Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
3.1. Patients’ Characteristics
3.2. Response to Treatment
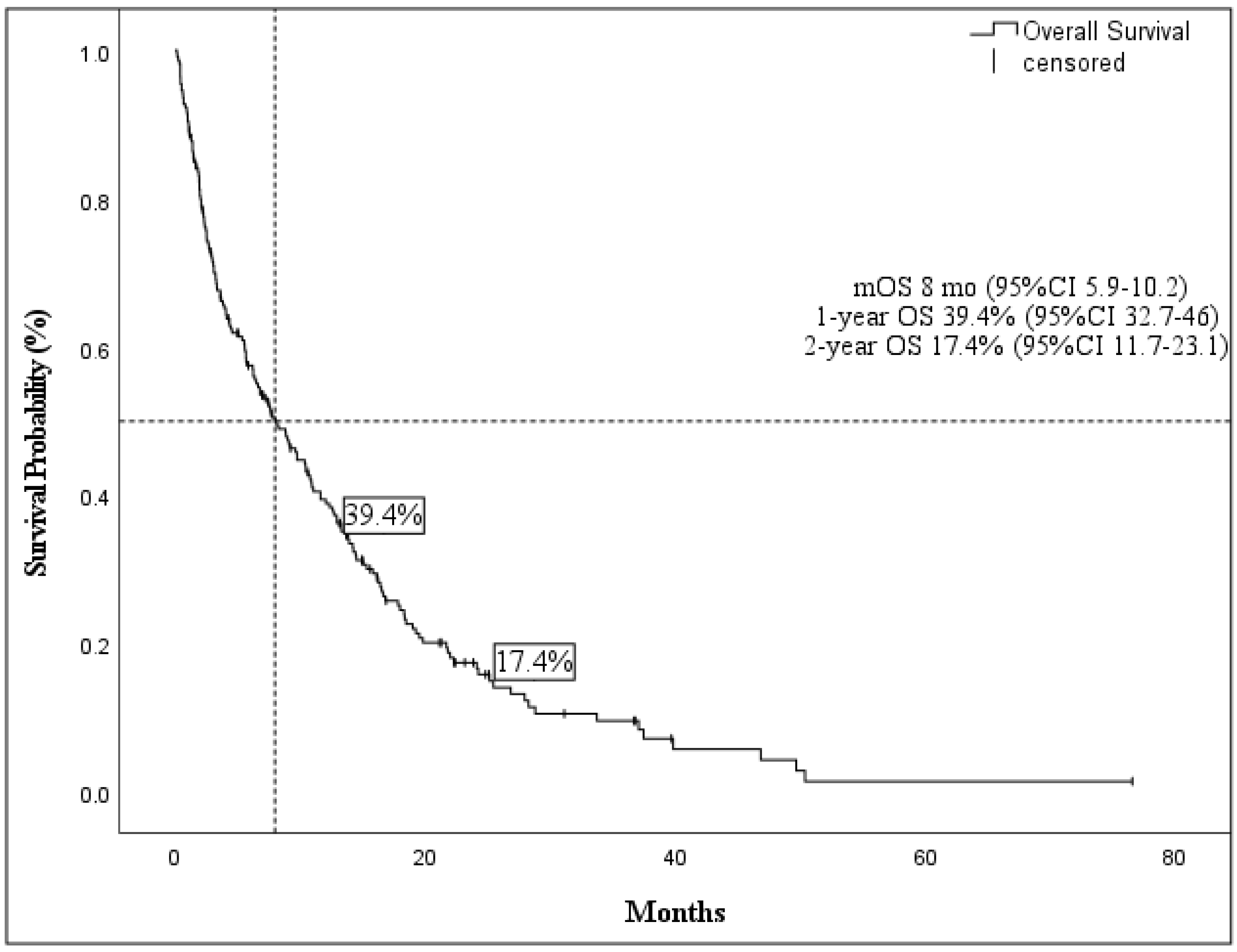
3.3. Overall Survival
3.4. Event-Free Survival
3.5. Long-Lasting Treated Patients
3.6. Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Rahul, E.; Gupta, I.; Chopra, A.; Ranjan, A.; Gupta, A.K.; Meena, J.P.; Viswanathan, G.K.; Bakhshi, S.; Misra, A.; et al. Molecular and genomic landscapes in secondary & therapy related acute myeloid leukemia. Am J Blood Res. 2021, 11, 472–497. [Google Scholar] [PubMed]
- Swerdlow, S.H.; International Agency for Research on Cancer, Curatori. WHO classification of tumours of haematopoietic and lymphoid tissues. In World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2008; p. 439. [Google Scholar]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.; Larson, R.A. Cancer and Leukemia Group B. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 24, 3895–3903. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Kantarjian, H.; Issa, J.P.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; De Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef]
- Steensma, D.P.; Baer, M.R.; Slack, J.L.; Buckstein, R.; Godley, L.A.; Garcia-Manero, G.; Albitar, M.; Larsen, J.S.; Arora, S.; Cullen, M.T.; et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: The alternative dosing for outpatient treatment (ADOPT) trial. J. Clin. Oncol. 2009, 27, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.-P.; Chou, W.-C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine vs patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with.30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- He, J.; Xiu, L.; De Porre, P.; Dass, R.; Thomas, X. Decitabine reduces transfusion dependence in older patients with acute myeloid leukemia: Results from a post hoc analysis of a randomized phase III study. Leuk Lymphoma. 2015, 56, 1033–1042. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Valente, M.A.; Hillege, H.L.; Navis, G.; Voors, A.A.; Dunselman, P.H.; Van Veldhuisen, D.J.; Damman, K. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur. J. Heart Fail. 2014, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Bonthapally, V.; Chawla, A.; Lefebvre, P.; Swords, R.; Lafeuille, M.-H.; Fortier, J.; Emond, B.; Duh, M.S.; Dezube, B.J. An Evaluation of Treatment Patterns and Outcomes in Elderly Patients Newly Diagnosed With Acute Myeloid Leukemia: A Retrospective Analysis of Electronic Medical Records From US Community Oncology Practices. Clin Lymphoma Myeloma Leuk. 2016, 16, 625–636.e3. [Google Scholar] [CrossRef] [PubMed]
- Derman, B.A.; Belli, A.J.; Battiwalla, M.; Hamadani, M.; Kansagra, A.; Lazarus, H.M.; Wang, C.K. Reality check: Real-world evidence to support therapeutic development in hematologic malignancies. Blood Rev. 2022, 53, 00913. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Salimi, T.; Epstein, R.S. Real-world use and outcomes of hypomethylating agent therapy in higher-risk myelodysplastic syndromes: Why are we not achieving the promise of clinical trials? Future Oncol. 2021, 17, 5163–5175. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Wang, R.; Wang, X.; Shallis, R.M.; Podoltsev, N.A.; Bewersdorf, J.P.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: A large population-based study in the United States. Blood Adv. 2020, 4, 2192–2201. [Google Scholar] [CrossRef]
- Récher, C.; Röllig, C.; Bérard, E.; Bertoli, S.; Dumas, P.Y.; Tavitian, S.; Kramer, M.; Serve, H.; Bornhäuser, M.; Platzbecker, U.; et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: A large patient data set study from European registries. Leukemia 2022, 36, 913–922. [Google Scholar] [CrossRef]
- Bocchia, M.; Candoni, A.; Borlenghi, E.; Defina, M.; Filì, C.; Cattaneo, C.; Sammartano, V.; Fanin, R.; Sciumè, M.; Sicuranza, A.; et al. Real-world experience with decitabine as a first-line treatment in 306 elderly acute myeloid leukaemia patients unfit for intensive chemotherapy. Hematol. Oncol. 