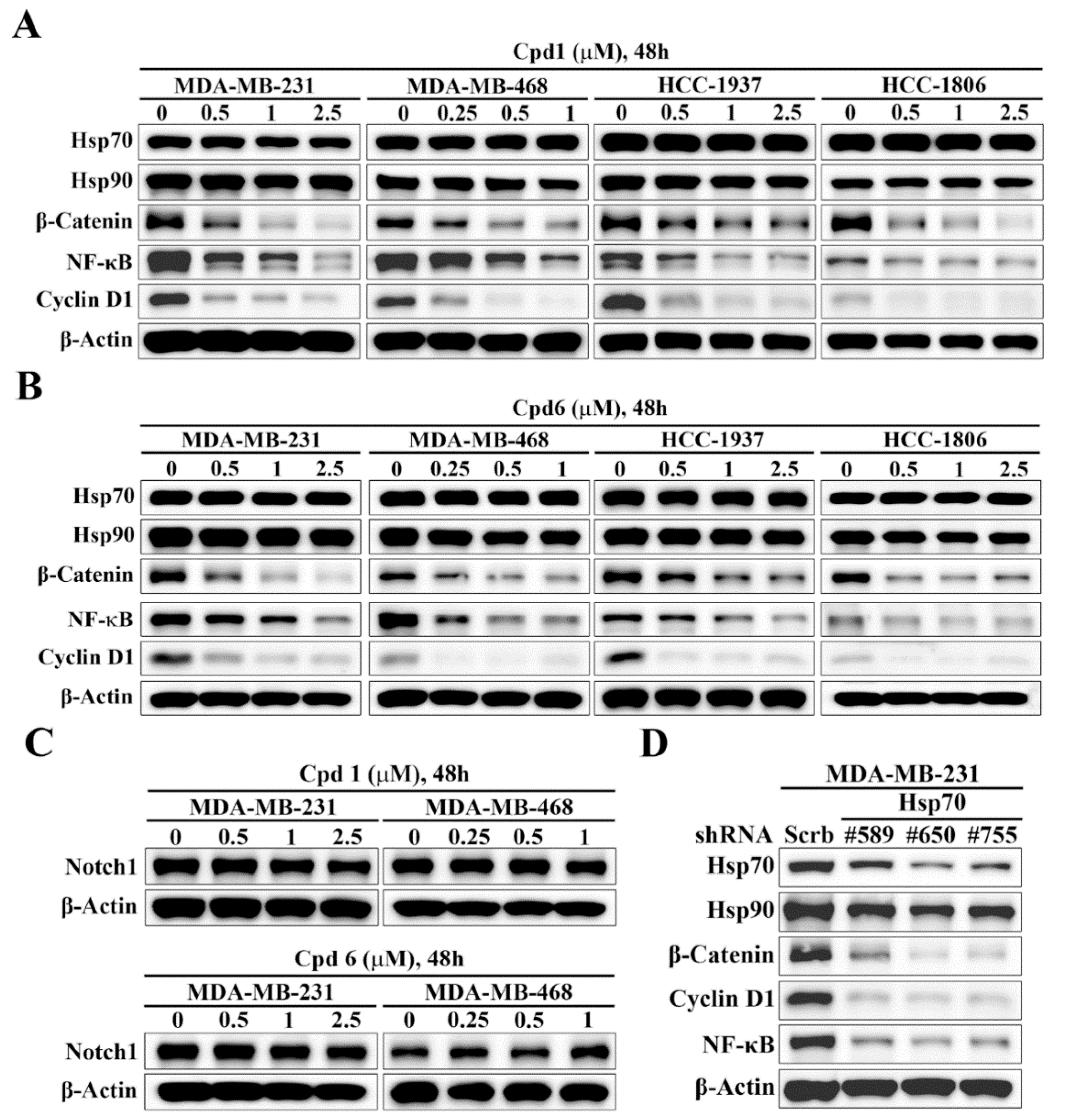
3.4. Hsp70 Inhibition Downregulates β-catenin and its Downstream Target Genes
The Notch and Wnt/β-catenin signaling pathways have been implicated in the regulation of self-renewal and survival of BCSCs [37]. To interrogate the mechanistic link between Hsp70 and BCSCs, we assessed the effects of the Hsp70 inhibitors compound 1 and compound 6 on the expressions of Notch1 and β-catenin in TNBC cells. Western blot analysis indicated that Hsp70 inhibitor-induced suppression of BCSCs in TNBC cells was associated with the inhibition of Wnt/β-catenin signaling, as manifested by parallel decreases in the expression levels of β-catenin and its downstream target genes, NFκB and Cyclin D1, in MDA-MB-231, MDA-MB-468, HCC-1937, and HCC-1806 cells (Figure 5A,B). In contrast, there were no appreciable changes in Notch1 expression in response to compound 1 and compound 6 in MDA-MB-231 and MDA-MB-468 cells (Figure 5C), indicating that Notch1 was not involved in Hsp70 inhibitor-mediated suppression of BCSCs in TNBC cells. The ability of Hsp70 to regulate β-catenin expression was confirmed by shRNA-mediated knockdown of Hsp70 in TNBC cells. Consistent with our premise, knockdown of Hsp70 was associated with reduced expression of β-catenin in MDA-MB-231 cells (Figure 5D).
