Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Patients
2.2. Statistical Analysis
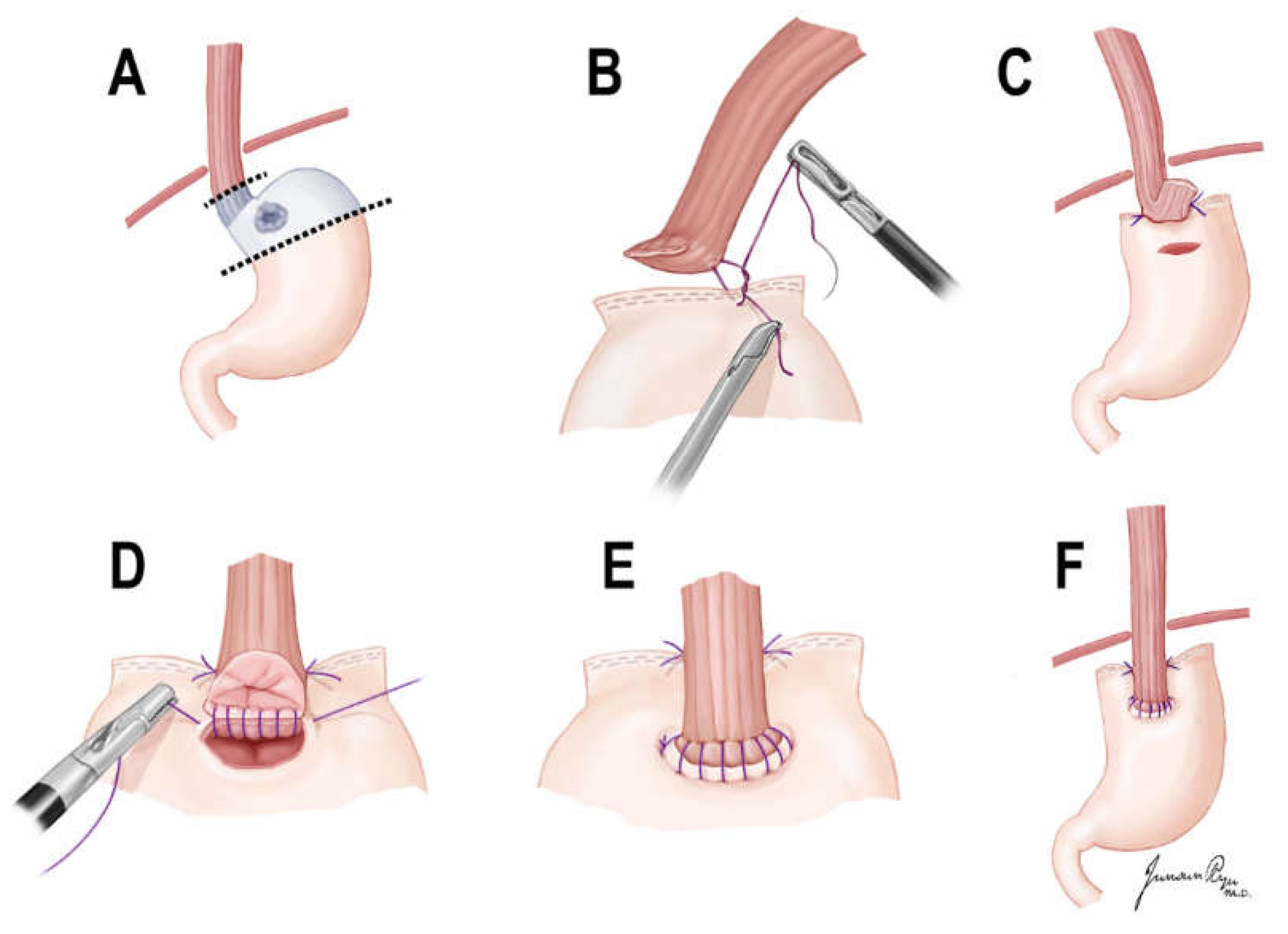
2.3. Surgical Techniques
3. Results
3.1. Patient Demographics and Clinicopathologic Findings of Patients
3.2. Postoperative Endoscopic Findings and Reflux Symptom in 1 Year Follow-Up
3.3. Short Term Outcomes of Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [CrossRef]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G.; The Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association; Group, D.W.; Panel, R. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef]
- Deans, C.; Yeo, M.S.; Soe, M.Y.; Shabbir, A.; Ti, T.K.; So, J.B. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J. Surg. 2011, 35, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Dassen, A.E.; Lemmens, V.E.; van de Poll-Franse, L.V.; Creemers, G.J.; Brenninkmeijer, S.J.; Lips, D.J.; Vd Wurff, A.A.; Bosscha, K.; Coebergh, J.W. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: A population-based study in the Netherlands. Eur. J. Cancer 2010, 46, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, T.; Takiguchi, S.; Hirao, M.; Imamura, H.; Kimura, Y.; Fujita, J.; Miyashiro, I.; Tamura, S.; Hiratsuka, M.; Kobayashi, K.; et al. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: A multi-institutional retrospective study. World J. Surg. 2014, 38, 1100–1106. [Google Scholar] [CrossRef]
- Katai, H.; Morita, S.; Saka, M.; Taniguchi, H.; Fukagawa, T. Long-term outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br. J. Surg. 2010, 97, 558–562. [Google Scholar] [CrossRef]
- Tanioka, T.; Waratchanont, R.; Fukuyo, R.; Saito, T.; Umebayashi, Y.; Kanemoto, E.; Kobayashi, K.; Nakagawa, M.; Inokuchi, M. Surgical and nutritional outcomes of laparoscopic proximal gastrectomy versus total gastrectomy: A meta-analysis. Surg. Endosc. 2020, 34, 1061–1069. [Google Scholar] [CrossRef]
- Toyomasu, Y.; Ogata, K.; Suzuki, M.; Yanoma, T.; Kimura, A.; Kogure, N.; Yanai, M.; Ohno, T.; Mochiki, E.; Kuwano, H. Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg. Endosc. 2017, 31, 1393–1401. [Google Scholar] [CrossRef]
- Kosuga, T.; Ichikawa, D.; Komatsu, S.; Okamoto, K.; Konishi, H.; Shiozaki, A.; Fujiwara, H.; Otsuji, E. Feasibility and Nutritional Benefits of Laparoscopic Proximal Gastrectomy for Early Gastric Cancer in the Upper Stomach. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S929–S935. [Google Scholar] [CrossRef]