2019, 37, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Scalzulli, E.; Colafigli, G.; Di Prima, A.; Diverio, D.; Mancini, M.; Latagliata, R.; Martelli, M.; Foà, R.; Breccia, M. Predictive factors for response and survival in elderly acute myeloid leukemia patients treated with hypomethylating agents: A real-life experience. Ann. Hematol. 2020, 99, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, E.; Imbergamo, S.; Candoni, A.; Liço, A.; Tanasi, I.; Mauro, E.; Mosna, F.; Leoncin, M.; Stulle, M.; Griguolo, D.; et al. Venetoclax in combination with hypomethylating agents in previously untreated patients with acute myeloid leukemia ineligible for intensive treatment: A real-life multicenter experience. Leuk. Res. 2022, 114, 106803. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.C.; Gutman, J.A.; Purev, E.; Nakic, M.; Tobin, J.; Chase, S.; Kaiser, J.; Lyle, L.; Boggs, C.; Halsema, K.; et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv. 2019, 3, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- He, P.-F.; Zhou, J.-D.; Yao, D.-M.; Ma, J.-C.; Wen, X.-M.; Zhang, Z.-H.; Lian, X.-Y.; Xu, Z.-J.; Qian, J.; Lin, J. Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Oncotarget 2017, 8, 41498–41507. [Google Scholar] [CrossRef]
- Wehmeyer, J.; Zaiss, M.; Losem, C.; Schmitz, S.; Niemeier, B.; Harde, J.; Hannig, C.V.; Harich, H.D.; Müller, J.; Klausmann, M.; et al. Impact of performance status and transfusion dependency on outcome of patients with myelodysplastic syndrome, acute myeloid leukemia and chronic myelomonocytic leukemia treated with azacitidine (PIAZA study). Eur. J. Haematol. 2018, 101, 766–773. [Google Scholar] [CrossRef]
- Röllig, C.; Kramer, M.; Schliemann, C.; Mikesch, J.H.; Steffen, B.; Krämer, A.; Noppeney, R.; Schäfer-Eckart, K.; Krause, S.W.; Hänel, M.; et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 2020, 136, 823–830. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Batty, G.N.; Kantarjian, H.; Issa, J.-P.; Jabbour, E.; Santos, F.P.; McCue, D.; Garcia-Manero, G.; Pierce, S.; O’Brien, S.; Cortés, J.E.; et al. Feasibility of Therapy With Hypomethylating Agents in Patients With Renal Insufficiency. Clin. Lymphoma Myeloma Leuk. 2010, 10, 205–210. [Google Scholar] [CrossRef]