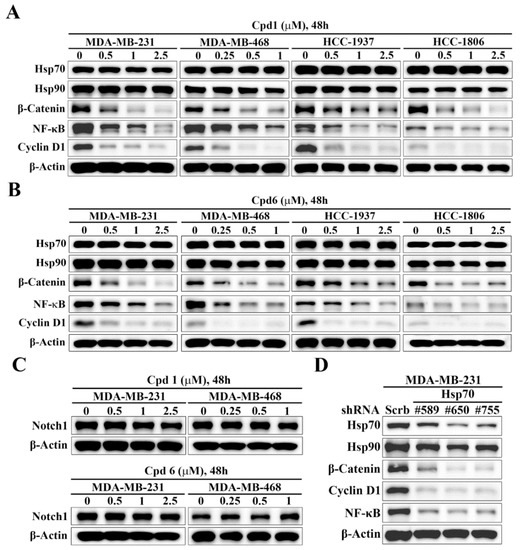
Figure 5.
Evidence that Hsp70 regulates the expression of β-catenin and its downstream target genes. Western blot analyses of the effect of (A) compound 1 and (B) compound 6 on the expressions of Hsp70, Hsp90, β-catenin, Cyclin D1, and NF-κB in MDA-MB-231, MDA-MB-468, HCC-1937, and HCC-1806 cells. (C) Western blot analyses of the effect of compound 1 (upper) and compound 6 (lower) on the expression of Notch1 in MDA-MB-231 and MDA-MB-468 cells. (D) Western blot analysis of the effect of stable knockdown of Hsp70 on the expressions of Hsp70, Hsp90, β-catenin, Cyclin D1, and NF-κB in MDA-MB-231 cells.
3.5. Evidence That Hsp70 Regulates BCSCs in TNBC via Modulating the Expression of β-catenin
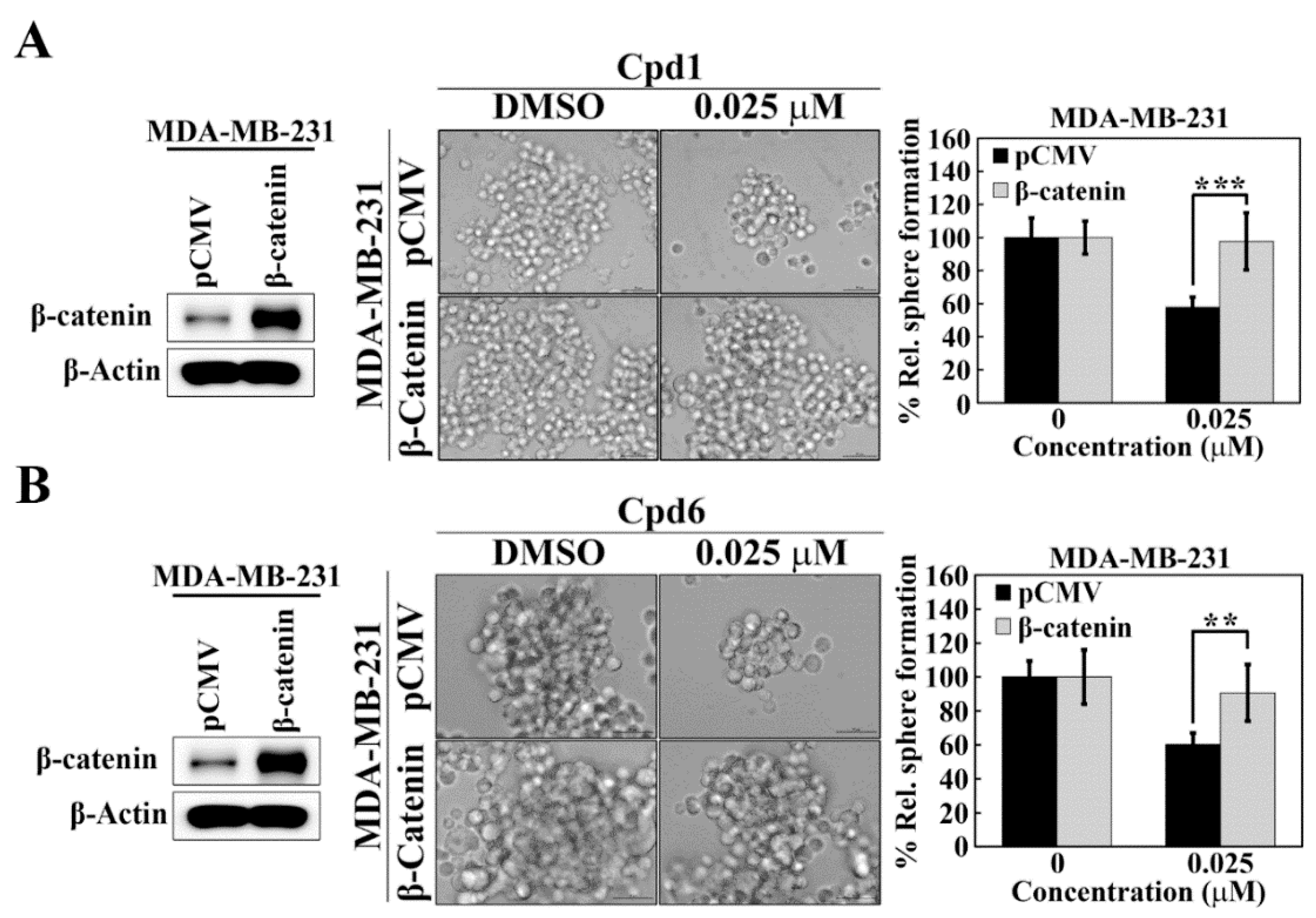
The causal relationship between Hsp70 and β-catenin in maintaining BCSCs in TNBC cells was further confirmed by ectopic expression of β-catenin in Hsp70 inhibitors-treated TNBC cells. As shown, MDA-MB-231 cells were transiently transfected with a β-catenin construct and control vector and the expression of β-catenin was demonstrated by Western blot analyses (Figure 6A,B). In addition, the mammosphere formation assay showed that overexpression of β-catenin abrogated the inhibitory effect of compound 1 (Figure 6A) and compound 6 (Figure 6B) on mammosphere formation in MDA-MB-231 cells, indicating that Hsp70 regulates BCSCs in TNBC cells, at least partially, via modulating the expression of β-catenin.
