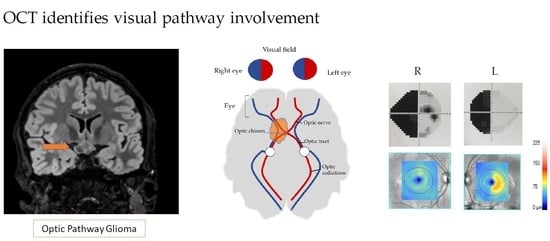
Optical Coherence Tomography Identifies Visual Pathway Involvement Earlier than Visual Function Tests in Children with MRI-Verified Optic Pathway Gliomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Glioma Diagnosis and Location
2.2. Visual Acuity
2.3. Visual Field
2.4. GC-IPL Layer
2.5. Statistical Analyses
2.6. Quantitative Categorizing Comparisons
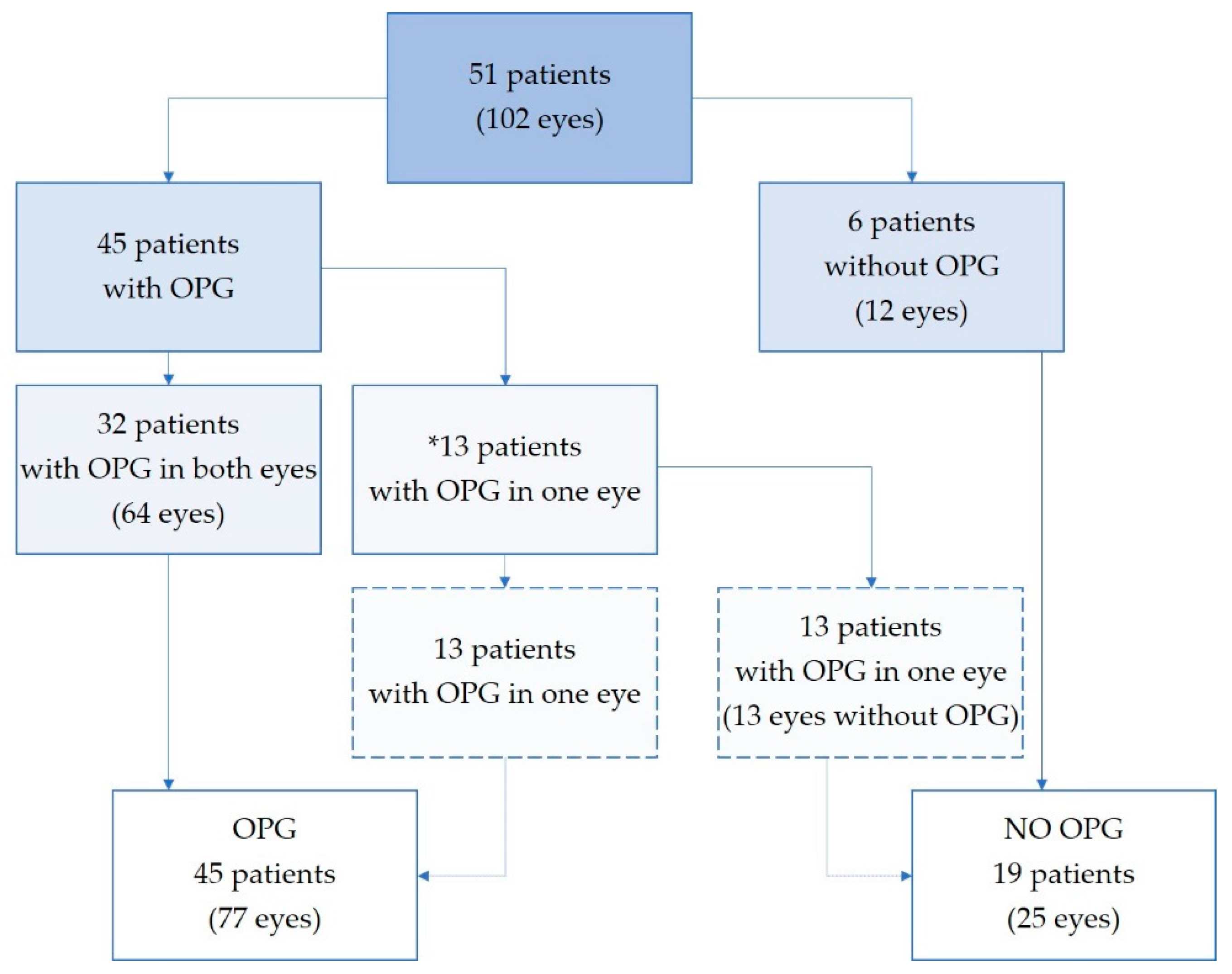
3. Results
3.1. MRI
3.2. Visual Acuity
3.3. Refraction
3.4. Visual Fields
3.5. OCT
3.6. OCT, VF, and VA
3.7. Quantitative Categorizing Comparisons
3.7.1. Optical Coherence Tomography and Visual Field Examinations
3.7.2. Optical Coherence Tomography vs. Magnetic Resonance Imaging
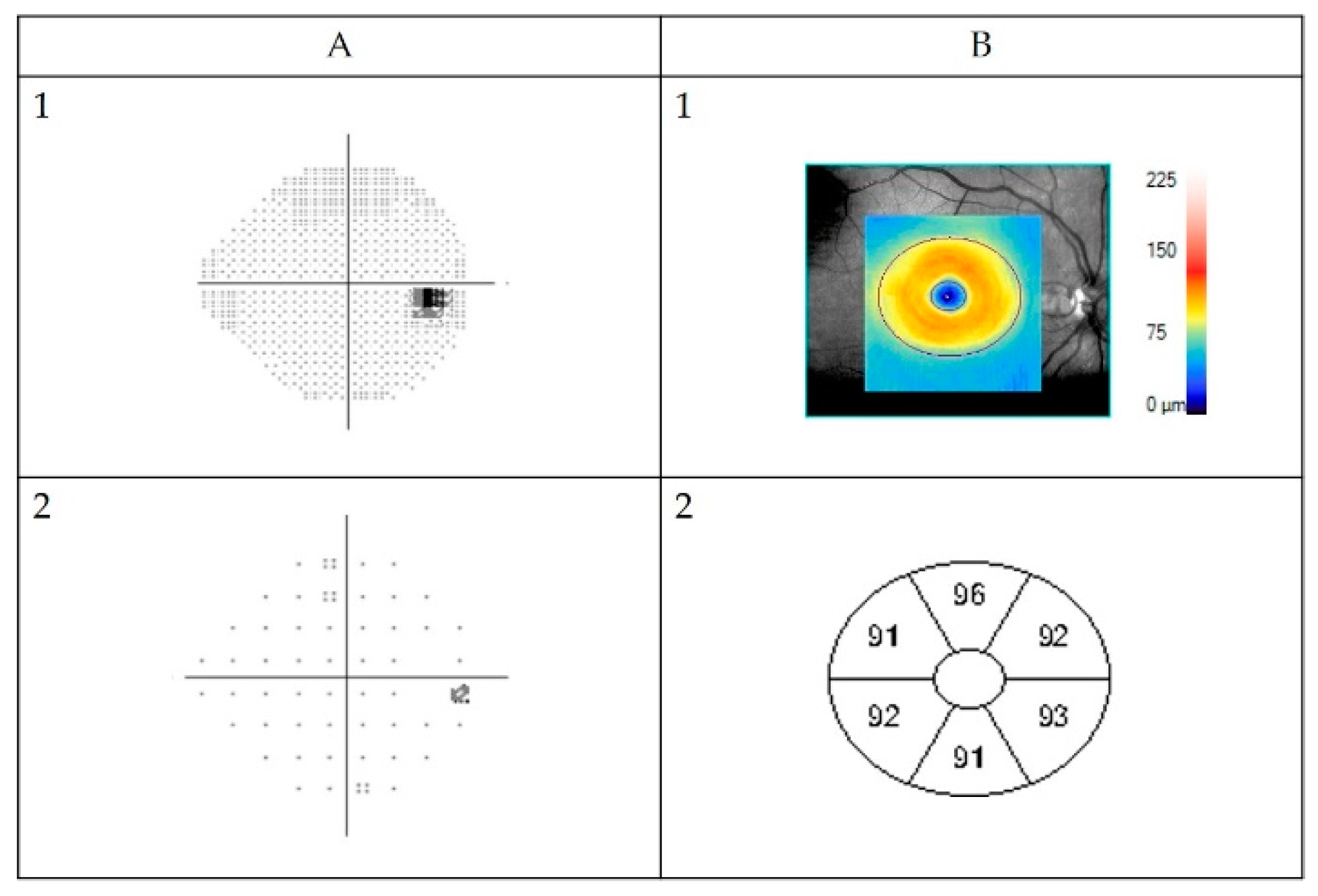
3.7.3. Representative Cases
Case A
Case B
Case C
Case D
Case E
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K.J.; Cullen, J.; Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Langer, C.E.; Turner, M.C.; McKean-Cowdin, R.; Fisher, J.L.; Lupo, P.J.; Partap, S.; et al. Childhood brain tumor epidemiology: A brain tumor epidemiology consortium review. Cancer Epidemiol. Prev. Biomark. 2014, 23, 2716–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Listernick, R.; Charrow, J.; Greenwald, M.; Mets, M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: A longitudinal study. J. Pediatrics 1994, 125, 63–66. [Google Scholar] [CrossRef]
- Blazo, M.A.; Lewis, R.A.; Chintagumpala, M.M.; Frazier, M.; McCluggage, C.; Plon, S.E. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. Am. J. Med. Genet. Part A 2004, 127A, 224–229. [Google Scholar] [CrossRef]
