Genetic Alterations in Mitochondrial DNA Are Complementary to Nuclear DNA Mutations in Pheochromocytomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumors
2.2. Next-Generation Sequencing
2.3. Mitochondrial DNA Analysis
2.3.1. Mitochondrial Genome Sequencing
2.3.2. Mitochondrial Copy-Number Variation and DNA Large Deletion
2.3.3. Evaluation of 6mA Methylation Sites
2.4. Sequencing of Nuclear Genes Involved in Mitochondrial DNA Integrity
2.5. Transcriptome Analysis
2.5.1. Microarray Gene Expression Analysis
2.5.2. Reverse Transcriptase-qPCR (RT-qPCR)
2.6. Mass Spectrometry and Protein Analysis
2.7. Statistical Analysis
3. Results
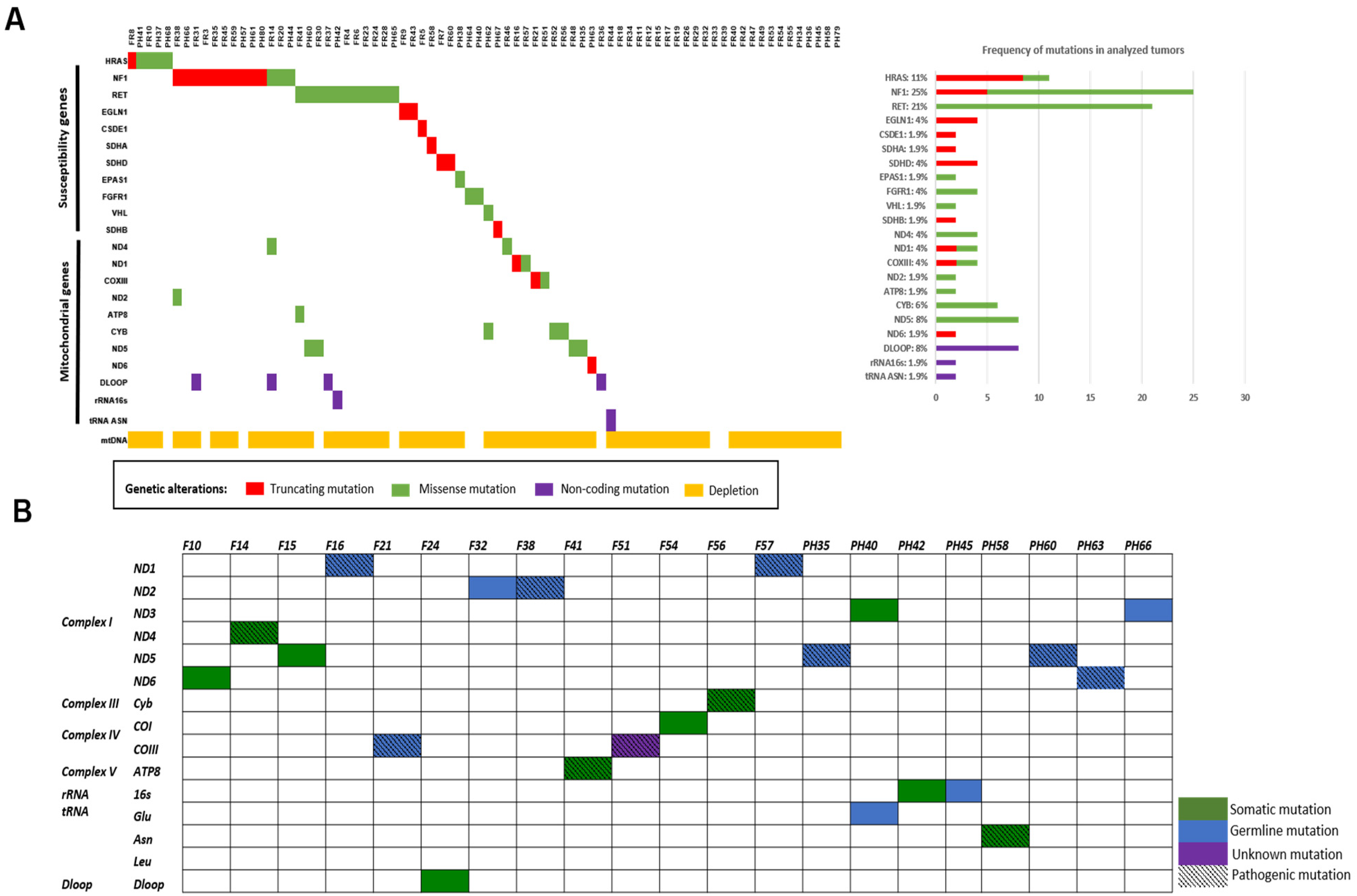
3.1. Novel Mutations in PCCs/PGLs Susceptibility Genes
3.2. Molecular Profiling and Gene Expression Patterns
3.3. Mitochondrial DNA Alterations in PCCs/PGLs
3.3.1. Novel Mitochondrial Mutations
3.3.2. Mitochondrial DNA Depletion and Large Deletions
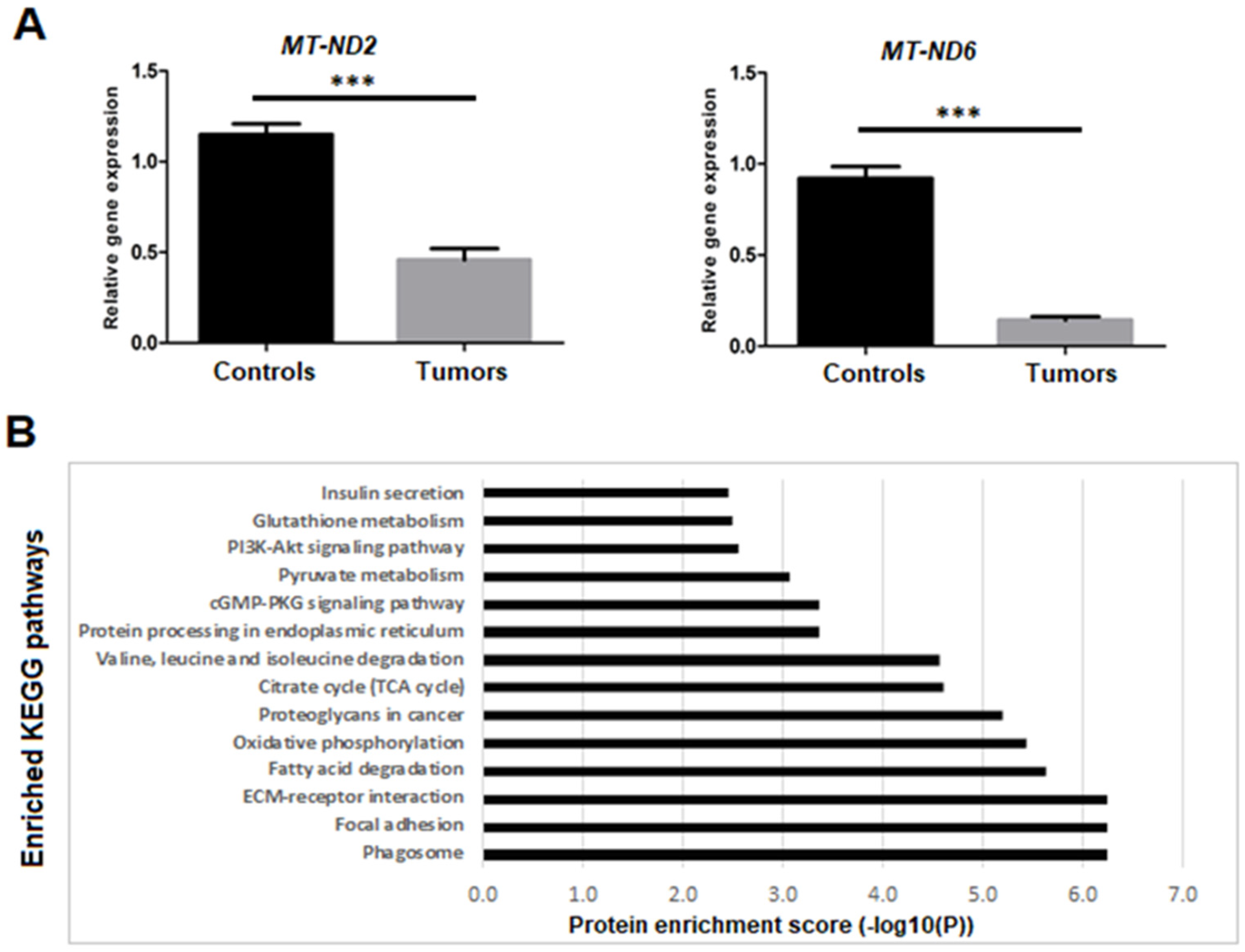
3.3.3. Dysregulation of Mitochondrial Genes and Proteins Expression
3.3.4. Absence of 6mA Methylation
3.4. Absence of Mutations in Mitochondrial DNA Integrity Genes
3.5. Dysregulation of Mitochondrial Biogenesis and Mitophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. DNA/RNA/Mitochondria Extraction
Appendix B. Haplogroups Analysis
Appendix C. Sanger Sequencing
Appendix D. In Silico Mutational Analysis
References
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Welander, J.; Söderkvist, P.; Gimm, O. Genetics and Clinical Characteristics of Hereditary Pheochromocytomas and Paragangliomas. Endocr. Relat. Cancer 2011, 18, R253–R276. [Google Scholar] [CrossRef]
- Pillai, S.; Gopalan, V.; Smith, R.A.; Lam, A.K.-Y. Updates on the Genetics and the Clinical Impacts on Phaeochromocytoma and Paraganglioma in the New Era. Crit. Rev. Oncol. Hematol. 2016, 100, 190–208. [Google Scholar] [CrossRef]