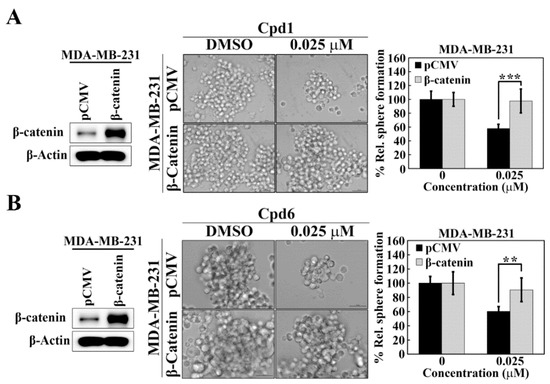
Figure 6.
β-Catenin is involved in Hsp70-mediated regulation of cancer stem cells in MDA-MB-231 cells. (A) Effects of ectopic expression of β-catenin versus pCMV vector control on mammosphere formation in compound 1 treated MDA-MB-231 cells. Left, Western blot analysis of β-catenin expression in ectopic expression of pCMV vector and β-catenin in MDA-MB-231 cells. Middle, representative images showing the effect of ectopic expression of β-catenin versus pCMV vector control on mammosphere formation in compound 1 treated MDA-MB-231 cells (100× magnification). Right, quantitative results of mammosphere formation. Data, means ± S.D., *** p < 0.001. (B) Effects of ectopic expression of β-catenin versus pCMV vector control on mammosphere formation in compound 6 treated MDA-MB-231 cells. Left, Western blot analysis of β-catenin expression in ectopic expression of pCMV vector and β-catenin in MDA-MB-231 cells. Middle, representative images showing the effect of ectopic expression of β-catenin versus pCMV vector control on mammosphere formation in compound 6 treated MDA-MB-231 cells (100× magnification). Right, quantitative results of mammosphere formation. Data, means ± S.D., ** p < 0.01.