- Dutton, J.J. Gliomas of the anterior visual pathway. Surv. Ophthalmol. 1994, 38, 427–452. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Giannini, C.; Asmann, Y.W.; Sharma, M.K.; Perry, A.; Tibbetts, K.M.; Jenkins, R.B.; Scheithauer, B.W.; Anant, S.; Jenkins, S.; et al. Gene expression profiling of NF-1-associated and sporadic pilocytic astrocytoma identifies aldehyde dehydrogenase 1 family member L1 (ALDH1L1) as an underexpressed candidate biomarker in aggressive subtypes. J. Neuropathol. Exp. Neurol. 2008, 67, 1194–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamji, M.F.; Benoit, B.G. Syndromic and sporadic pediatric optic pathway gliomas: Review of clinical and histopathological differences and treatment implications. Neurosurg. Focus 2007, 23, E3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Zhong, Y. Demographic and prognostic factors of optic nerve astrocytoma: A retrospective study of surveillance, epidemiology, and end results (SEER). BMC Cancer 2021, 21, 976. [Google Scholar] [CrossRef]
- Magli, A.; Forte, R.; Cinalli, G.; Esposito, F.; Parisi, S.; Capasso, M.; Papparella, A. Functional changes after treatment of optic pathway paediatric low-grade gliomas. Eye 2013, 27, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Avery, R.A.; Fisher, M.J.; Liu, G.T. Optic pathway gliomas. J. Neuroophthalmol. 2011, 31, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassiman, C.; Legius, E.; Spileers, W.; Casteels, I. Ophthalmological assessment of children with neurofibromatosis type 1. Eur. J. Pediatrics 2013, 172, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Listernick, R.; Louis, D.N.; Packer, R.J.; Gutmann, D.H. Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann. Neurol. 1997, 41, 143–149. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh-Meyer, H.V.; Wong, A.; Papchenko, T.; Matheos, K.; Stylli, S.; Nichols, A.; Frampton, C.; Daniell, M.; Savino, P.J.; Kaye, A.H. Optical coherence tomography predicts visual outcome for pituitary tumors. J. Clin. Neurosci. 2015, 22, 1098–1104. [Google Scholar] [CrossRef]