- Tabebi, M.; Söderkvist, P.; Jensen, L.D. Hypoxia Signaling and Circadian Disruption in and by Pheochromocytoma. Front. Endocrinol. 2018, 9, 612. [Google Scholar] [CrossRef]
- Bausch, B.; Schiavi, F.; Ni, Y.; Welander, J.; Patocs, A.; Ngeow, J.; Wellner, U.; Malinoc, A.; Taschin, E.; Barbon, G.; et al. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017, 3, 1204. [Google Scholar] [CrossRef]
- Flynn, A.; Dwight, T.; Harris, J.; Benn, D.; Zhou, L.; Hogg, A.; Catchpoole, D.; James, P.; Duncan, E.L.; Trainer, A.; et al. Pheo-Type: A Diagnostic Gene-Expression Assay for the Classification of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2016, 101, 1034–1043. [Google Scholar] [CrossRef]
- Challen, C.; Brown, H.; Cai, C.; Betts, G.; Paterson, I.; Sloan, P.; West, C.; Birch-Machin, M.; Robinson, M. Mitochondrial DNA Mutations in Head and Neck Cancer Are Infrequent and Lack Prognostic Utility. Br. J. Cancer 2011, 104, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.R.; Thomas, K.J. The role of mitochondria in the development and progression of lung cancer. Comput. Struct. Biotechnol. J. 2013, 6, e201303019. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Li, J.; Zhang, W.; Liao, Y.; Chen, J.; Sun, Z. Correlational Study on Mitochondrial DNA Mutations as Potential Risk Factors in Breast Cancer. Oncotarget 2016, 7, 31270–31283. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Moraes, C.T. Mechanisms Linking MtDNA Damage and Aging. Free Radic. Biol. Med. 2015, 85, 250–258. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA Copy Number Variation across Human Cancers. eLife Sci. 2016, 5, e10769. [Google Scholar] [CrossRef]
- De Paepe, B. Mitochondrial Markers for Cancer: Relevance to Diagnosis, Therapy, and Prognosis and General Understanding of Malignant Disease Mechanisms. Available online: https://www.hindawi.com/journals/isrn/2012/217162/ (accessed on 3 July 2019).