3.6. Hsp70 Inhibition via Pharmacological Inhibitors of Hsp70 or Genetic Knockdown Hampers MDA-MB-231 Tumorigenicity and Tumor Growth
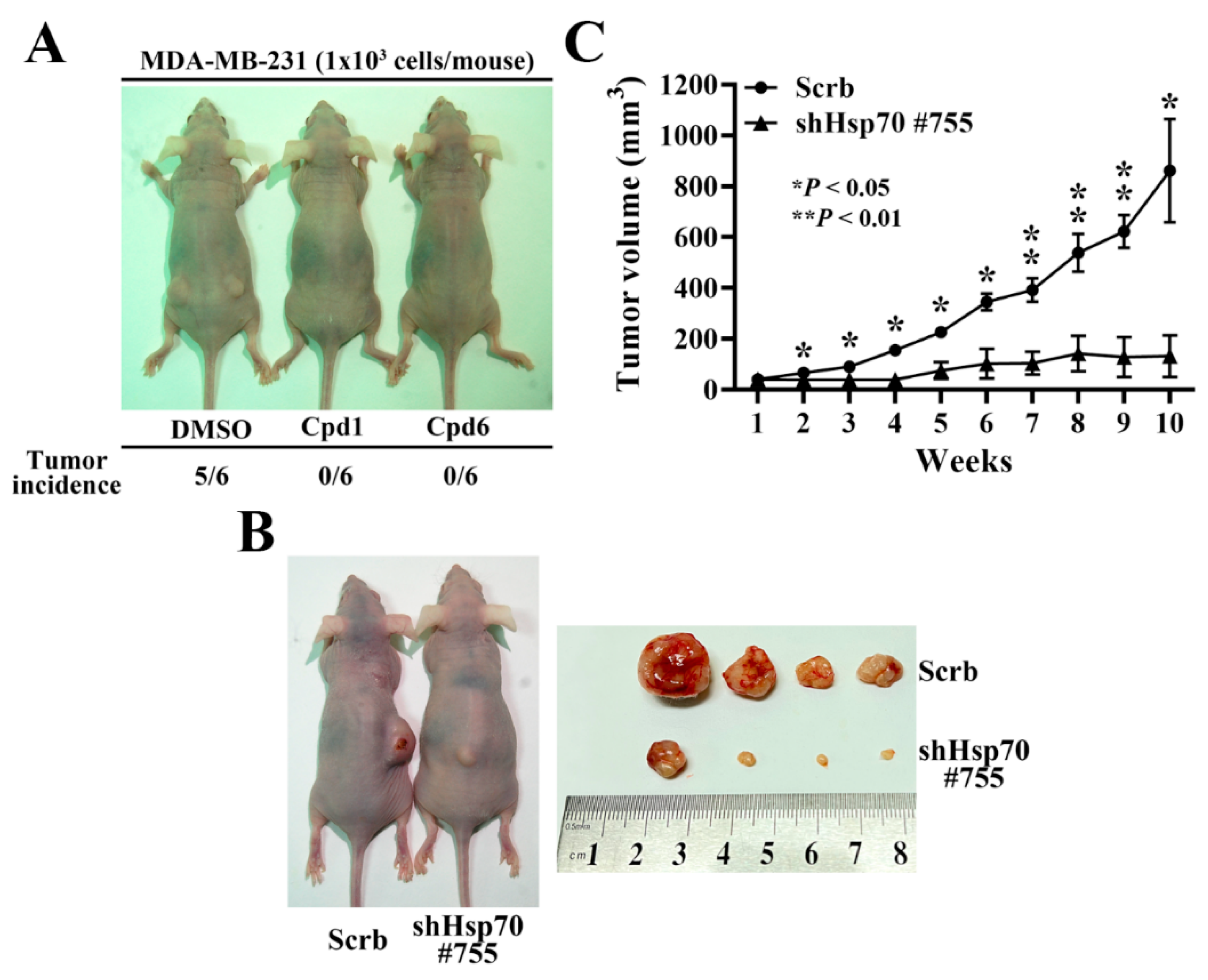
In order to further confirm the role of Hsp70 implicated in regulating BCSCs, compound 1, compound 6, and the isolated stable Hsp70 knockdown clone (shHsp70 #755) of MDA-MB-231 cells were used to investigate the in vivo efficacy of Hsp70 inhibition in suppressing tumorigenesis and xenograft tumor growth, respectively. Since CSCs possess strong tumor-initiating capacity, we examined the effect of compound 1 and compound 6 on tumor initiation by injecting 1 × 103 living cells, which were collected from 2.5 μM compound 1 or compound 6 treated MDA-MB-231 cells for 48 h, versus the same numbers of control DMSO treated cells subcutaneously into both flanks of the nude mice (n = 6 for each group). After 12 weeks post-injection, the tumor incidence in mice receiving DMSO control group was 83.3% compared to the respective Hsp70 inhibitors treated groups, in which none of mice developed tumor (Figure 7A). In addition, in a separate experiment, the suppressive effect of Hsp70 knockdown on tumor growth was evaluated by injecting nude mice with 1 × 106 shHsp70 #755 or control cells (n = 4) (Figure 7B). Consistent with the results on tumor initiation, knockdown of Hsp70 significantly reduced MDA-MB-231 xenograft tumor growth, as indicated by tumor volume, relative to the control (84% inhibition) (Figure 7C).