- Jindahra, P.; Petrie, A.; Plant, G.T. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009, 132 Pt 3, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.; Sanchez-Dalmau, B.F.; Villoslada, P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS ONE 2014, 9, e97444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, L.; Lennartsson, F.; Nilsson, M. Retinal ganglion cell topography predicts visual field function in spastic cerebral palsy. Dev. Med. Child Neurol. 2020, 62, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, L.; Lennartsson, F.; Nilsson, M. Ganglion Cell Topography Indicates Pre- or Postnatal Damage to the Retro-Geniculate Visual System, Predicts Visual Field Function and May Identify Cerebral Visual Impairment in Children—A Multiple Case Study. Neuroophthalmology 2019, 43, 363–370. [Google Scholar] [CrossRef]
- Herro, A.M.; Lam, B.L. Retrograde degeneration of retinal ganglion cells in homonymous hemianopsia. Clin. Ophthalmol. 2015, 9, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Dodgshun, A.J.; Elder, J.E.; Hansford, J.R.; Sullivan, M.J. Long-term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer 2015, 121, 4190–4196. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.; Jaspan, T.; Milano, G.; Gregson, R.; Parker, T.; Ritzmann, T.; Benson, C.; Walker, D. Radiological classification of optic pathway gliomas: Experience of a modified functional classification system. Br. J. Radiol. 2008, 81, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Maresky, H.S.; Ely, A.B.; Bartischovsky, T.; Coret-Simon, J.; Morad, Y.; Rozowsky, S.; Klar, M.; Negieva, S.; Bekerman, I.; Tal, S. MRI measurements of the normal pediatric optic nerve pathway. J. Clin. Neurosci. 2018, 48, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Mncube, S.S.; Goodier, M.D. Normal measurements of the optic nerve, optic nerve sheath and optic chiasm in the adult population. SA J. Radiol. 2019, 23, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyvarinen, L.; Nasanen, R.; Laurinen, P. New visual acuity test for pre-school children. Acta Ophthalmol. 1980, 58, 507–511. [Google Scholar] [CrossRef]