- Levine, L.B.; Roddy, J.V.; Kim, M.; Li, J.; Phillips, G.; Walker, A.R. A comparison of toxicities in acute myeloid leukemia patients with and without renal impairment treated with decitabine. J. Oncol. Pharm. Pract. 2018, 24, 290–298. [Google Scholar] [CrossRef]
- Aiello, F.; Dueñas, E.P.; Musso, C.G. Senescent Nephropathy: The New Renal Syndrome. Healthcare 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Short, N.J.; DiNardo, C.; Stein, E.M.; Daver, N.; E Perl, A.; Wang, E.S.; Wei, A.; Tallman, M. Harnessing the benefits of available targeted therapies in acute myeloid leukaemia. Lancet Haematol. 2021, 8, e922–e933. [Google Scholar] [CrossRef]


| Characteristics | No. of Patients = 220 |
|---|---|
| Male, n (%) | 119 (54) |
| Female, n (%) | 101 (46) |
| Median age at diagnosis (IQR) | 78.2 (75–86.2) |
| Hb g/dL (median, IQR) | 8.8 (7.9–10.0) |
| WBC × 109/L (median, IQR) | 3.24 (1.6–9.8) |
| Platelet count × 109/L (median, range) | 56 (32–92.5) |
| BMI, n (%) | |
| <25 ≥25 | 97 (44.1) 123 (55.9) |
| ECOG PS, n (%) | |
| <2 ≥2 | 132 (60) 88 (40) |
| eGFR, n (%) | |
| <60 mL/min ≥60 mL/min | 37 (16.8) 183 (83.2) |
| BM blast percentage, n (%) | |
| 20–30% 30–50% >50% | 78 (35.5) 74 (33.6) 68 (30.9) |
| CCI, n (%) | |
| <3 ≥3 | 44 (20) 176 (80) |
| AML type, n (%) | |
| De novo-AML s-AML | 135 (61.4) 85 (38.6) |
| ELN risk stratification, data available n (%) | 205 (93.1) |
| Favorable Intermediate Poor/adverse | 17 (8.3) 100 (48.8) 88 (42.9) |
| Infection at diagnosis, n (%) | |
| No Yes | 171 (77.7) 49 (22.3) |
| Transfusion requirement, n (%) | |
| No Yes | 83 (37.7) 137 (62.3) |
| Median No of cycles (IQR) | 5 (2–12) |
| TDT, n (%) <15 days 15–30 days >30 days | 85 (38.6) 64 (29.1) 60 (32.3) |
| Variable | Median OS, Months (95% CI) | p Value * | Median EFS, Months (95% CI) | p Value * |
|---|---|---|---|---|
| Age, years | ||||
| <80 | 10.5 (7.4–13.5) | 0.001 | 8.7 (5.0–9.6) | 0.002 |
| ≥80 | 6.2 (3.0–9.5) | 4.0 ( 2.1–5.8) | ||
| Gender | ||||
| Male | 7.1 (4.0–10.1) | 0.752 | 5.6 (2.1–9.1) | 0.552 |
| Female | 9.0 (6.3–11.8) | 7.8 (5.1–10.5) | ||
| ECOG | ||||