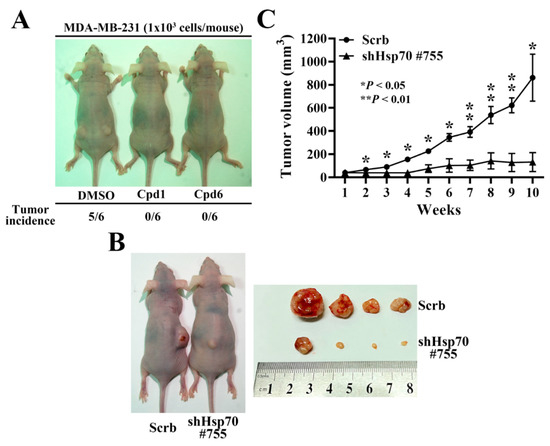
Figure 7.
In vivo efficacy of Hsp70 inhibitors and stable Hsp70 knockdown in suppressing MDA-MB-231 tumorigenicity and xenograft tumor growth in nude mice. (A) Comparison of the tumor incidence between Hsp70 inhibitors treated and control DMSO treated MDA-MB-231 cells. 1 × 103 DMSO, compound 1, or compound 6 treated MDA-MB-231 cells were subcutaneously injected into both flanks of the mice. (B) Representative images of stable control knockdown (Scrb) and Hsp70 knockdown clone (shHsp70 #755) on subcutaneous MDA-MB-231 xenograft tumor-bearing mice (left) and dissected tumor samples (right). (C) Suppressive effect of Hsp70 knockdown (shHsp70 #755) on subcutaneous MDA-MB-231 xenograft tumor volumes (means ± S.E.M., n = 4). * p < 0.05, ** p < 0.01.
4. Discussion
TNBC, representing about 15 – 20% of all breast cancers, is one of the most challenging cancers to treat. In spite of the available treatment options for TNBC remains limited, significant progress has been made over the past decades toward the development of novel emerging targeted or combination therapies [2,38,39]. Recently, CSCs have been considered the key driver of tumor progression and metastasis, and to be responsible for cancer relapse and drug resistance [40]. BCSCs have been known to contribute to tumor development, recurrence and metastasis of breast cancer, and therapeutic resistance in the clinic [41]. TNBC cells have a greater mammosphere-forming capacity and more aggressive phenotypes, which are associated with the presence of BCSCs, than non-TNBC cells [42,43]. Clinically, histological analyses also revealed that breast cancer tissues from TNBC patients exhibited enriched CD44+/CD24− and ALDH1+ expression levels compared to non-TNBC tissues [42,43,44]. Accordingly, many therapeutic strategies targeting the CSCs phenotypes have shown inhibitory effects on tumor initiation, therapy resistance, and metastasis in TNBC cells [3].
Three major signaling pathways, including Wnt/β-catenin, Notch, and Hedgehog, have critical roles in regulating the self-renewal and differentiation of BCSCs. A number of studies have suggested that dysregulated Wnt/β-catenin signaling was correlated with breast cancer development and progression [45,46]. Increased Wnt/β-catenin signaling has also been identified as a driving force of TNBC tumorigenesis [47]. Moreover, aberrant activation of Wnt/β-catenin signaling or transcriptional activity of β-catenin has been implicated in the self-renewal activity and invasive potential of BCSCs [48]. Yang Y. et al. demonstrated that suppression of the Wnt/β-catenin signaling pathway notably inhibited BCSCs self-renewal by a natural product [49]. Compared to other cells, the higher level of Wnt/β-catenin signaling pathway contributes to the high invasiveness and drug resistance of BCSCs. Therefore, inhibition of Wnt/β-catenin signaling or transcriptional activity of β-catenin may be a potential option for TNBC therapy by reducing BCSC stemness [3].