- Kirches, E. MtDNA As a Cancer Marker: A Finally Closed Chapter? Curr. Genom. 2017, 18, 255–267. [Google Scholar] [CrossRef][Green Version]
- Neuhaus, J.F.G.; Baris, O.R.; Kittelmann, A.; Becker, K.; Rothschild, M.A.; Wiesner, R.J. Catecholamine Metabolism Induces Mitochondrial DNA Deletions and Leads to Severe Adrenal Degeneration during Aging. Neuroendocrinology 2017, 104, 72–84. [Google Scholar] [CrossRef]
- Neuhaus, J.F.G.; Baris, O.R.; Hess, S.; Moser, N.; Schröder, H.; Chinta, S.J.; Andersen, J.K.; Kloppenburg, P.; Wiesner, R.J. Catecholamine Metabolism Drives Generation of Mitochondrial DNA Deletions in Dopaminergic Neurons. Brain 2014, 137, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Lenders, J.W.M.; Duh, Q.-Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.-P.; Grebe, S.K.G.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Comino-Méndez, I.; de Cubas, A.A.; Bernal, C.; Álvarez-Escolá, C.; Sánchez-Malo, C.; Ramírez-Tortosa, C.L.; Pedrinaci, S.; Rapizzi, E.; Ercolino, T.; Bernini, G.; et al. Tumoral EPAS1 (HIF2A) Mutations Explain Sporadic Pheochromocytoma and Paraganglioma in the Absence of Erythrocytosis. Hum. Mol. Genet. 2013, 22, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libé, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative Genomic Analysis Reveals Somatic Mutations in Pheochromocytoma and Paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Welander, J.; Larsson, C.; Bäckdahl, M.; Hareni, N.; Sivlér, T.; Brauckhoff, M.; Söderkvist, P.; Gimm, O. Integrative Genomics Reveals Frequent Somatic NF1 Mutations in Sporadic Pheochromocytomas. Hum. Mol. Genet. 2012, 21, 5406–5416. [Google Scholar] [CrossRef]
- Currás-Freixes, M.; Inglada-Pérez, L.; Mancikova, V.; Montero-Conde, C.; Letón, R.; Comino-Méndez, I.; Apellániz-Ruiz, M.; Sánchez-Barroso, L.; Aguirre Sánchez-Covisa, M.; Alcázar, V.; et al. Recommendations for Somatic and Germline Genetic Testing of Single Pheochromocytoma and Paraganglioma Based on Findings from a Series of 329 Patients. J. Med. Genet. 2015, 52, 647–656. [Google Scholar] [CrossRef]
- Castro-Vega, L.J.; Buffet, A.; De Cubas, A.A.; Cascón, A.; Menara, M.; Khalifa, E.; Amar, L.; Azriel, S.; Bourdeau, I.; Chabre, O.; et al. Germline Mutations in FH Confer Predisposition to Malignant Pheochromocytomas and Paragangliomas. Hum. Mol. Genet. 2014, 23, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Welander, J.; Łysiak, M.; Brauckhoff, M.; Brunaud, L.; Söderkvist, P.; Gimm, O. Activating FGFR1 Mutations in Sporadic Pheochromocytomas. World J. Surg. 2018, 42, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Brière, J.-J.; Libé, R.; Vescovo, L.; Rivière, J.; Tissier, F.; Jouanno, E.; Jeunemaitre, X.; Bénit, P.; Tzagoloff, A.; et al. SDHA Is a Tumor Suppressor Gene Causing Paraganglioma. Hum. Mol. Genet. 2010, 19, 3011–3020. [Google Scholar] [CrossRef]
- Yao, L.; Schiavi, F.; Cascon, A.; Qin, Y.; Inglada-Pérez, L.; King, E.E.; Toledo, R.A.; Ercolino, T.; Rapizzi, E.; Ricketts, C.J.; et al. Spectrum and Prevalence of FP/TMEM127 Gene Mutations in Pheochromocytomas and Paragangliomas. JAMA 2010, 304, 2611–2619. [Google Scholar] [CrossRef]
- Yang, C.; Zhuang, Z.; Fliedner, S.M.J.; Shankavaram, U.; Sun, M.G.; Bullova, P.; Zhu, R.; Elkahloun, A.G.; Kourlas, P.J.; Merino, M.; et al. Germ-Line PHD1 and PHD2 Mutations Detected in Patients with Pheochromocytoma/Paraganglioma-Polycythemia. J. Mol. Med. 2015, 93, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wilzén, A.; Rehammar, A.; Muth, A.; Nilsson, O.; Tešan Tomić, T.; Wängberg, B.; Kristiansson, E.; Abel, F. Malignant Pheochromocytomas/Paragangliomas Harbor Mutations in Transport and Cell Adhesion Genes. Int. J. Cancer 2016, 138, 2201–2211. [Google Scholar] [CrossRef]
- Bayley, J.-P.; Kunst, H.P.M.; Cascon, A.; Sampietro, M.L.; Gaal, J.; Korpershoek, E.; Hinojar-Gutierrez, A.; Timmers, H.J.L.M.; Hoefsloot, L.H.; Hermsen, M.A.; et al. SDHAF2 Mutations in Familial and Sporadic Paraganglioma and Phaeochromocytoma. Lancet Oncol. 2010, 11, 366–372. [Google Scholar] [CrossRef]
- Luchetti, A.; Walsh, D.; Rodger, F.; Clark, G.; Martin, T.; Irving, R.; Sanna, M.; Yao, M.; Robledo, M.; Neumann, H.P.H.; et al. Profiling of Somatic Mutations in Phaeochromocytoma and Paraganglioma by Targeted Next Generation Sequencing Analysis. Int. J. Endocrinol. 2015, 2015, 138573. [Google Scholar] [CrossRef]
