Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
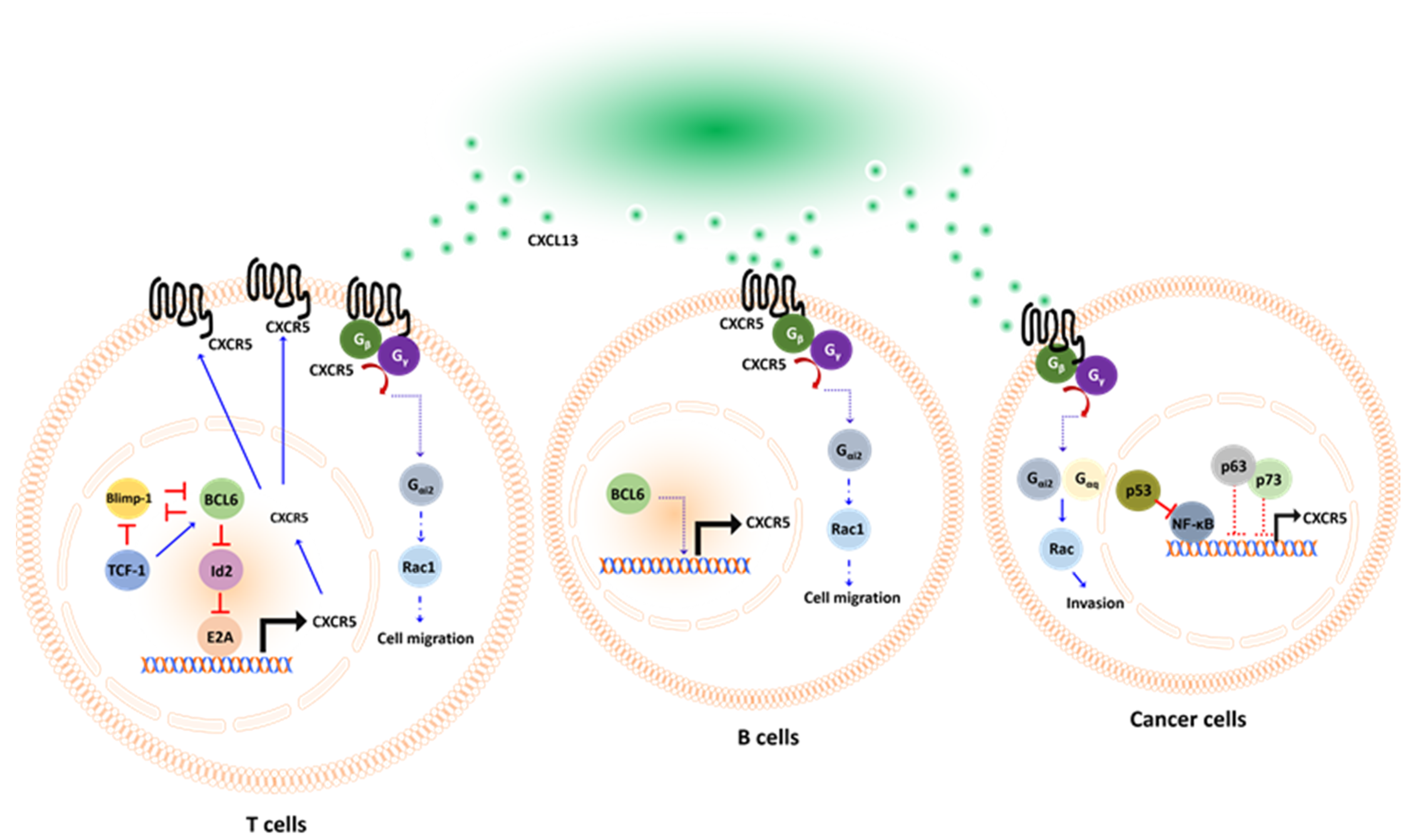
2. The CXCL13/CXCR5 Signaling Axis
3. The Expression and Implications of CXCL13/CXCR5
4. The CXCL13/CXCR5 Signaling Axis in the ICI Response of Preclinical Models
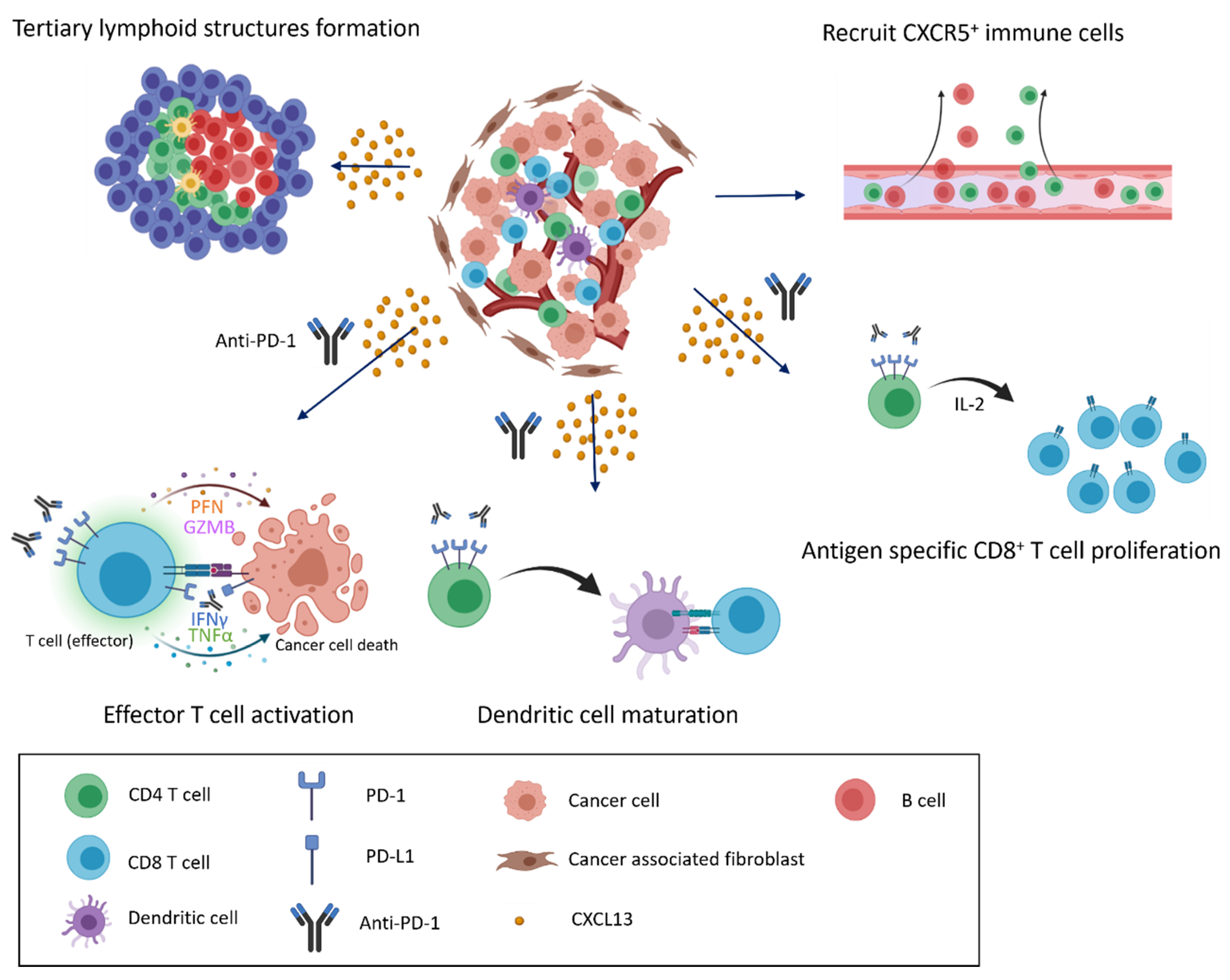
5. CXCL13/CXCR5 Axis for ICI Response in Clinical Tumors
5.1. Breast Cancer
5.2. Bladder Cancer
5.3. Non-Small Cell Lung Cancer
5.4. Hepatocellular Carcinoma
5.5. Pan-Cancers
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Obermajer, N.; Kalinski, P. Quantitative evaluation of tumor-specific T cells in tumors and lymphoid tissues. Methods Enzymol. 2019, 635, 149–166. [Google Scholar] [PubMed]
- Aerts, J.G.; Hegmans, J.P. Tumor-Specific Cytotoxic T Cells Are Crucial for Efficacy of Immunomodulatory Antibodies in Patients with Lung Cancer. Cancer Res. 2013, 73, 2381–2388. [Google Scholar] [CrossRef] [Green Version]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells with Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacqueline, C.; Finn, O.J. Antibodies specific for disease-associated antigens (DAA) expressed in non-malignant diseases reveal potential new tumor-associated antigens (TAA) for immunotherapy or immunoprevention. Semin. Immunol. 2020, 47, 101394. [Google Scholar] [CrossRef]
- Gao, J.-Q.; Okada, N.; Mayumi, T.; Nakagawa, S. Immune Cell Recruitment and Cell-Based System for Cancer Therapy. Pharm. Res. 2008, 25, 752–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leber, T. Uber die Entstehung der Entzundung und die entzundungerregeden Scadliekeiten. Fortschr. Med. 1888, 4, 460. [Google Scholar]
- McCutcheon, M. Chemotaxis in Leukocytes. Physiol. Rev. 1946, 26, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Hereld, D. Moving toward understanding eukaryotic chemotaxis. Eur. J. Cell Biol. 2006, 85, 905–913. [Google Scholar] [CrossRef]
- Bernardini, G.; Zabel, B.A. Editorial: The Role of Chemoattractants in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2671. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bao, Z.; Tang, P.; Gong, W.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell. Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Molon, B.; Gri, G.; Bettella, M.; Gómez-Moutón, C.; Lanzavecchia, A.; Martínez-A, C.; Mañes, S.; Viola, A. T cell costimulation by chemokine receptors. Nat. Immunol. 2005, 6, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Sarukhan, A.; Bronte, V.; Molon, B. The pros and cons of chemokines in tumor immunology. Trends Immunol. 2012, 33, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Fein, M.R.; He, X.-Y.; Almeida, A.S.; Bružas, E.; Pommier, A.; Yan, R.; Eberhardt, A.; Fearon, D.T.; van Aelst, L.; Wilkinson, J.E.; et al. Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J. Exp. Med. 2020, 217, e20181551. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zheng, M.; Li, Y.-M.; Fan, X.-Y.; Wang, J.-C.; Li, Z.-C.; Yang, H.-J.; Yu, J.-M.; Cui, J.; Jiang, J.-L.; et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics 2019, 9, 3659–3673. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadiere, C.; Mami-Chouaib, F. Role of Chemokines and Chemokine Receptors in Shaping the Effector Phase of the Antitumor Immune Response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Zlotnik, A. The Biology of Chemokines and their Receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef]
- Lacalle, R.A.; Blanco, R.; Carmona-Rodríguez, L.; Martín-Leal, A.; Mira, E.; Mañes, S. Chemokine Receptor Signaling and the Hallmarks of Cancer. Int. Rev. Cell Mol. Biol. 2017, 331, 181–244. [Google Scholar]
- Wang, Y.; Xu, P.; Qiu, L.; Zhang, M.; Huang, Y.; Zheng, J. CXCR7 Participates in CXCL12-mediated Cell Cycle and Proliferation Regulation in Mouse Neural Progenitor Cells. Curr. Mol. Med. 2016, 16, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlahakis, S.R.; Villasis-Keever, A.; Gomez, T.; Vanegas, M.; Vlahakis, N.; Paya, C.V. G Protein-Coupled Chemokine Receptors Induce Both Survival and Apoptotic Signaling Pathways. J. Immunol. 2002, 169, 5546–5554. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, S.H.; Baribaud, F.; Coughlan, C.M.; Sunshine, M.J.; Lee, V.M.Y.; Doms, R.W.; Littman, D.R.; Raper, J.A. The Chemokine Stromal Cell-Derived Factor-1 Promotes the Survival of Embryonic Retinal Ganglion Cells. J. Neurosci. 2003, 23, 4601–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, Á.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef] [Green Version]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.; Wang, B.; Yu, H.; Lin, J.; Xia, K.; Hou, W.; Yang, M.; Chen, J.; Yang, M.; Wang, X.; et al. Serum CCL27 predicts the response to Bacillus Calmette-Guerin immunotherapy in non-muscle-invasive bladder cancer. OncoImmunology 2020, 9, 1776060. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansel, K.M.; Ngo, V.; Hyman, P.L.; Luther, S.; Forster, R.; Sedgwick, J.D.; Browning, J.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef]