Previous reports have shown that Hsp70 is highly elevated in several types of tumors [50], and constitutively high expression of Hsp70 correlates with increased tumor grades and poor prognosis [19]. Additionally, Hsp70 overexpression has also been found to correlate with more aggressive tumor phenotypes, including cancer cells metastasis and therapy resistance [51,52]. Accumulating evidence also revealed that upregulated Hsp70 expression was found in CSC-like cells [53,54,55], and inhibition of Hsp70 reduced the stemness phenotypes in different tumor types [30,55,56]. Therefore, targeting Hsp70 could be an effective strategy for cancer therapy by eliminating both cancer cells and CSCs. In an attempt for developing the novel therapeutic strategy for TNBC, we provide evidence in this study that inhibition of Hsp70 with Hsp70 inhibitors could not only inhibit cancer cell viability and proliferation, but also suppress mammosphere formation through reducing the expression of β-catenin and its downstream target genes, NFκB and Cyclin D1, in TNBC. Moreover, we used the Hsp70 inhibitors treated TNBC cells and a stable Hsp70 knockdown clone of MDA-MB-231 cells to demonstrate the in vivo efficacy of Hsp70 inhibition in suppressing tumorigenesis and xenograft tumor growth. Together, these findings suggest that the inhibition of Hsp70 may represent a potential therapeutic strategy against breast cancer cells and BCSCs in TNBC. Further studies to evaluate the potential clinical role of Hsp70 in TNBC and the development of derivatives of Hsp70 inhibitors with improved efficacy and safety profiles are warranted.
5. Conclusions
Substantial evidence has shown that Hsp70 is overexpressed in multiple types of cancers, including breast, colon, liver, prostate, esophagus, and cervix, suggesting its involvement in tumorigenesis. This study further demonstrated that Hsp70 represents a potential anti-CSCs target in TNBC cells. Accordingly, two putative Hsp70 inhibitors, compound 1 and compound 6, exhibited in vitro and in vivo efficacy in suppressing CSCs through the inhibition of β-catenin in TNBC cells. From a therapeutic perspective, compound 1 and compound 6 might help foster new therapeutic strategies for TNBC treatment to eliminate BCSCs by targeting Hsp70.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14194898/s1, File S1: Original western blot images.
Author Contributions
P.-C.C., C.-H.T. and J.-R.W. conceived the study and designed experiments. M.-T.L., Y.-C.L. and P.-C.C. performed the experiments and C.-H.T. and P.-C.C. conducted data collection and analysis. P.-C.C., C.-H.T., J.-R.W. and H.-W.L. wrote the manuscript, and all authors revised and confirmed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grands TTCRD110-18 from Taichung Tzu Chi Hospital for Dr. Chia-Hung Tsai, and was also financially supported by the grants CMU108-Z-07, CMU109-Z-07 and CMU110-Z-07 from China Medical University, and Drug Development Center, China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Institutional Review Board Statement
All animal experiments were performed in accordance with the relevant guidelines and regulations and approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (CMUIACUC-2019-110).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Acknowledgments
The authors thank Dilip Detroja for providing technical assistance in the synthesis of compound 1 and compound 6.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, M.; Tsodikov, A.; Bauer, K.R.; Parise, C.A.; Caggiano, V. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: The California Cancer Registry, 1999–2004. Cancer 2008, 112, 737–747. [Google Scholar] [CrossRef]
- Lee, A.; Djamgoz, M.B.A. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, J.H.; Nam, J.S. Targeting Cancer Stem Cells in Triple-Negative Breast Cancer. Cancers 2019, 11, 965. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Valent, P.; Bonnet, D.; De Maria, R.; Lapidot, T.; Copland, M.; Melo, J.V.; Chomienne, C.; Ishikawa, F.; Schuringa, J.J.; Stassi, G.; et al. Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 2012, 12, 767–775. [Google Scholar] [CrossRef]