- Wadt, K.; Choi, J.; Chung, J.-Y.; Kiilgaard, J.; Heegaard, S.; Drzewiecki, K.T.; Trent, J.M.; Hewitt, S.M.; Hayward, N.K.; Gerdes, A.-M.; et al. A Cryptic BAP1 Splice Mutation in a Family with Uveal and Cutaneous Melanoma, and Paraganglioma. Pigment Cell Melanoma Res. 2012, 25, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.A.; Zöller, B.; Hedelius, A.; Wang, X.; Stenman, E.; Sundquist, J.; Sundquist, K. Quantification of Mitochondrial DNA Copy Number in Suspected Cancer Patients by a Well Optimized ddPCR Method. Biomol. Detect. Quantif. 2017, 13, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef]
- Douvlataniotis, K.; Bensberg, M.; Lentini, A.; Gylemo, B.; Nestor, C.E. No Evidence for DNA N6-Methyladenine in Mammals. Sci. Adv. 2020, 6, eaay3335. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wu, T.; Cui, X.; Zhu, P.; Tan, C.; Dou, X.; Hsu, K.-W.; Lin, Y.-T.; Peng, P.-H.; Zhang, L.-S.; et al. N6-Deoxyadenosine Methylation in Mammalian Mitochondrial DNA. Mol. Cell 2020, 78, 382–395.e8. [Google Scholar] [CrossRef]
- Josephson, H.; Ntzouni, M.; Skoglund, C.; Linder, S.; Turkina, M.V.; Vikström, E. Pseudomonas Aeruginosa N-3-Oxo-Dodecanoyl-Homoserine Lactone Impacts Mitochondrial Networks Morphology, Energetics, and Proteome in Host Cells. Front. Microbiol. 2020, 11, 1069. [Google Scholar] [CrossRef]
- Fokkema, I.F.A.C.; Taschner, P.E.M.; Schaafsma, G.C.P.; Celli, J.; Laros, J.F.J.; den Dunnen, J.T. LOVD v.2.0: The next Generation in Gene Variant Databases. Hum. Mutat. 2011, 32, 557–563. [Google Scholar] [CrossRef]
- Welander, J.; Andreasson, A.; Brauckhoff, M.; Bäckdahl, M.; Larsson, C.; Gimm, O.; Söderkvist, P. Frequent EPAS1/HIF2α Exons 9 and 12 Mutations in Non-Familial Pheochromocytoma. Endocr. Relat. Cancer 2014, 21, 495–504. [Google Scholar] [CrossRef]
- Levinsohn, J.L.; Tian, L.C.; Boyden, L.M.; McNiff, J.M.; Narayan, D.; Loring, E.S.; Yun, D.; Sugarman, J.L.; Overton, J.D.; Mane, S.M.; et al. Whole Exome Sequencing Reveals Somatic Mutations in HRAS and KRAS Which Cause Nevus Sebaceus. J. Investig. Dermatol. 2013, 133, 827. [Google Scholar] [CrossRef]
- Van der Lee, R.; Szklarczyk, R.; Smeitink, J.; Smeets, H.J.M.; Huynen, M.A.; Vogel, R. Transcriptome Analysis of Complex I-Deficient Patients Reveals Distinct Expression Programs for Subunits and Assembly Factors of the Oxidative Phosphorylation System. BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Reznik, E.; Wang, Q.; La, K.; Schultz, N.; Sander, C. Mitochondrial Respiratory Gene Expression Is Suppressed in Many Cancers. eLife 2017, 6, e21592. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.H.; Kamalidehghan, B.; Houshmand, M.; Meng, G.Y.; Sadeghizadeh, M.; Aryani, O.; Nafissi, S. Gene Expression Profiling of Mitochondrial Oxidative Phosphorylation (OXPHOS) Complex I in Friedreich Ataxia (FRDA) Patients. PLoS ONE 2014, 9, e94069. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.F.; Rebelo-Guiomar, P.; D’Souza, A.R.; Powell, C.A.; Van Haute, L.; Minczuk, M. Regulation of Mammalian Mitochondrial Gene Expression: Recent Advances. Trends Biochem. Sci. 2017, 42, 625–639. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dasgupta, S.; Sidransky, D. Mitochondrial Subversion in Cancer. Cancer Prev. Res. 2011, 4, 638–654. [Google Scholar] [CrossRef]
- Kim, A. Mitochondrial DNA Somatic Mutation in Cancer. Toxicol. Res. 2014, 30, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Yusoff, A.A.; Mohd Nasir, K.N.; Haris, K.; Mohd Khair, S.Z.N.; Abdul Ghani, A.R.I.; Idris, Z.; Abdullah, J.M. Detection of Somatic Mutations in the Mitochondrial DNA Control Region D-Loop in Brain Tumors: The First Report in Malaysian Patients. Oncol. Lett. 2017, 14, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Tarnopolsky, M.; Greenamyre, J.; Johns, D. A Frameshift Mitochondrial Complex I Gene Mutation in a Patient with Dystonia and Cataracts: Is the Mutation Pathogenic? J. Med. Genet. 2001, 38, 58–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urra, F.A.; Muñoz, F.; Lovy, A.; Cárdenas, C. The Mitochondrial Complex(I)Ty of Cancer. Front. Oncol. 2017, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic Pathways and the Electron Transport Chain: A DangeROS Liaison. Br. J. Cancer 2019, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Bénit, P.; Chrétien, D.; Bouchereau, J.; Schiff, M.; El-Khoury, R.; Tzagoloff, A.; Rustin, P. Mitochondrial Cytochrome c Oxidase Deficiency. Clin. Sci. 2016, 130, 393–407. [Google Scholar] [CrossRef]