- Förster, R.; Mattis, A.E.; Kremmer, E.; Wolf, E.; Brem, G.; Lipp, M. A Putative Chemokine Receptor, BLR1, Directs B Cell Migration to Defined Lymphoid Organs and Specific Anatomic Compartments of the Spleen. Cell 1996, 87, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- del Molino del Barrio, I.; Kirby, J.; Ali, S. The Role of Chemokine and Glycosaminoglycan Interaction in Chemokine-Mediated Migration In Vitro and In Vivo. Methods Enzymol. 2016, 570, 309–333. [Google Scholar]
- Meijer, J.; Zeelenberg, I.S.; Sipos, B.; Roos, E. The CXCR5 Chemokine Receptor Is Expressed by Carcinoma Cells and Promotes Growth of Colon Carcinoma in the Liver. Cancer Res. 2006, 66, 9576–9582. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhang, X.; Guo, H.; Fu, L.; Pan, G.; Sun, Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol. Cell. Biochem. 2015, 400, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qian, L.; Chen, X.; Ding, B. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 13217–13224. [Google Scholar]
- Thommen, D.S.; Koelzer, V.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Razis, E.; Kalogeras, K.T.; Kotsantis, I.; Koliou, G.-A.; Manousou, K.; Wirtz, R.; Veltrup, E.; Patsea, H.; Poulakaki, N.; Dionysopoulos, D.; et al. The Role of CXCL13 and CXCL9 in Early Breast Cancer. Clin. Breast Cancer 2020, 20, e36–e53. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, X.; Zhang, W.; Chen, E.; Xu, J.; Wang, F.; Cao, G.; Ju, Z.; Jin, D.; Huang, X.; et al. CXCL-13 Regulates Resistance to 5-Fluorouracil in Colorectal Cancer. Cancer Res. Treat. 2020, 52, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Ruan, H.; Song, Z.; Cao, Q.; Wang, K.; Bao, L.; Liu, D.; Tong, J.; Yang, H.; Chen, K.; et al. Identification of CXCL13 as a potential biomarker in clear cell renal cell carcinoma via comprehensive bioinformatics analysis. Biomed. Pharmacother. 2019, 118, 109264. [Google Scholar] [CrossRef]
- Jiao, F.; Sun, H.; Yang, Q.; Sun, H.; Wang, Z.; Liu, M.; Chen, J. Association of CXCL13 and Immune Cell Infiltration Signature in Clear Cell Renal Cell Carcinoma. Int. J. Med Sci. 2020, 17, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Razis, E.; Kalogeras, K.T.; Kotoula, V.; Eleftheraki, A.; Nikitas, N.; Kronenwett, R.; Timotheadou, E.; Christodoulou, C.; Pectasides, D.; Gogas, H.; et al. Improved Outcome of High-Risk Early HER2 Positive Breast Cancer with High CXCL13-CXCR5 Messenger RNA Expression. Clin. Breast Cancer 2012, 12, 183–193. [Google Scholar] [CrossRef]
- Biswas, S.; Sengupta, S.; Chowdhury, S.R.; Jana, S.; Mandal, G.; Mandal, P.K.; Saha, N.; Malhotra, V.; Gupta, A.; Kuprash, D.V.; et al. CXCL13–CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res. Treat. 2014, 143, 265–276. [Google Scholar] [CrossRef]
- Qi, X.-W.; Xia, S.-H.; Yin, Y.; Jin, L.-F.; Pu, Y.; Hua, D.; Wu, H.-R. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1916–1924. [Google Scholar] [PubMed]
- Singh, R.; Gupta, P.; Kloecker, G.H.; Singh, S.; Lillard, J.W., Jr. Expression and clinical significance of CXCR5/CXCL13 in human non-small cell lung carcinoma. Int. J. Oncol. 2014, 45, 2232–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, R.; Blando, J.M.; Perez, C.J.; Abba, M.; Benavides, F.; Kazanietz, M.G. Protein Kinase C Epsilon Cooperates with PTEN Loss for Prostate Tumorigenesis through the CXCL13-CXCR5 Pathway. Cell Rep. 2017, 19, 375–388. [Google Scholar] [CrossRef]
- El-Haibi, C.P.; Singh, R.; Gupta, P.; Sharma, P.K.; Greenleaf, K.N.; Singh, S.; Lillard, J.W., Jr. Antibody Microarray Analysis of Signaling Networks Regulated by Cxcl13 and Cxcr5 in Prostate Cancer. J. Proteom. Bioinform. 2012, 5, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Ammirante, M.; Shalapour, S.; Kang, Y.; Jamieson, C.A.M.; Karin, M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. USA 2014, 111, 14776–14781. [Google Scholar] [CrossRef] [Green Version]
- Sambandam, Y.; Sundaram, K.; Liu, A.; Kirkwood, K.; Ries, W.L.; Reddy, S.V. CXCL13 activation of c-Myc induces RANK ligand expression in stromal/preosteoblast cells in the oral squamous cell carcinoma tumor-bone microenvironment. Oncogene 2012, 32, 97–105. [Google Scholar] [PubMed] [Green Version]