| 0–1 | 9.8 (7.5–12.0) | <0.001 | 10.9 (7.5–14.3) | 0.051 |
| ≥2 | 2.3 (1.3–3.8) | 5.7 (3.5–7.9) | ||
| BM blast count at diagnosis | ||||
| 20–29% | 13.7 (9.8–17.6) | <0.001 | 12.1 (8.7–15.5) | <0.001 |
| 30–50% | 8.9 (5.0–12.7) | 6.2 (2.6–9.7) | ||
| >50% | 4.1 (1.7–6.5) | 3.7 (2.2–5.2) | ||
| Type of AML | ||||
| de novo AML | 9.0 (6.5–11.5) | 0.206 | 8.7 (5.9–11.6) | 0.005 |
| s-AML | 6.8 (3.3–10.3) | 4.2 (2.0–6.4) | ||
| Infectious at AML diagnosis | ||||
| No | 9.7 (7.3–12.1) | 0.203 | 8.7 (6.5–10.9) | 0.126 |
| Yes | 4.6 (1.7–8.0) | 3.4 (1.9–4.8) | ||
| eGFR (ml/min/1.73 m2) | ||||
| ≥60 | 9.2 (6.9–11.4) | 0.124 | 8.6 (6.7–10.5) | 0.196 |
| <60 | 3.1 (1–5.3) | 2.6 (1.5–3.7) | ||
| BMI at diagnosis | ||||
| <25 | 10.7 (7.4–14.0) | 0.120 | 8.6 (5.9–11.3) | 0.630 |
| ≥25 | 6.2 (5.1–7.4) | 5.7 (3.6–7.7) | ||
| CCI | ||||
| <3 | 14.3 (7.9–20.7) | 0.029 | 13.3 (8.0–18.7) | 0.007 |
| ≥3 | 6.7 (5.1–8.2) | 5.2 (3.0–7.4) | ||
| Transfusion dependency at diagnosis | ||||
| No | 12.1 (9.8–14.3) | 0.138 | 9.3 (7.1–11.6) | 0.391 |
| Yes | 6.0 (3.0–9.0) | 5.0 (2.1–7.8) | ||
| Complex karyotype | ||||
| No | 11.0 (6.8–15.1) | 0.003 | 9.8 (7.8–11.8) | <0.001 |
| Yes | 3.3 (1.3–5.3) | 2.1 (1.3–2.9) | ||
| ELN risk classification | ||||
| Favorable | 19.5 (8.1–31.0) | <0.001 | 19.4 (8.8–30.1) | <0.001 |
| Intermediate | 10.7 (6.5–14.8) | 10.6 (7.5–13.7) | ||
| Adverse | 4.4 (1.7–7.0) | 3.1 (1.6–4.4) | ||
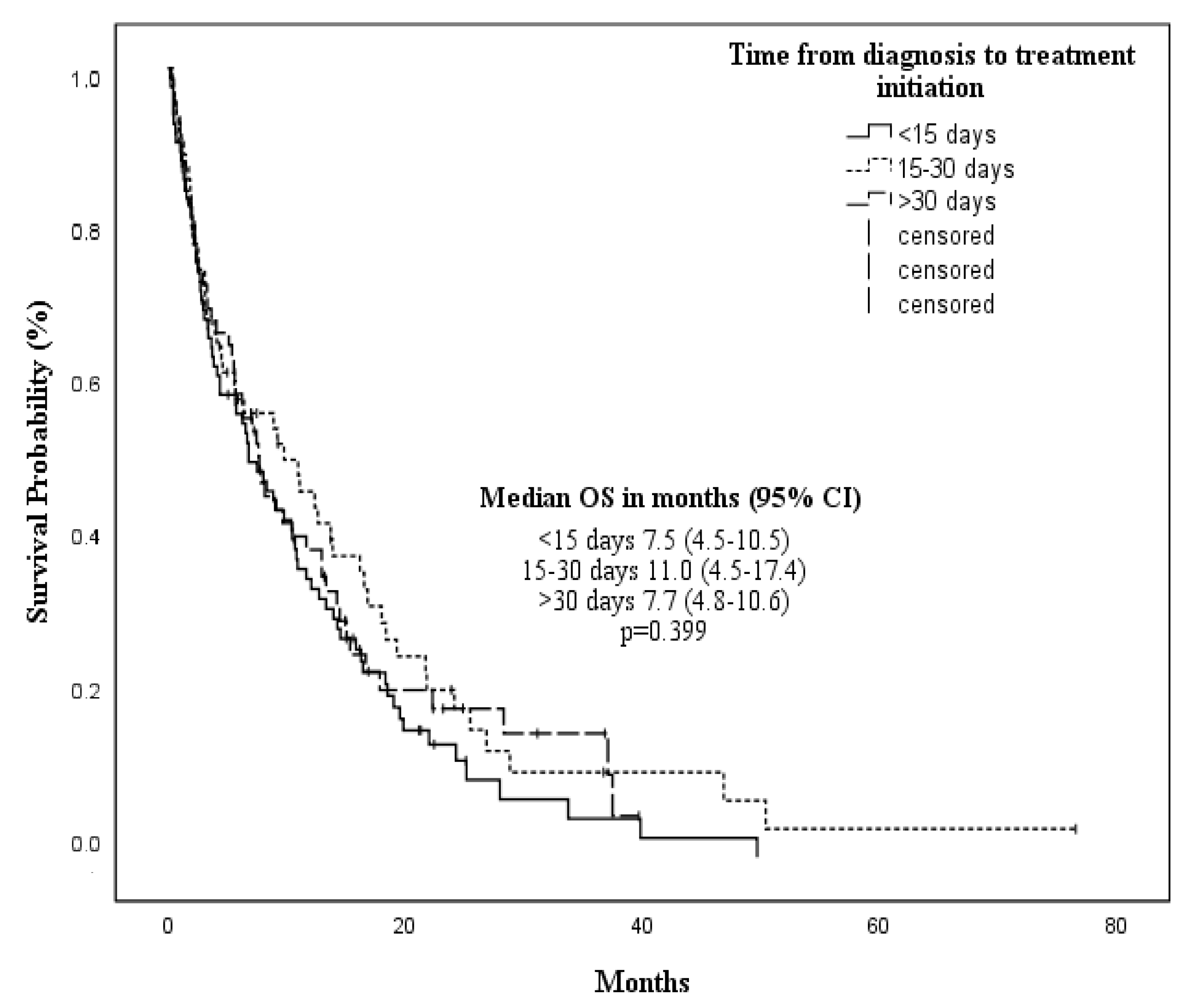
| Time from diagnosis to treatment initiation | ||||
| <15 days | 7.5 (4.5–10.5) | 0.399 | 5.8 (2.5–9.1) | 0.211 |
| 15—30 days | 11.0 (4.5–17.4) | 9.8 (7.9–11.7) | ||
| >30 days | 7.7 (4.8–10.6) | 5.7 (2.6–8.8) | ||
| Type of HMA | ||||
| Aza | 8.3 (5.8–10.8) | 0.810 | 7.3 (4.5–10.1) | 0.947 |
| Dec | 7.8 (3.3–12.3) | 6.2 (1.7–10.6) | ||
| BM blast count after 4th cycle | ||||
| <30% | 16.2 (12.3–20.0) | 0.034 | 14.3 (11.4–17.1) | 0.069 |
| ≥30% | 9.1 (3.7–14.5) | 8.9 (4.6–13.1) | ||
| Response after 4th cycle | ||||
| CR | 19.5 (12.9–26.2) | 0.011 | 17.5 (12.2–22.8) | 0.026 |
| PR | 15.3 (11.6–19.1) | 14.6 (10.8–18.4) | ||