- Lant, J.T.; Berg, M.D.; Heinemann, I.U.; Brandl, C.J.; O’Donoghue, P. Pathways to Disease from Natural Variations in Human Cytoplasmic TRNAs. J. Biol. Chem. 2019, 294, 5294–5308. [Google Scholar] [CrossRef]
- Smith, A.L.M.; Whitehall, J.C.; Bradshaw, C.; Gay, D.; Robertson, F.; Blain, A.P.; Hudson, G.; Pyle, A.; Houghton, D.; Hunt, M.; et al. Age-Associated Mitochondrial DNA Mutations Cause Metabolic Remodeling That Contributes to Accelerated Intestinal Tumorigenesis. Nat. Cancer 2020, 1, 976–989. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.F.; Millet, A.R.; Tigano, M.; Dubois, S.M.; Crimmins, H.; Babin, L.; Charpentier, M.; Piganeau, M.; Brunet, E.; Sfeir, A. Single-Molecule Analysis of MtDNA Replication Uncovers the Basis of the Common Deletion. Mol. Cell 2017, 65, 527–538. [Google Scholar] [CrossRef]
- Nie, H.; Shu, H.; Vartak, R.; Milstein, A.C.; Mo, Y.; Hu, X.; Fang, H.; Shen, L.; Ding, Z.; Lu, J.; et al. Mitochondrial Common Deletion, a Potential Biomarker for Cancer Occurrence, Is Selected against in Cancer Background: A Meta-Analysis of 38 Studies. PLoS ONE 2013, 8, e67953. [Google Scholar] [CrossRef]
- Russell, O.; Turnbull, D. Mitochondrial DNA Disease—Molecular Insights and Potential Routes to a Cure. Exp. Cell Res. 2014, 325, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Alila-Fersi, O.; Tabebi, M.; Maalej, M.; Belguith, N.; Keskes, L.; Mkaouar-Rebai, E.; Fakhfakh, F. First Description of a Novel Mitochondrial Mutation in the MT-TI Gene Associated with Multiple Mitochondrial DNA Deletion and Depletion in Family with Severe Dilated Mitochondrial Cardiomyopathy. Biochem. Biophys. Res. Commun. 2018, 497, 1049–1054. [Google Scholar] [CrossRef]
- Tasdogan, A.; McFadden, D.G.; Mishra, P. Mitochondrial DNA Haplotypes as Genetic Modifiers of Cancer. Trends Cancer 2020, 6, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, H.; Ji, P.; Zhu, X.; Yuan, H.; Jin, G.; Dai, J.; Hu, Z.; Su, Y.; Ma, H. Mitochondrial DNA Copy Number Is Associated with Risk of Head and Neck Squamous Cell Carcinoma in Chinese Population. Cancer Med. 2018, 7, 2776–2782. [Google Scholar] [CrossRef]
- Su, X.; Wang, W.; Ruan, G.; Liang, M.; Zheng, J.; Chen, Y.; Wu, H.; Fahey, T.J.; Guan, M.; Teng, L. A Comprehensive Characterization of Mitochondrial Genome in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2016, 17, 1594. [Google Scholar] [CrossRef]
- Weerts, M.J.A.; Sieuwerts, A.M.; Smid, M.; Look, M.P.; Foekens, J.A.; Sleijfer, S.; Martens, J.W.M. Mitochondrial DNA Content in Breast Cancer: Impact on in Vitro and in Vivo Phenotype and Patient Prognosis. Oncotarget 2016, 7, 29166–29176. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, Y.; Huang, T.; Yan, C.; Yu, F.; Du, J.; Dai, J.; Ma, H.; Jin, G. High Mitochondrial DNA Copy Number Was Associated with an Increased Gastric Cancer Risk in a Chinese Population. Mol. Carcinog. 2017, 56, 2593–2600. [Google Scholar] [CrossRef]
- Errichiello, E.; Venesio, T. Mitochondrial DNA Variations in Tumors: Drivers or Passengers? In Mitochondrial DNA: New Insights; Books on Demand: Norderstedt, Germany, 2018. [Google Scholar] [CrossRef]
- Tan, Z.; Luo, X.; Xiao, L.; Tang, M.; Bode, A.M.; Dong, Z.; Cao, Y. The Role of PGC1α in Cancer Metabolism and Its Therapeutic Implications. Mol. Cancer Ther. 2016, 15, 774–782. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial Biogenesis and Clearance: A Balancing Act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional Control of Mitochondrial Biogenesis: The Central Role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Feilchenfeldt, J.; Bründler, M.A.; Soravia, C.; Tötsch, M.; Meier, C.A. Peroxisome Proliferator-Activated Receptors (PPARs) and Associated Transcription Factors in Colon Cancer: Reduced Expression of PPARgamma-Coactivator 1 (PGC-1). Cancer Lett. 2004, 203, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ba, Y.; Zhang, C.; Zhang, Y.; Zhang, C. [Down-regulation of PGC-1alpha expression in human hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi 2008, 30, 593–597. [Google Scholar]