- Pandruvada, S.; Yuvaraj, S.; Liu, X.; Sundaram, K.; Shanmugarajan, S.; Ries, W.L.; Norris, J.S.; London, S.D.; Reddy, S.V. Role of CXC chemokine ligand 13 in oral squamous cell carcinoma associated osteolysis in athymic mice. Int. J. Cancer 2009, 126, 2319–2329. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Cai, Y.; Chen, H.; Chen, Z.; Zhu, D.; Zhong, Q.; Xie, W. CXCL13/CXCR5 Axis Predicts Poor Prognosis and Promotes Progression Through PI3K/AKT/mTOR Pathway in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2018, 8, 682. [Google Scholar] [CrossRef] [Green Version]
- del Grosso, F.; Coco, S.; Scaruffi, P.; Stigliani, S.; Valdora, F.; Benelli, R.; Salvi, S.; Boccardo, S.; Truini, M.; Croce, M.; et al. Role of CXCL13-CXCR5 Crosstalk Between Malignant Neuroblastoma Cells and Schwannian Stromal Cells in Neuroblastic Tumors. Mol. Cancer Res. 2011, 9, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, I.; Cocco, C.; Morandi, F.; Prigione, I.; Pistoia, V. CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunol. Immunother. 2008, 57, 541–548. [Google Scholar] [CrossRef]
- Cha, Z.; Qian, G.; Zang, Y.; Gu, H.; Huang, Y.; Zhu, L.; Li, J.; Liu, Y.; Tu, X.; Song, H.; et al. Circulating CXCR5+CD4+ T cells assist in the survival and growth of primary diffuse large B cell lymphoma cells through interleukin 10 pathway. Exp. Cell Res. 2017, 350, 154–160. [Google Scholar] [CrossRef]
- Charbonneau, B.; Wang, A.H.; Maurer, M.; Asmann, Y.W.; Zent, C.S.; Link, B.; Ansell, S.M.; Weiner, G.; Ozsan, N.; Feldman, A.; et al. CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunol. Immunother. 2013, 62, 1475–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, G.; Lipp, M. Signal transduction by the chemokine receptor CXCR5: Structural requirements for G protein activation analyzed by chimeric CXCR1/CXCR5 molecules. Biol. Chem. 2001, 382, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- El-Haibi, C.P.; Sharma, P.; Singh, R.; Gupta, P.; Taub, D.D.; Singh, S.; Lillard, J.J.W. Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Mol. Cancer 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyama, Y.; Kano, H.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Dynamic regulation of GDP binding to G proteins revealed by magnetic field-dependent NMR relaxation analyses. Nat. Commun. 2017, 8, 14523. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-B.; Moratz, C.; Huang, N.-N.; Kelsall, B.; Cho, H.; Shi, C.-S.; Schwartz, O.; Kehrl, J.H. Rgs1 and Gnai2 Regulate the Entrance of B Lymphocytes into Lymph Nodes and B Cell Motility within Lymph Node Follicles. Immunity 2005, 22, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.-Y.; Park, C.; Kehrl, J.H. Impaired trafficking of Gnai2+/− and Gnai2−/− T lymphocytes: Implications for T cell movement within lymph nodes. J. Immunol. 2007, 179, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Tybulewicz, V.L.J.; Henderson, R.B. Rho family GTPases and their regulators in lymphocytes. Nat. Rev. Immunol. 2009, 9, 630–644. [Google Scholar] [CrossRef]
- Bellamri, N.; Viel, R.; Morzadec, C.; Lecureur, V.; Joannes, A.; de Latour, B.; Llamas-Gutierrez, F.; Wollin, L.; Jouneau, S.; Vernhet, L. TNF-α and IL-10 Control CXCL13 Expression in Human Macrophages. J. Immunol. 2020, 204, 2492–2502. [Google Scholar] [CrossRef]
- Al-Kufaidy, R.; Vazquez-Tello, A.; BaHammam, A.S.; Al-Muhsen, S.; Hamid, Q.; Halwani, R. IL-17 enhances the migration of B cells during asthma by inducing CXCL13 chemokine production in structural lung cells. J. Allergy Clin. Immunol. 2017, 139, 696. [Google Scholar] [CrossRef] [Green Version]
- Dejardin, E.; Droin, N.M.; Delhase, M.; Haas, E.; Cao, Y.; Makris, C.; Li, Z.-W.; Karin, M.; Ware, C.F.; Green, D.R. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 2002, 17, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Franzoso, G.; Carlson, L.; Poljak, L.; Shores, E.W.; Epstein, S.; Leonardi, A.; Grinberg, A.; Tran, T.; Scharton-Kersten, T.; Anver, M. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 1998, 187, 147–159. [Google Scholar] [CrossRef]
- Li, H.; Xia, J.Q.; Zhu, F.S.; Xi, Z.H.; Pan, C.Y.; Gu, L.M.; Tian, Y.Z. LPS promotes the expression of PD-L1 in gastric cancer cells through NF-κB activation. J. Cell. Biochem. 2018, 119, 9997–10004. [Google Scholar] [CrossRef]
- Gowrishankar, K.; Gunatilake, D.; Gallagher, S.J.; Tiffen, J.; Rizos, H.; Hersey, P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS ONE 2015, 10, e0123410. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Li, Y.; Wang, Z.; Bai, H.; Duan, J.; Wang, S.; Wang, L.; Wang, J. Hypoxia-inducible factor-1α and nuclear factor-κB play important roles in regulating programmed cell death ligand 1 expression by epidermal growth factor receptor mutants in non-small-cell lung cancer cells. Cancer Sci. 2019, 110, 1665–1675. [Google Scholar] [CrossRef]