- Liu, S.; Wicha, M.S. Targeting breast cancer stem cells. J. Clin. Oncol. 2010, 28, 4006–4012. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer stem cells behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Q.; Zou, Y.; Chen, H.; Qi, L.; Chen, Y. Stem Cells and Cellular Origins of Breast Cancer: Updates in the Rationale, Controversies, and Therapeutic Implications. Front. Oncol. 2019, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Gaspar, C.; Richer, W.; Franken, P.F.; Sacchetti, A.; Joosten, R.; Idali, A.; Brandao, J.; Decraene, C.; Fodde, R. Cancer stemness in Wnt-driven mammary tumorigenesis. Carcinogenesis 2014, 35, 2–13. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Subramanyam, D.; Reedijk, M.; Sridhar, S.S. Notch signaling pathway as a therapeutic target in breast cancer. Mol. Cancer Ther. 2011, 10, 9–15. [Google Scholar] [CrossRef]
- Habib, J.G.; O’Shaughnessy, J.A. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016, 5, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones 2018, 23, 303–315. [Google Scholar] [CrossRef]
- Michels, A.A.; Kanon, B.; Konings, A.W.; Ohtsuka, K.; Bensaude, O.; Kampinga, H.H. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 1997, 272, 33283–33289. [Google Scholar] [CrossRef] [PubMed]
- Stege, G.J.; Li, G.C.; Li, L.; Kampinga, H.H.; Konings, A.W. On the role of hsp72 in heat-induced intranuclear protein aggregation. Int. J. Hyperth. 1994, 10, 659–674. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Clark, G.M.; Tandon, A.K.; Fuqua, S.A.; Welch, W.J.; McGuire, W.L. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J. Natl. Cancer Inst. 1993, 85, 570–574. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Chi, J.G.; Lee, H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J. Korean Med. Sci. 2005, 20, 829–834. [Google Scholar] [CrossRef]
- Alaiya, A.A.; Oppermann, M.; Langridge, J.; Roblick, U.; Egevad, L.; Brindstedt, S.; Hellstrom, M.; Linder, S.; Bergman, T.; Jornvall, H.; et al. Identification of proteins in human prostate tumor material by two-dimensional gel electrophoresis and mass spectrometry. Cell Mol. Life Sci. 2001, 58, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Han, H.S.; Choi, H.K.; Lee, Y.J.; Kim, Y.J.; Han, M.Y.; Park, Y.M. Differential, stage-dependent expression of Hsp70, Hsp110 and Bcl-2 in colorectal cancer. J. Gastroenterol. Hepatol. 2003, 18, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Joo, I.S.; Song, S.Y.; Kim, D.S.; Bae, D.S.; Lee, J.H. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol. Oncol. 1999, 74, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Park, Y.M.; Kim, J.I.; Shim, E.H.; Kim, C.W.; Jang, J.J.; Kim, S.H.; Lee, W.H. T cell lymphoma in transgenic mice expressing the human Hsp70 gene. Biochem. Biophys. Res. Commun. 1996, 218, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Gurbuxani, S.; Schmitt, E.; Cande, C.; Parcellier, A.; Hammann, A.; Daugas, E.; Kouranti, I.; Spahr, C.; Pance, A.; Kroemer, G.; et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 2003, 22, 6669–6678. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Maingret, L.; Puig, P.E.; Rerole, A.L.; Ghiringhelli, F.; Hammann, A.; Solary, E.; Kroemer, G.; Garrido, C. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res. 2006, 66, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Li, X.; Moses, M.A.; Gilbert, L.A.; Kalyanaraman, C.; Young, Z.T.; Chernova, M.; Journey, S.N.; Weissman, J.S.; Hann, B.; et al. Exploration of Benzothiazole Rhodacyanines as Allosteric Inhibitors of Protein-Protein Interactions with Heat Shock Protein 70 (Hsp70). J. Med. Chem. 2018, 61, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.Y.; Gabai, V.L. Hsp70 in cancer: Back to the future. Oncogene 2015, 34, 4153–4161. [Google Scholar] [CrossRef]
- Gong, J.; Weng, D.; Eguchi, T.; Murshid, A.; Sherman, M.Y.; Song, B.; Calderwood, S.K. Targeting the hsp70 gene delays mammary tumor initiation and inhibits tumor cell metastasis. Oncogene 2015, 34, 5460–5471. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kumar, V.; Lee, D.Y.; Chen, Y.; Wu, Y.C.; Gao, J.Y.; Chu, P.C. Development of Novel Rhodacyanine-Based Heat Shock Protein 70 Inhibitors. Curr. Med. Chem. 2021, 28, 5431–5446. [Google Scholar] [CrossRef] [PubMed]
- Yaglom, J.A.; Wang, Y.; Li, A.; Li, Z.; Monti, S.; Alexandrov, I.; Lu, X.; Sherman, M.Y. Cancer cell responses to Hsp70 inhibitor JG-98: Comparison with Hsp90 inhibitors and finding synergistic drug combinations. Sci. Rep. 2018, 8, 3010. [Google Scholar] [CrossRef]
- Mosser, D.D.; Morimoto, R.I. Molecular chaperones and the stress of oncogenesis. Oncogene 2004, 23, 2907–2918. [Google Scholar] [CrossRef]