- Zhang, Y.; Ba, Y.; Liu, C.; Sun, G.; Ding, L.; Gao, S.; Hao, J.; Yu, Z.; Zhang, J.; Zen, K.; et al. PGC-1alpha Induces Apoptosis in Human Epithelial Ovarian Cancer Cells through a PPARgamma-Dependent Pathway. Cell Res. 2007, 17, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic Regulation of Mitochondrial Dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Wieder, S.Y.; Serasinghe, M.N.; Sung, J.C.; Choi, D.C.; Birge, M.B.; Yao, J.L.; Bernstein, E.; Celebi, J.T.; Chipuk, J.E. Activation of the Mitochondrial Fragmentation Protein DRP1 Correlates with BRAF(V600E) Melanoma. J. Invest. Dermatol. 2015, 135, 2544–2547. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial Dynamics Regulates Migration and Invasion of Breast Cancer Cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Bae, G.E.; Kim, K.-H.; Lee, S.-I.; Chung, C.; Lee, D.; Lee, T.H.; Kwon, I.S.; Yeo, M.-K. Prognostic Significance of LC3B and P62/SQSTM1 Expression in Gastric Adenocarcinoma. Anticancer Res. 2019, 39, 6711–6722. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic Regulation of P62 Is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Wang, J.; Wang, X.; Lu, M.; Guo, Y. Involvement of PINK1/Parkin-Mediated Mitophagy in AGE-Induced Cardiomyocyte Aging. Int. J. Cardiol. 2017, 227, 201–208. [Google Scholar] [CrossRef]
- Yoo, S.-M.; Jung, Y.-K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [CrossRef]
- Herrnstadt, C.; Howell, N. An Evolutionary Perspective on Pathogenic MtDNA Mutations: Haplogroup Associations of Clinical Disorders. Mitochondrion 2004, 4, 791–798. [Google Scholar] [CrossRef]
- Richard, C.; Richard, C.; Pennarun, E.; Kivisild, T.; Tambets, K.; Tolk, H.-V.; Metspalu, E.; Reidla, M.; Chevalier, S.; Giraudet, S.; et al. An MtDNA Perspective of French Genetic Variation. Ann. Hum. Biol. 2007, 34, 68–79. [Google Scholar] [CrossRef]
- Welander, J.; Andreasson, A.; Juhlin, C.C.; Wiseman, R.W.; Bäckdahl, M.; Höög, A.; Larsson, C.; Gimm, O.; Söderkvist, P. Rare Germline Mutations Identified by Targeted Next-Generation Sequencing of Susceptibility Genes in Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2014, 99, E1352–E1360. [Google Scholar] [CrossRef]
- Calabrese, C.; Simone, D.; Diroma, M.A.; Santorsola, M.; Guttà, C.; Gasparre, G.; Picardi, E.; Pesole, G.; Attimonelli, M. MToolBox: A Highly Automated Pipeline for Heteroplasmy Annotation and Prioritization Analysis of Human Mitochondrial Variants in High-Throughput Sequencing. Bioinformatics 2014, 30, 3115–3117. [Google Scholar] [CrossRef]
- Castellana, S.; Fusilli, C.; Mazzoccoli, G.; Biagini, T.; Capocefalo, D.; Carella, M.; Vescovi, A.L.; Mazza, T. High-Confidence Assessment of Functional Impact of Human Mitochondrial Non-Synonymous Genome Variations by APOGEE. PLoS Comput. Biol. 2017, 13, e1005628. [Google Scholar] [CrossRef]
- Martín-Navarro, A.; Gaudioso-Simón, A.; Álvarez-Jarreta, J.; Montoya, J.; Mayordomo, E.; Ruiz-Pesini, E. Machine Learning Classifier for Identification of Damaging Missense Mutations Exclusive to Human Mitochondrial DNA-Encoded Polypeptides. BMC Bioinform. 2017, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Niroula, A.; Vihinen, M. PON-Mt-TRNA: A Multifactorial Probability-Based Method for Classification of Mitochondrial TRNA Variations. Nucleic Acids Res. 2016, 44, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Elson, J.L.; Smith, P.M.; Greaves, L.C.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.A.; Taylor, R.W.; Vila-Sanjurjo, A. The Presence of Highly Disruptive 16S RRNA Mutations in Clinical Samples Indicates a Wider Role for Mutations of the Mitochondrial Ribosome in Human Disease. Mitochondrion 2015, 25, 17–27. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]



| Sample | Gene | Mutation | Protein Variation | Mutation Status | Previously Reported |
|---|---|---|---|---|---|
| French cohort | |||||
| F3 | NF1 | c.2044C>T | Q682X | Unknown | Yes (rs1597712392) |
| F4 | RET | c.2753T>C | M918T | Somatic | Yes (rs74799832) |
| F5 | CSDE1 | c.1660C>T | R554X | Unknown | No |
| F6 | RET | c.1900T>C | C634R | Germline | Yes (rs75076352) |
| F7 † | SDHD | c.205_217 del | S69fs | Unknown | No |
| F8 | HRAS | c.37G>C | G13R | Germline | Yes (rs104894228) |
| F9 | EGLN1 | c.153G>A | W51X | Unknown | No |