- Choi, J.; Diao, H.; Faliti, C.E.; Truong, J.; Rossi, M.; Bélanger, S.; Yu, B.; Goldrath, A.W.; Pipkin, M.E.; Crotty, S. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol. 2020, 21, 777–789. [Google Scholar] [CrossRef]
- Miyazaki, M.; Rivera, R.R.; Miyazaki, K.; Lin, Y.; Agata, Y.; Murre, C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat. Immunol. 2011, 12, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.A.; Chen, Y.; Ong, H.S.; Wu, D.; Man, K.; Deleage, C.; Minnich, M.; Meckiff, B.; Wei, Y.; Hou, Z.; et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 2016, 17, 1187–1196. [Google Scholar] [CrossRef]
- Celis, F.P.; Taborda, N.A.; Rugeles, M.T. Follicular CD8+ T Cells: Origin, Function and Importance during HIV Infection. Front. Immunol. 2017, 8, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, L.A.; Bélanger, S.; Omilusik, K.D.; Cho, S.; Scott-Browne, J.P.; Nance, J.P.; Goulding, J.; Lasorella, A.; Lu, L.-F.; Crotty, S. Id2 reinforces TH 1 differentiation and inhibits E2A to repress T FH differentiation. Nat. Immunol. 2016, 17, 834–843. [Google Scholar] [CrossRef]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yu, D. TCF-1 at the Tfh and Th1 Divergence. Trends Immunol. 2015, 36, 758–760. [Google Scholar] [CrossRef]
- Shao, P.; Li, F.; Wang, J.; Chen, X.; Liu, C.; Xue, H.-H. Cutting Edge: Tcf1 Instructs T Follicular Helper Cell Differentiation by Repressing Blimp1 in Response to Acute Viral Infection. J. Immunol. 2019, 203, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shin, H.M.; Moseman, E.A.; Ji, Y.; Huang, B.; Harly, C.; Sen, J.M.; Berg, L.J.; Gattinoni, L.; McGavern, D.B.; et al. TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell Rep. 2015, 12, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.S.; Gullicksrud, J.A.; Xing, S.; Zeng, Z.; Shan, Q.; Li, F.; Love, P.E.; Peng, W.; Xue, H.-H.; Crotty, S. LEF-1 and TCF-1 orchestrate T FH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 2015, 16, 980–990. [Google Scholar] [CrossRef] [Green Version]
- Mitkin, N.; Hook, C.D.; Schwartz, A.M.; Biswas, S.; Kochetkov, D.V.; Muratova, A.M.; Afanasyeva, M.; Kravchenko, J.E.; Bhattacharyya, A.; Kuprash, D.V. p53-dependent expression of CXCR5 chemokine receptor in MCF-7 breast cancer cells. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.; Alagesan, P.; Desai, N.K.; Pack, T.; Wu, J.-H.; Inoue, A.; Freedman, N.J.; Rajagopal, S. C-X-C Motif Chemokine Receptor 3 Splice Variants Differentially Activate Beta-Arrestins to Regulate Downstream Signaling Pathways. Mol. Pharmacol. 2017, 92, 136–150. [Google Scholar] [CrossRef]
- Thompson, B.D.; Jin, Y.; Wu, K.H.; Colvin, R.A.; Luster, A.D.; Birnbaumer, L.; Wu, M.X. Inhibition of Gαi2 activation by Gαi3 in CXCR3-mediated signaling. J. Biol. Chem. 2007, 282, 9547–9555. [Google Scholar] [CrossRef] [Green Version]
- Caggia, S.; Chunduri, H.; Millena, A.C.; Perkins, J.N.; Venugopal, S.V.; Vo, B.T.; Li, C.; Tu, Y.; Khan, S.A. Novel role of Giα2 in cell migration: Downstream of PI3-kinase–AKT and Rac1 in prostate cancer cells. J. Cell. Physiol. 2019, 234, 802–815. [Google Scholar] [CrossRef]
- Denecke, B.; Meyerdierks, A.; Böttger, E.C. RGS1 Is Expressed in Monocytes and Acts as a GTPase-activating Protein for G-protein-coupled Chemoattractant Receptors. J. Biol. Chem. 1999, 274, 26860–26868. [Google Scholar] [CrossRef] [Green Version]
- Legler, D.F.; Loetscher, M.; Roos, R.S.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. B Cell–attracting Chemokine 1, a Human CXC Chemokine Expressed in Lymphoid Tissues, Selectively Attracts B Lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998, 187, 655–660. [Google Scholar] [CrossRef]
- Rouanne, M.; Arpaia, N.; Marabelle, A. CXCL13 shapes tertiary lymphoid structures and promotes response to immunotherapy in bladder cancer. Eur. J. Cancer 2021, 151, 245–248. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Peske, J.D.; Woods, A.N.; Leick, K.M.; Mauldin, I.S.; Meneveau, M.O.; Young, S.J.; Lindsay, R.S.; Melssen, M.M.; Cyranowski, S.; et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 2021, 36, 109422. [Google Scholar] [CrossRef]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. Cxc Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu-Trantien, C.; Migliori, E.; Buisseret, L.; de Wind, A.; Brohée, S.; Garaud, S.; Noël, G.; Chi, V.L.D.; Lodewyckx, J.-N.; Naveaux, C.; et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017, 2, e91487. [Google Scholar] [CrossRef] [Green Version]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Nakayamada, S.; Kanno, Y.; Takahashi, H.; Jankovic, D.; Lu, K.T.; Johnson, T.A.; Sun, H.-W.; Vahedi, G.; Hakim, O.; Handon, R.; et al. Early Th1 Cell Differentiation Is Marked by a Tfh Cell-like Transition. Immunity 2011, 35, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Lönnberg, T.; Svensson, V.; James, K.R.; Fernandez-Ruiz, D.; Sebina, I.; Montandon, R.; Soon, M.S.; Fogg, L.G.; Nair, A.S.; Liligeto, U.; et al. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci. Immunol. 2017, 2, eaal2192. [Google Scholar] [CrossRef] [Green Version]