- Moutakis, K.; Stigmar, G.; Hall-Lindberg, J. Using the KM visual acuity chart for more reliable evaluation of amblyopia compared to the HVOT method. Acta Ophthalmol. Scand. 2004, 82, 547–551. [Google Scholar] [CrossRef]
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Donahue, S.P.; Porter, A. SITA visual field testing in children. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2001, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.E.; Cumberland, P.M.; Walters, B.C.; Russell-Eggitt, I.; Rahi, J.S.; OPTIC Study Group. Study of Optimal Perimetric Testing in Children (OPTIC): Feasibility, Reliability and Repeatability of Perimetry in Children. PLoS ONE 2015, 10, e0130895. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Thomas, R.; Muliyil, J.P.; George, R. Role of frequency doubling perimetry in detecting neuro-ophthalmic visual field defects. Am. J. Ophthalmol. 2001, 131, 734–741. [Google Scholar] [CrossRef]
- Arnljots, U.; Nilsson, M.; Hed Myrberg, I.; Åden, U.; Hellgren, K. Profile of macular ganglion cell-inner plexiform layer thickness in healthy 6.5 year- old Swedish children. BMC Ophthalmol. 2020, 20, 329. [Google Scholar] [CrossRef]
- Rosner, B.; Glynn, R.J.; Lee, M.L. Extension of the rank sum test for clustered data: Two-group comparisons with group membership defined at the subunit level. Biometrics 2006, 62, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, X.; Lee, M.L.T.; Rosner, B.; Yan, J. Wilcoxon Rank-Based Tests for Clustered Data with R Package clusrank. J. Stat. Softw. 2020, 96, 1–26. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 6 October 2021).