| F10 | HRAS | c.181C>A | Q61K | Somatic | Yes (rs28933406) |
| F14 | NF1 | c.1885G>A | G629R | Germline | Yes (rs199474738) |
| F20 | NF1 | c.1466A>G | Y489C | Somatic | Yes (rs137854557) |
| F23 | RET | c.1853G>A | C618Y | Somatic | Yes(rs79781594) |
| F24 | RET | c.1900T>C | C634R | Germline | Yes (rs75076352) |
| F27 † | HRAS | c.182A>T | Q61L | Somatic | Yes (rs121913233) |
| F28 | RET | c.2753T>C | M918T | Somatic | Yes (rs74799832) |
| F30 | RET | c.1832G>A | C611Y | Germline | Yes (rs377767397) |
| F31 | NF1 | c.5704_5705 insC | L1902fs | Somatic | No |
| F35 | NF1 | c.3674_3688 del | A1226_V1230 del | Somatic | No |
| F37 | RET | c.2753T>C | M918T | Somatic | Yes (rs74799832) |
| F38 | NF1 | c.7301_7302 delAG | Q2434fs | Somatic | No |
| F41 | RET | c.1902C>G | C634W | Somatic | Yes (rs77709286) |
| F43 † | EGLN1 | c.607_619 del | N203fs | Germline | No |
| F45 | NF1 | c.1904_1907 del | P635fs | Germline | No |
| F58 | SDHA | c.1432_1432+1delGG | 428? | Germline | Yes (rs878854627) |
| F59 | NF1 | c.6841G>T | G2281X | Somatic | Yes [38] |
| F60 | SDHD | c.187_188 delTC | L64fs | Unknown | Yes (rs387906358) |
| Swedish cohort | |||||
| PH 37 | HRAS | c.182A>G | Q61R | Somatic | Yes (rs121913233) |
| PH 38 | EPAS1 | c.1235T>A | I412N | Somatic | Yes [39] |
| PH 40 | FGFR1 | c.1638C>T | R546K | Somatic | Yes [25] |
| PH 41 | HRAS | c.37G>C | G13R | Somatic | Yes [40] |
| PH 42 | RET | c.1893_1898delCGAGCT | Asp631_Leu633delinsGlu | Somatic | Yes (rs121913307) |
| PH 44 | NF1 | c.1340T>C | L447P | Somatic | Yes [22] |
| PH 57 | NF1 | c.4798_4799delAA | K1600fs | Somatic | Yes [25] |
| PH 60 | RET | c.2753T>C | M918T | Somatic | Yes (rs74799832) |
| PH 61 | NF1 | c.2806A>T | K936X | Somatic | Yes [25] |
| PH 62 | VHL | c.284C>G | P95R | Somatic | Yes [25] |
| PH 64 | FGFR1 | c.1638C>T | R546K | Somatic | Yes (rs779707422) |
| PH 65 | RET | c.2753T>C | M918T | Germline | Yes (rs74799832) |
| PH 66 | NF1 | c.289C>T | Q97X | Somatic | Yes (rs1597635615) |
| PH 67 | SDHB | c.664delT | G228fs | Germline | Yes [25] |
| PH 68 | HRAS | c.37G>C | G13R | Somatic | Yes (rs104894228) |
| PH80 | NF1 | c.3158C>G | S1053X | Unknown | Yes (rs1597717610) |
| Gene | Mutation | Protein Variation | HM/HT State (%) | Mutation Status | Predicted Mutation Impact | mt CNV (%) | mt Large Deletion | Phenotype | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MitImpact2 a | mfold b | PON-mt-tRNA c | |||||||||
| French cohort | |||||||||||
| F10 | ND6 | 14173T>C | Val167= | HM | Germline | NA | NA | NA | 41.6 | No | Novel |
| F14 | ND4 | 12068A>G | Met437Val | HM | Germline | Deleterious | NA | NA | 18.6 | No | Novel † |
| Dloop | 16093T>C | non coding | HT (60.3) | Germline | NA | NA | NA | Breast, Thyroid, and Prostate cancers | |||
| F15 | ND5 | 14143A>G | Thr603Ala | HM | Germline | Neutral | NA | NA | 40.9 | No | Novel |
| F16 | ND1 | 3563G>A | Trp86X * | HT (39) | Somatic | NA | NA | NA | 29.6 | No | Novel † |
| F21 | COXIII | 9553G>A | Trp116X * | HT (39.7) | Somatic | NA | NA | NA | 20.8 | No | Novel † |
| F24 | Dloop | 16076C>T | non coding | HT (42.2) | Germline | NA | NA | NA | 35.9 | No | Novel |
| F31 | Dloop | 16183delA | non coding | HT (56) | Somatic | NA | NA | NA | 22.0 | No | Colon cancer |
| F32 | ND2 | 4725A>C | Met86Leu | HT (65.6) | Somatic | Neutral | NA | NA | 17.1 | No | Novel |
| F36 | Dloop | 16093T>C | non coding | HT (67) | Germline | NA | NA | NA | 188.9 | No | Breast, Thyroid, and Prostate cancers |
| F37 | Dloop | 16183delA | non coding | HM | Germline | NA | NA | NA | 9.6 | No | Colon cancer |
| F38 | ND2 | 4789G>A | Gly107Glu | HT (74.2) | Somatic | Deleterious | NA | NA | 15.0 | No | Novel † |
| F41 | ATP8 | 8466A>T | His34Leu | HM | Germline | Deleterious | NA | NA | 19.5 | No | Novel † |
| F44 | Dloop | 16218C>T | non coding | HM | Germline | NA | NA | NA | 17.6 | No | Ovarian and Prostate cancers |
| F46 | ND5 | 13135G>A | Ala267Thr | HM | Germline | Deleterious | NA | NA | 40.9 | No | Cervical and head and neck cancers |
| F48 | ND5 | 12338T>C | Met1Thr | HM | Germline | Deleterious | NA | NA | 3.5 | No | Colon cancer |
| F51 | COXIII | 9349T>C | Leu48Pro | HT (35.9) | Unknown | Deleterious | NA | NA | 22.2 | No | Novel † |
| F52 | CYB | 15789C>T | Thr348Ile | HM | Unknown | Deleterious | NA | NA | 14.6 | No | Breast cancer |