- Oestreich, K.J.; Huang, A.C.; Weinmann, A.S. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 2011, 208, 1001–1013. [Google Scholar] [CrossRef]
- Lüthje, K.; Kallies, A.; Shimohakamada, Y.; Belz, G.; Light, A.; Tarlinton, D.; Nutt, S. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012, 13, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Pagán, A.J.; Igyártó, B.Z.; Taylor, J.J.; Jenkins, M.K. Opposing Signals from the Bcl6 Transcription Factor and the Interleukin-2 Receptor Generate T Helper 1 Central and Effector Memory Cells. Immunity 2011, 35, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noël, G.; Fontsa, M.L.; Garaud, S.; de Silva, P.; de Wind, A.; Eynden, G.G.V.D.; Salgado, R.; Boisson, A.; Locy, H.; Thomas, N.; et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Investig. 2021, 131, e139905. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, M.A.; Eto, D.; Locci, M.; Cho, M.; Davidson, T.; Haddad, E.K.; Crotty, S. Bcl6 and Maf Cooperate to Instruct Human Follicular Helper CD4 T Cell Differentiation. J. Immunol. 2012, 188, 3734–3744. [Google Scholar] [CrossRef] [Green Version]
- Denton, A.E.; Innocentin, S.; Carr, E.J.; Bradford, B.M.; Lafouresse, F.; Mabbott, N.A.; Mörbe, U.; Ludewig, B.; Groom, J.R.; Good-Jacobson, K.L.; et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J. Exp. Med. 2019, 216, 621–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balança, C.-C.; Salvioni, A.; Scarlata, C.-M.; Michelas, M.; Martinez-Gomez, C.; Gomez-Roca, C.; Sarradin, V.; Tosolini, M.; Valle, C.; Pont, F.; et al. PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 2021, 6, e142513. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Kobayashi, S.; Miyagawa-Hayashino, A.; Okahata, A.; Doi, K.; Nishitani, K.; Murata, K.; Ito, H.; Tsuruyama, T.; Haga, H.; et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat. Commun. 2018, 9, 3762. [Google Scholar] [CrossRef]
- Workel, H.H.; Lubbers, J.M.; Arnold, R.; Prins, T.M.; van der Vlies, P.; de Lange, K.; Bosse, T.; van Gool, I.C.; Eggink, F.A.; Wouters, M.C.; et al. A Transcriptionally Distinct CXCL13+CD103+CD8+ T-cell Population Is Associated with B-cell Recruitment and Neoantigen Load in Human Cancer. Cancer Immunol. Res. 2019, 7, 784–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tang, L.; Guo, L.; Chen, C.; Gu, S.; Zhou, Y.; Ye, G.; Li, X.; Wang, W.; Liao, X.; et al. CXCL13-mediated recruitment of intrahepatic CXCR5+CD8+ T cells favors viral control in chronic HBV infection. J. Hepatol. 2020, 72, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, K.S.; Amé-Thomas, P.; Tarte, K.; Gondois-Rey, F.; Granjeaud, S.; Orlanducci, F.; Foucher, E.D.; Broussais, F.; Bouabdallah, R.; Fest, T.; et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with T(FH) features in Hodgkin lymphomas. Blood Adv. 2018, 2, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Vermi, W.; Lonardi, S.; Bosisio, D.; Uguccioni, M.; Danelon, G.; Pileri, S.; Fletcher, C.; Sozzani, S.; Zorzi, F.; Arrigoni, G.; et al. Identification of CXCL13 as a new marker for follicular dendritic cell sarcoma. J. Pathol. 2008, 216, 356–364. [Google Scholar] [CrossRef]
- Vissers, J.L.M.; Hartgers, F.C.; Lindhout, E.; Figdor, C.G.; Adema, G.J. BLC (CXCL13) is expressed by different dendritic cell subsets in vitro and in vivo. Eur. J. Immunol. 2001, 31, 1544–1549. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Lindqvist, M.; Heit, A.; Wu, J.E.; Reiss, S.M.; Kendric, K.; Bélanger, S.; Kasturi, S.P.; Landais, E.; Akondy, R.S.; et al. CXCL13 is a plasma biomarker of germinal center activity. Proc. Natl. Acad. Sci. USA 2016, 113, 2702–2707. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Xu, Y.; Liu, Y.; Zhu, L.; Wang, L.; Cui, X.; Lu, J.; Zhang, Y.; Zhou, L.; Chen, M.; et al. Gene Expression Subtyping Reveals Immune alterations:TCGA Database for Prognosis in Ovarian Serous Cystadenocarcinoma. Front. Mol. Biosci. 2021, 8, 619027. [Google Scholar]