- Liu, K.; Kang, M.; Li, J.; Qin, W.; Wang, R. Prognostic value of the mRNA expression of members of the HSP90 family in non-small cell lung cancer. Exp. Ther. Med. 2019, 17, 2657–2665. [Google Scholar] [CrossRef]
- Lawson, J.C.; Blatch, G.L.; Edkins, A.L. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res. Treat. 2009, 118, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Chiotaki, R.; Polioudaki, H.; Theodoropoulos, P.A. Cancer stem cells in solid and liquid tissues of breast cancer patients: Characterization and therapeutic perspectives. Curr. Cancer Drug Targets 2015, 15, 256–269. [Google Scholar] [CrossRef]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Jhan, J.R.; Andrechek, E.R. Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics 2017, 18, 1595–1609. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci 2018, 25, 20. [Google Scholar] [CrossRef]
- Pires, B.R.; IS, D.E.A.; Souza, L.D.; Rodrigues, J.A.; Mencalha, A.L. Targeting Cellular Signaling Pathways in Breast Cancer Stem Cells and its Implication for Cancer Treatment. Anticancer Res. 2016, 36, 5681–5691. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.; Wang, H.; Shi, X.; Fan, Y.; Ding, X.; Lin, C.; Zhan, Q.; Qian, H.; Xu, B. Enriched CD44(+)/CD24(-) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC). Cancer Lett. 2014, 353, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, F.; Wang, H.; Lin, C.; Fan, Y.; Zhang, X.; Qian, H.; Xu, B. Stem cell marker aldehyde dehydrogenase 1 (ALDH1)-expressing cells are enriched in triple-negative breast cancer. Int. J. Biol. Markers 2013, 28, e357–e364. [Google Scholar] [CrossRef] [PubMed]
- Honeth, G.; Bendahl, P.O.; Ringner, M.; Saal, L.H.; Gruvberger-Saal, S.K.; Lovgren, K.; Grabau, D.; Ferno, M.; Borg, A.; Hegardt, C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008, 10, R53. [Google Scholar] [CrossRef] [PubMed]
- Zardawi, S.J.; O’Toole, S.A.; Sutherland, R.L.; Musgrove, E.A. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol. Histopathol. 2009, 24, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.B.; Hong, I.S.; Kim, R.J.; Lee, S.Y.; Park, S.J.; Lee, E.S.; Park, J.H.; Yun, C.H.; Chung, J.U.; Lee, K.J.; et al. Wnt/beta-Catenin Small-Molecule Inhibitor CWP232228 Preferentially Inhibits the Growth of Breast Cancer Stem-like Cells. Cancer Res. 2015, 75, 1691–1702. [Google Scholar] [CrossRef]
- Xu, J.; Prosperi, J.R.; Choudhury, N.; Olopade, O.I.; Goss, K.H. beta-Catenin is required for the tumorigenic behavior of triple-negative breast cancer cells. PLoS ONE 2015, 10, e0117097. [Google Scholar] [CrossRef]
- Jang, G.B.; Kim, J.Y.; Cho, S.D.; Park, K.S.; Jung, J.Y.; Lee, H.Y.; Hong, I.S.; Nam, J.S. Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 2015, 5, 12465. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hao, E.; Pan, X.; Tan, D.; Du, Z.; Xie, J.; Hou, X.; Deng, J.; Wei, K. Gomisin M2 from Baizuan suppresses breast cancer stem cell proliferation in a zebrafish xenograft model. Aging 2019, 11, 8347–8361. [Google Scholar] [CrossRef]
- Murphy, M.E. The HSP70 family and cancer. Carcinogenesis 2013, 34, 1181–1188. [Google Scholar] [CrossRef]
- Juhasz, K.; Lipp, A.M.; Nimmervoll, B.; Sonnleitner, A.; Hesse, J.; Haselgruebler, T.; Balogi, Z. The complex function of hsp70 in metastatic cancer. Cancers 2013, 6, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Yan, G.; You, B.; Sun, J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008, 68, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shin, H.S.; Choi, G.; Lee, Y.C. Proteomic analysis of CD44(+) and CD44(-) gastric cancer cells. Mol. Cell Biochem. 2014, 396, 213–220. [Google Scholar] [CrossRef]
- Ronci, M.; Catanzaro, G.; Pieroni, L.; Po, A.; Besharat, Z.M.; Greco, V.; Levi Mortera, S.; Screpanti, I.; Ferretti, E.; Urbani, A. Proteomic analysis of human Sonic Hedgehog (SHH) medulloblastoma stem-like cells. Mol. Biosyst. 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.Y.; Le, H.T.; Min, H.Y.; Pei, H.; Lim, Y.; Song, I.; Nguyen, Y.T.K.; Hong, S.; Han, B.W.; Lee, H.Y. Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics 2021, 11, 2932–2952. [Google Scholar] [CrossRef]
- Gupta, N.; Jagadish, N.; Surolia, A.; Suri, A. Heat shock protein 70-2 (HSP70-2) a novel cancer testis antigen that promotes growth of ovarian cancer. Am. J. Cancer Res. 2017, 7, 1252–1269. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).