| F54 | COXI | 6072A>G | Ile57Val | HM | Germline | Neutral | NA | NA | 11.8 | No | Novel |
| F56 | Cyb | 15471T>C | Leu24Ser | HM | Germline | Deleterious | NA | NA | 7.7 | No | Novel † |
| F57 | ND1 | 4164A>C | Met286Ile | HT (73.9) | Somatic | Deleterious | NA | NA | 10.0 | No | Novel † |
| Swedish cohort | |||||||||||
| PH35 | ND5 | 13498G>A | Gly388Ser | HT (35.3) | Somatic | Deleterious | NA | NA | 29.0 | Yes | Novel † |
| PH40 | ND3 | 10113A>G | Ile19Val | HM | Germline | Neutral | NA | NA | 57.9 | Yes | Novel |
| tRNAGlu | 14721G>A | NA | HT (57.3) | Somatic | NA | NA | Neutral | Novel | |||
| PH42 | rRNA16s | 2222T>C | non coding | HM | Germline | NA | Deleterious | NA | 36.5 | Yes | Pancreatic cancer |
| PH45 | rRNA 16s | 1969G>A | NA | HT (36.6) | Somatic | NA | Neutral | NA | 46.6 | Yes | Novel |
| PH58 | tRNAAsn | 5658T>C | NA | HT (30.8) | Germline | NA | NA | Deleterious | 32.7 | Yes | Novel † |
| PH60 | ND5 | 13345G>A | Ala337Thr | HT (88.7) | Somatic | Deleterious | NA | NA | 28.3 | Yes | Novel † |
| PH62 | CYB | 15672T>C | Met309Thr | HM | Germline | Deleterious | NA | NA | 20.2 | Yes | Breast and Thyroid cancers |
| PH63 | ND6 | 14603GinsT | Ser24Tyrfsx11 | HT (30.5) | Somatic | NA | NA | NA | 35.3 | Yes | Novel † |
| PH66 | ND3 | 10068G>A | Ala4Thr | HT (33.3) | Somatic | Neutral | NA | NA | 29.0 | No | Novel |
| Identified Proteins | Gene Name | Fold Change | p-Value |
|---|---|---|---|
| mt-OXPHOS | |||
| NADH-ubiquinone oxidoreductase chain 4 | ND4 | 1.8 | 0.3 |
| NADH-ubiquinone oxidoreductase chain 5 | ND5 | 1.6 | 0.71 |
| Cytochrome c oxidase subunit 2 | COX2 | 0.9 | 0.61 |
| ATP synthase subunit | ATP6 | 1.2 | 0.52 |
| ATP synthase protein 8 | ATP8 | * | 0.12 |
| Other Mitochondrial Proteins | |||
| Dynamin-1-like protein | DNM1L | 7.4 | 0.011 |
| Protein-L-isoaspartate O-methyltransferase | PCMT1 | 5.9 | 0.0029 |
| Voltage-dependent anion-selective channel protein 3 | VDAC3 | 2.6 | 0.045 |
| ATP synthase subunit gamma | ATP5F1C | 1.9 | 0.028 |
| ATP synthase subunit d, mitochondrial | ATP5H | 1.9 | 0.019 |
| ATP synthase subunit gamma | ATP5F1C | 1.9 | 0.028 |
| ATP synthase subunit d, mitochondrial | ATP5H | 1.9 | 0.019 |
| NADH dehydrogenase (ubiquinone) 1 alpha subcomplex subunit 13 | NDUFA13 | 1.8 | 0.045 |
| NADH dehydrogenase (ubiquinone) 1 alpha subcomplex subunit 5 | NDUFA5 | 1.3 | 0.028 |
| Cytochrome c oxidase subunit 7C | COX7C | 0.7 | 0.023 |
| Cytochrome b-c1 complex subunit 6 | UQCRH | 0.6 | 0.0033 |
| Trifunctional enzyme subunit alpha | HADHA | 0.6 | 0.0066 |
| Isocitrate dehydrogenase (NAD) subunit alpha | IDH3A | 0.4 | 0.00086 |
| Phosphoglycerate kinase 1 | PGK1 | 0.4 | 0.004 |
| Electron transfer flavoprotein–ubiquinone oxidoreductase | ETFDH | 0.3 | 0.0028 |
| Glutathione S-transferase | GSTM3 | 0.2 | 0.045 |
| Pyruvate carboxylase | PC | 0.1 | 0.024 |
| Succinate-CoA ligase (GDP-forming) subunit beta | SUCLG2 | 0.06 | 0.00049 |
| Acetyl-CoA acetyltransferase | ACAT1 | 0.02 | 0.00023 |
| NADPH: adrenodoxin oxidoreductase, | FDXR | 0.004 | <0.00010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabebi, M.; Łysiak, M.; Dutta, R.K.; Lomazzi, S.; Turkina, M.V.; Brunaud, L.; Gimm, O.; Söderkvist, P. Genetic Alterations in Mitochondrial DNA Are Complementary to Nuclear DNA Mutations in Pheochromocytomas. Cancers 2022, 14, 269. https://doi.org/10.3390/cancers14020269
Tabebi M, Łysiak M, Dutta RK, Lomazzi S, Turkina MV, Brunaud L, Gimm O, Söderkvist P. Genetic Alterations in Mitochondrial DNA Are Complementary to Nuclear DNA Mutations in Pheochromocytomas. Cancers. 2022; 14(2):269. https://doi.org/10.3390/cancers14020269
Chicago/Turabian StyleTabebi, Mouna, Małgorzata Łysiak, Ravi Kumar Dutta, Sandra Lomazzi, Maria V. Turkina, Laurent Brunaud, Oliver Gimm, and Peter Söderkvist. 2022. "Genetic Alterations in Mitochondrial DNA Are Complementary to Nuclear DNA Mutations in Pheochromocytomas" Cancers 14, no. 2: 269. https://doi.org/10.3390/cancers14020269
APA StyleTabebi, M., Łysiak, M., Dutta, R. K., Lomazzi, S., Turkina, M. V., Brunaud, L., Gimm, O., & Söderkvist, P. (2022). Genetic Alterations in Mitochondrial DNA Are Complementary to Nuclear DNA Mutations in Pheochromocytomas. Cancers, 14(2), 269. https://doi.org/10.3390/cancers14020269