- Zanetti, C.; Kumar, R.; Ender, J.; Godavarthy, P.S.; Hartmann, M.; Hey, J.; Breuer, K.; Weissenberger, E.S.; Minciacchi, V.R.; Karantanou, C.; et al. The age of the bone marrow microenvironment influences B-cell acute lymphoblastic leukemia progression via CXCR5-CXCL13. Blood 2021, 138, 1870–1884. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, M.; He, Y.; Peng, J.; Zhang, X.; Wang, C.; Xia, X.; Song, W. CXC Chemokines as Therapeutic Targets and Prognostic Biomarkers in Skin Cutaneous Melanoma Microenvironment. Front. Oncol. 2021, 11, 619003. [Google Scholar] [CrossRef]
- Lv, Y.; Lv, D.; Lv, X.; Xing, P.; Zhang, J.; Zhang, Y. Immune Cell Infiltration-Based Characterization of Triple-Negative Breast Cancer Predicts Prognosis and Chemotherapy Response Markers. Front. Genet. 2021, 12, 616469. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Zhan, X. Comprehensive Analysis of Tumor Microenvironment Identified Prognostic Immune-Related Gene Signature in Ovarian Cancer. Front. Genet. 2021, 12, 616073. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Gong, S.; Zhou, H.; Yu, L.; Liang, M.; Shi, R.; Wu, Z.; Zhang, J.; Li, S. Analysis of the Prognosis and Therapeutic Value of the CXC Chemokine Family in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 10, 570736. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.M.; Xiong, L.; et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020, 12, eabc4220. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Hamanishi, J.; Ukita, M.; Yamanoi, K.; Takamatsu, S.; Abiko, K.; Murakami, R.; Miyamoto, T.; Suzuki, H.; Ueda, A.; et al. Tertiary lymphoid structures are associated with favorable survival outcomes in patients with endometrial cancer. Cancer Immunol. Immunother. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.-C. Tumor-Associated Tertiary Lymphoid Structures: A Cancer Biomarker and a Target for Next-generation Immunotherapy. Adv. Exp. Med. Biol. 2021, 1329, 51–68. [Google Scholar]
- Delvecchio, F.R.; Fincham, R.E.; Spear, S.; Clear, A.; Roy-Luzarraga, M.; Balkwill, F.R.; Gribben, J.G.; Bombardieri, M.; Hodivala-Dilke, K.; Capasso, M.; et al. Pancreatic Cancer Chemotherapy Is Potentiated by Induction of Tertiary Lymphoid Structures in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1543–1565. [Google Scholar] [CrossRef]
- Wennhold, K.; Thelen, M.; Lehmann, J.; Schran, S.; Preugszat, E.; Garcia-Marquez, M.; Lechner, A.; Shimabukuro-Vornhagen, A.; Ercanoglu, M.S.; Klein, F.; et al. CD86+ Antigen-Presenting B Cells Are Increased in Cancer, Localize in Tertiary Lymphoid Structures, and Induce Specific T-cell Responses. Cancer Immunol. Res. 2021, 9, 1098–1108. [Google Scholar] [CrossRef]
- Nebhan, C.A.; Cortellini, A.; Ma, W.; Ganta, T.; Song, H.; Ye, F.; Irlmeier, R.; Debnath, N.; Saeed, A.; Radford, M.; et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors Among Patients Aged 80 Years or Older with Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021, 7, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Lu, J.; Zhang, G.; Wang, Y.; He, M.; Xu, Q.; Xu, C.; Liu, H. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 2021, 9, e001136. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Larsen, M.S.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Li, J.; Jiang, K.; Wang, R.; Ge, J.L.; Yang, H.; Liu, S.J.; Jia, L.T.; Wang, L.; Chen, B.L. CDK4/6 inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics 2020, 10, 10619–10633. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.; Liu, R.; Chen, Y.; Ren, C.; Du, S. TOX correlates with prognosis, immune infiltration, and T cells exhaustion in lung adenocarcinoma. Cancer Med. 2020, 9, 6694–6709. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Liao, P.; Yan, L.-Y.; Zhao, Q.-Y.; Xie, Z.-Y.; Dong, J.; Sun, H.-T. Correlation of MKI67 with prognosis, immune infiltration, and T cell exhaustion in hepatocellular carcinoma. BMC Gastroenterol. 2021, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Terranova-Barberio, M.; Pawlowska, N.; Dhawan, M.; Moasser, M.; Chien, A.J.; Melisko, M.E.; Rugo, H.; Rahimi, R.; Deal, T.; Daud, A.; et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat. Commun. 2020, 11, 3584. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti–PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef]