| SD | 8.9 (6.0–11.7) | 8.5 (4.6–12.3) | ||
| Best response | ||||
| CR | 22.0 (15.6–28.4) | 0.001 | 19.5 (14.7–24.2) | <0.001 |
| PR | 15.3 (11.0–19.8) | 13.2 (8.9–17.5) | ||
| SD | 8.9 (5.7–12.1) | 8.7 (6.5–10.9) | ||
| Transfusion independence | ||||
| Yes | 19.3 (15.4–23.2) | <0.001 | 17.7 (14.0–21.3) | <0.001 |
| No | 3.3 (0.8–5.8) | 4.2 (2.6–5.8) | ||
| Treatment-related complication | ||||
| No | 10.8 (0.6–27.3) | 0.699 | 8.5 (6.1–10.9) | 0.870 |
| Yes | 5.7 (3.7–7.6) | 6.6 (2.6–10.6) |
| Covariate | HR (95% CI) | p Value |
|---|---|---|
| BM blast count at diagnosis ≥50% | 1.69 (0.99–2.90) | 0.054 |
| Age at diagnosis ≥80 years | 2.26 (1.23–4.16) | 0.009 |
| CCI ≥3 | 1.97 (1.05–3.69) | 0.034 |
| Presence of CK | 2.89 (1.51–5.49) | 0.001 |
| Type of best response ≥PR | 0.22 (0.12–0.40) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molica, M.; Mazzone, C.; Niscola, P.; Carmosino, I.; Di Veroli, A.; De Gregoris, C.; Bonanni, F.; Perrone, S.; Cenfra, N.; Fianchi, L.; et al. Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers 2022, 14, 4897. https://doi.org/10.3390/cancers14194897
Molica M, Mazzone C, Niscola P, Carmosino I, Di Veroli A, De Gregoris C, Bonanni F, Perrone S, Cenfra N, Fianchi L, et al. Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers. 2022; 14(19):4897. https://doi.org/10.3390/cancers14194897
Chicago/Turabian StyleMolica, Matteo, Carla Mazzone, Pasquale Niscola, Ida Carmosino, Ambra Di Veroli, Cinzia De Gregoris, Fabrizio Bonanni, Salvatore Perrone, Natalia Cenfra, Luana Fianchi, and et al. 2022. "Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience" Cancers 14, no. 19: 4897. https://doi.org/10.3390/cancers14194897
APA StyleMolica, M., Mazzone, C., Niscola, P., Carmosino, I., Di Veroli, A., De Gregoris, C., Bonanni, F., Perrone, S., Cenfra, N., Fianchi, L., Piccioni, A. L., Spadea, A., Luzi, G., Mengarelli, A., Cudillo, L., Maurillo, L., Pagano, L., Breccia, M., Rigacci, L., & De Fabritiis, P. (2022). Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers, 14(19), 4897. https://doi.org/10.3390/cancers14194897