- Gu, S.; Glaug, N.; Cnaan, A.; Packer, R.J.; Avery, R.A. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1402–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepokur, M.; Sarici, A.M. Investigation of retinal nerve fiber layer thickness and ganglion cell layer-inner plexiform layer thickness in patients with optic pathway gliomas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1757–1765. [Google Scholar] [CrossRef]
- Na, J.H.; Lee, K.; Lee, J.R.; Baek, S.; Yoo, S.J.; Kook, M.S. Detection of macular ganglion cell loss in preperimetric glaucoma patients with localized retinal nerve fibre defects by spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2013, 41, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Karti, O.; Nalbantoglu, O.; Abali, S.; Ayhan, Z.; Tunc, S.; Kusbeci, T.; Ozkan, B. Retinal Ganglion Cell Loss in Children With Type 1 Diabetes Mellitus Without Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Tieger, M.G.; Hedges, T.R., III; Ho, J.; Erlich-Malona, N.K.; Vuong, L.N.; Athappilly, G.K.; Mendoza-Santiesteban, C.E. Ganglion Cell Complex Loss in Chiasmal Compression by Brain Tumors. J. Neuroophthalmol. 2017, 37, 7–12. [Google Scholar] [CrossRef]
- Saidha, S.; Syc, S.B.; Durbin, M.K.; Eckstein, C.; Oakley, J.D.; Meyer, S.A.; Conger, A.; Frohman, T.C.; Newsome, S.; Ratchford, J.N.; et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult. Scler. J. 2011, 17, 1449–1463. [Google Scholar] [CrossRef]
- Walter, S.D.; Ishikawa, H.; Galetta, K.M.; Sakai, R.E.; Feller, D.J.; Henderson, S.B.; Wilson, J.A.; Maguire, M.G.; Galetta, S.L.; Frohman, E.; et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012, 119, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Bowd, C.; Zangwill, L.M.; Weinreb, R.N.; Medeiros, F.A.; Belghith, A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am. J. Ophthalmol. 2017, 175, 37–44. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnljots, U.; Nilsson, M.; Sandvik, U.; Myrberg, I.H.; Munoz, D.M.; Blomgren, K.; Hellgren, K. Optical Coherence Tomography Identifies Visual Pathway Involvement Earlier than Visual Function Tests in Children with MRI-Verified Optic Pathway Gliomas. Cancers 2022, 14, 318. https://doi.org/10.3390/cancers14020318
Arnljots U, Nilsson M, Sandvik U, Myrberg IH, Munoz DM, Blomgren K, Hellgren K. Optical Coherence Tomography Identifies Visual Pathway Involvement Earlier than Visual Function Tests in Children with MRI-Verified Optic Pathway Gliomas. Cancers. 2022; 14(2):318. https://doi.org/10.3390/cancers14020318
Chicago/Turabian StyleArnljots, Urszula, Maria Nilsson, Ulrika Sandvik, Ida Hed Myrberg, Daniel Martin Munoz, Klas Blomgren, and Kerstin Hellgren. 2022. "Optical Coherence Tomography Identifies Visual Pathway Involvement Earlier than Visual Function Tests in Children with MRI-Verified Optic Pathway Gliomas" Cancers 14, no. 2: 318. https://doi.org/10.3390/cancers14020318
APA StyleArnljots, U., Nilsson, M., Sandvik, U., Myrberg, I. H., Munoz, D. M., Blomgren, K., & Hellgren, K. (2022). Optical Coherence Tomography Identifies Visual Pathway Involvement Earlier than Visual Function Tests in Children with MRI-Verified Optic Pathway Gliomas. Cancers, 14(2), 318. https://doi.org/10.3390/cancers14020318