- Tian, S.; Wang, F.; Zhang, R.; Chen, G. Global Pattern of CD8+ T-Cell Infiltration and Exhaustion in Colorectal Cancer Predicts Cancer Immunotherapy Response. Front. Pharmacol. 2021, 12, 715721. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Ou, D.-L.; Bai, L.-Y.; Chen, C.-W.; Lin, L.; Huang, S.-F.; Cheng, A.-L.; Jeng, Y.-M.; Hsu, C. Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Liver Cancer 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Bassez, A.; Vos, H.; van Dyck, L.; Floris, G.; Arijs, I.; Desmedt, C.; Boeckx, B.; Bempt, M.V.; Nevelsteen, I.; Lambein, K.; et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med. 2021, 27, 820–832. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Friedlaender, A.; Kim, C.; Addeo, A. Rethinking the Optimal Duration of Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer Throughout the COVID-19 Pandemic. Front. Oncol. 2020, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, R.; He, D.; Zhang, Y.; Chen, H.; Zhu, Y.; Cheng, Y.; Liu, R.; Zhu, R.; Gong, L.; et al. CD8+ T effector and immune checkpoint signatures predict prognosis and responsiveness to immunotherapy in bladder cancer. Oncogene 2021, 40, 6223–6234. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Lin, L.-I.; Cheng, Y.-C.; Feng, Z.-R.; Shao, Y.-Y.; Cheng, A.-L.; Ou, D.-L. Cyclin E1 Inhibition can Overcome Sorafenib Resistance in Hepatocellular Carcinoma Cells Through Mcl-1 Suppression. Clin. Cancer Res. 2016, 22, 2555–2564. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.-W.; Ho, Y.-J.; Yang, Y.-J.; Liao, H.-A.; Ciou, S.-C.; Lin, L.-I.; Ou, D.-L. Zebrafish as a disease model for studying human hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 12042–12058. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Edeline, J.; Masi, G.; Ma, Y.T.; Wang, W.; Wege, H.; Fei, C.; Ling, C.; Ma, X.; Zhang, P.; et al. 360 Tumor-immune signatures associated with response or resistance to tislelizumab in patients with previously treated advanced hepatocellular carcinoma (HCC). J. Immunother. Cancer 2021, 9, A387. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Gooley, T.A.; Chien, J.W.; Pergam, S.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.J.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; et al. Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response with Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, Y.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res. 2013, 73, 3591–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.-T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and In Vivo Toxicology in Non-Human Primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Balança, C.-C.; Scarlata, C.-M.; Michelas, M.; Devaud, C.; Sarradin, V.; Franchet, C.; Gomez, C.M.; Gomez-Roca, C.; Tosolini, M.; Heaugwane, D.; et al. Dual Relief of T-lymphocyte Proliferation and Effector Function Underlies Response to PD-1 Blockade in Epithelial Malignancies. Cancer Immunol. Res. 2020, 8, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Shen, J.; Zhang, G.; Chen, X.; Wu, J.; Chen, W. CD40 controls CXCR5-induced recruitment of myeloid-derived suppressor cells to gastric cancer. Oncotarget 2015, 6, 38901–38911. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Takemoto, Y.; Deng, H.; Middelhoff, M.; Friedman, R.A.; Chu, T.H.; Churchill, M.J.; Ma, Y.; Nagar, K.K.; Tailor, Y.H.; et al. Histidine decarboxylase (HDC)-expressing granulocytic myeloid cells induce and recruit Foxp3+ regulatory T cells in murine colon cancer. OncoImmunology 2017, 6, e1290034. [Google Scholar] [CrossRef] [Green Version]
- Amarnath, S.; Costanzo, C.M.; Mariotti, J.; Ullman, J.L.; Telford, W.G.; Kapoor, V.; Riley, J.L.; Levine, B.L.; June, C.H.; Fong, T.; et al. Regulatory T Cells and Human Myeloid Dendritic Cells Promote Tolerance via Programmed Death Ligand-1. PLoS Biol. 2010, 8, e1000302. [Google Scholar] [CrossRef] [PubMed]

| Target in the Axis | Treatment | Disease | Experimental Method | Method of Detection | Value | Outcome |
|---|---|---|---|---|---|---|
| CXCR5+ CD8+ T cells | IL-21 Anti-PD-1 | HBV-related HCC | Ex vivo from patients; in vivo in mice | RNA-seq qPCR IHC ELISA Western | Favorable | CXCR5+CD8+ T cells are recruited to the liver, aiding antibody production and controlling the viral load. Anti-PD-1 and IL-21 treatment restore CXCR5+CD8+ T cell function [98]. |
| PD-1hi CXCL13+ CD39+CD4+ T cells | Anti-PD-1 | Head and neck cancer, cervical cancer, and ovarian cancer | Ex vivo from patients | scRNA-Seq | Favorable | PD-1 blockade evokes CD39+CD4+ T cell function and improves dendritic cell maturation and CD8+ T cell proliferation [95]. |
| CXCL13+ immune cells | Anti-PD-1 CXCL13 | Ovarian cancer | In vivo in mice (subcutaneous) | Immunofluorescence IHC ELISA | Favorable | CXCL13 increases CD8+ T cell infiltration at the tumor site and upregulates effector cytokine levels. CXCL13 enhances the anti-PD-1 response [117]. |
| CXCR5+ CXCL13+ B cells | Anti-PD-1 Anti-CTLA4 | Metastatic melanoma | Patients’ tumor samples | IHC Immunofluorescence | Favorable | The co-occurrence of CD20+ B cells and CD8+ T cells is associated with better survival. Tertiary lymphoid structure formation containing CD8+ T cells and CD20+ B cells predicts clinical outcomes for immune checkpoint inhibitors [118]. |
| ID8 cells (cancer cells) secreting CXCL13 | Combination of CDK4/6i and anti-PD-1 | Ovarian cancer | In vivo in mice (ip) | RT Profiler PCR array | Favorable | CDK4/6 inhibition (abemaciclib) enhances CD8+ T cell, and B cell infiltration in a murine ovarian cancer model induces pro-inflammatory responses and increases CXCL13 secretion, which recruits additional lymphocytes to the tumor microenvironment. CDK4/6 inhibition and anti-PD-1 combination improve treatment efficacy in ovarian cancer [119]. |
| Cancer-associated fibroblasts expressing CXCL13 | Anti-PD-L1 Anti-CTLA4 | Melanoma and colon adenocarcinoma | In vivo in mice (ip, subcutaneous) | Real-time PCR Immunofluorescence | Favorable | Cancer-associated fibroblasts depend on tumor necrosis factor receptor signaling to orchestrate tumor-associated TLS development, and CD8+ T cells organize cancer-associated fibroblasts into reticular networks. The number and size of tumor-associated TLSs with discrete B and T cells are associated with favorable responses to immune checkpoint blockade [83]. |
| Target in the Axis | Treatment | Disease | Method of Detection | Number of Patients Investigated | Value | Outcome |
|---|---|---|---|---|---|---|
| CXCL13+PD1+CD8+ T cells | Anti-PD-1 | Non-small cell lung cancer | Transcriptome analysis | Peripheral blood of healthy donors (n = 6) Fraction of PD-1bright within CD8+ TILs (n = 24) | Favorable | The presence of PD-1+CD8+ T cells can predict PD-1 blockade response and survival rate [34]. |
| CXCL13 | Anti-PD-1 Anti-PD-L1 | Metastatic urothelial carcinoma and bladder cancer | Whole-exome sequencing data analysis TCGA analysis | CheckMate275 (n = 270) IMvigor210 (n = 310) | Favorable | CXCL13 expression plus ARID1A mutation work together to predict a favorable response to anti-PD-1 blockade [109]. |
| CXCL13 | Anti-PD-L1 | Bladder cancer | Single-sample GSEA Gene ontology analysis KEGG analysis WGCNA | IMvigor210 (n = 310) | Favorable | CXCL13 expression plus TLS formation predict a favorable response to anti-PD-1 blockade [130]. |
| CXCL13+/LAG3+CD8+ T cells | Anti-PD-1 Anti-PD-L1 | Hepatocellular carcinoma | Multiplex immunofluorescence staining TCGA-LIHC analysis Nanostring RNA analysis | Cohort 1 (n = 24) Cohort 2 (n = 18) | Favorable | CXCL13 expression plus exhausted T cells marker expression predict a favorable response to anti-PD-1 blockade [125]. |
| CXCL13+CD8+ T cells CXCL13+CD4+ T cells | Anti-PD-1 Nab-Paclitaxel | Triple-negative breast cancer | ATAC-seq RNA-seq Single-cell RNA seq Whole-exome sequencing IHC | n = 22 | Favorable | High levels of baseline CXCL13+ T cells predict favorable response to anti-PD-L1 plus nab-paclitaxel combination therapy [131]. |
| CXCL13 in CD8+ T cells | Anti-PD-L1, Anti-PD-1, Anti-CTLA4 | Seven cancer types | Single-cell RNA-seq ATAC-seq | n = 1008 | Favorable | CXCL13 expression is a marker of clonal neoantigen-specific CD8+ TILs that selectively expresses in CPI responders (“CR/PR”). [132]. |
| CXCL13 in tumor cells | Anti-PD-1 | Pan-cancer | Nanostring RNA analysis IHC Gene expression profiles | NCT01295827 (n = 1260) NCT01848834 (n =297) NCT02054806 (n = 477) | Favorable | T cells expanded signature including CXCL13 and 17 other genes are necessary for clinical response to PD-1 checkpoint blockade [133]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-H.; Jian, C.-Z.; Lin, L.-I.; Low, G.-S.; Ou, P.-Y.; Hsu, C.; Ou, D.-L. Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer. Cancers 2022, 14, 294. https://doi.org/10.3390/cancers14020294
Hsieh C-H, Jian C-Z, Lin L-I, Low G-S, Ou P-Y, Hsu C, Ou D-L. Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer. Cancers. 2022; 14(2):294. https://doi.org/10.3390/cancers14020294
Chicago/Turabian StyleHsieh, Ching-Hung, Cheng-Zhe Jian, Liang-In Lin, Guan-Sian Low, Ping-Yun Ou, Chiun Hsu, and Da-Liang Ou. 2022. "Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer" Cancers 14, no. 2: 294. https://doi.org/10.3390/cancers14020294
APA StyleHsieh, C.-H., Jian, C.-Z., Lin, L.-I., Low, G.-S., Ou, P.-Y., Hsu, C., & Ou, D.-L. (2022). Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer. Cancers, 14(2), 294. https://doi.org/10.3390/cancers14020294