- Takiguchi, N.; Takahashi, M.; Ikeda, M.; Inagawa, S.; Ueda, S.; Nobuoka, T.; Ota, M.; Iwasaki, Y.; Uchida, N.; Kodera, Y.; et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): A nationwide multi-institutional study. Gastric Cancer 2015, 18, 407–416. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.H.; Park, D.J.; Kim, H.H. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 2013, 16, 282–289. [Google Scholar] [CrossRef]
- Ushimaru, Y.; Fujiwara, Y.; Shishido, Y.; Yanagimoto, Y.; Moon, J.H.; Sugimura, K.; Omori, T.; Miyata, H.; Yano, M. Clinical Outcomes of Gastric Cancer Patients Who Underwent Proximal or Total Gastrectomy: A Propensity Score-Matched Analysis. World J. Surg. 2018, 42, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, K.B.; Kwon, O.K.; Yu, W. Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Choda, Y.; Otsuka, S.; Ueyama, S.; Tanaka, N.; Muraoka, A.; Hato, S.; Kimura, T.; Tanakaya, K.; Kikuchi, S.; et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann. Gastroenterol. Surg. 2019, 3, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Han, W.H.; Eom, B.W.; Yoon, H.M.; Ryu, J.; Kim, Y.W. Spade-Shaped Anastomosis Following a Proximal Gastrectomy Using a Double Suture to Fix the Posterior Esophageal Wall to the Anterior Gastric Wall (SPADE Operation): Case-Control Study of Early Outcomes. J. Gastric Cancer 2020, 20, 72–80. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Bennett, J.R.; Blum, A.L.; Dent, J.; De Dombal, F.T.; Galmiche, J.P.; Lundell, L.; Margulies, M.; Richter, J.E.; Spechler, S.J.; et al. The Endoscopic Assessment of Esophagitis: A Progress Report on Observer Agreement. Gastroenterology 1996, 111, 85–92. [Google Scholar] [CrossRef]
- Kubo, M.S.M.; Gotoda, T.; Ono, H.; Fujishiro, M.; Saito, D.; Sano, T.; Katai, H. Endoscopic evaluation of the remnant stomach after gastrectomy: Proposal for a new classification. Gastric Cancer 2002, 5, 83–89. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Jung, D.H.; Lee, Y.; Kim, D.W.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg. Endosc. 2017, 31, 3961–3969. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jung, D.H.; Son, S.Y.; Lee, C.M.; Park, D.J.; Kim, H.H. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014, 17, 562–570. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, K.H.; Lee, S.H.; Choi, C.W.; Kim, S.J.; In Choi, C.; Kim, D.H.; Kim, D.H.; Hwang, S.H. Can Proximal Gastrectomy with Double-Tract Reconstruction Replace Total Gastrectomy? A Propensity Score Matching Analysis. J. Gastrointest. Surg. 2020, 24, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Shaibu, Z.; Chen, Z.; Mzee, S.A.S.; Theophilus, A.; Danbala, I.A. Effects of reconstruction techniques after proximal gastrectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2020, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Hayami, M.; Hiki, N.; Nunobe, S.; Mine, S.; Ohashi, M.; Kumagai, K.; Ida, S.; Watanabe, M.; Sano, T.; Yamaguchi, T. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann. Surg. Oncol. 2017, 24, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Tanabe, K.; Yamamoto, Y.; Ohta, H.; Saito, R.; Ohdan, H. Laparoscopic proximal gastrectomy with hinged double flap method using knotless barbed absorbable sutures: A case series. Int. J. Surg. Case Rep. 2018, 51, 165–169. [Google Scholar] [CrossRef]
- Park, D.J.; Park, Y.S.; Ahn, S.H.; Kim, H.H. Laparoscopic Proximal Gastrectomy as a Surgical Treatment for Upper Third Early Gastric Cancer. Korean J. Gastroenterol. 2017, 70, 134–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, M.; Yamaue, H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: A review of the literature published from 2000 to 2014. Surg. Today 2016, 46, 517–527. [Google Scholar] [CrossRef]
- Tomita, R.; Fujisaki, S.; Tanjoh, K.; Fukuzawa, M. A novel operative technique on proximal gastrectomy reconstructed by interposition of a jejunal J pouch with preservation of the vagal nerve and lower esophageal sphincter. Hepatogastroenterology 2001, 48, 1186–1191. [Google Scholar] [PubMed]
- Huh, Y.J.; Lee, H.J.; Oh, S.Y.; Lee, K.G.; Yang, J.Y.; Ahn, H.S.; Suh, Y.S.; Kong, S.H.; Lee, K.U.; Yang, H.K. Clinical Outcome of Modified Laparoscopy-Assisted Proximal Gastrectomy Compared to Conventional Proximal Gastrectomy or Total Gastrectomy for Upper-Third Early Gastric Cancer with Special References to Postoperative Reflux Esophagitis. J. Gastric Cancer 2015, 15, 191–200. [Google Scholar] [CrossRef]

| Variable | Value [Number (%)] |
|---|---|
| Age † (year) | 60.5 ± 10.7 (33–82) |
| BMI † (kg/m2) | 24.6 ± 2.9 (18.7–32.0) |
| Sex | |
| Male | 29 (85.3%) |
| Female | 5 (14.7%) |
| Location of tumor | |
| Cardia | 16 (47.1%) |
| Fundus | 2 (5.9%) |
| High body | 16 (47.1%) |
| Size of tumor ‡ (cm) | 2.0 (1.5–2.8) |
| Histology | |
| WD | 7 (21.2%) |
| MD | 13 (39.4%) |
| PD | 6 (18.2%) |
| SRC | 7 (21.2%) |
| ASA score | |
| 1 | 10 (29.4%) |
| 2 | 20 (58.8%) |
| 3 | 4 (11.8%) |
| c T classification | |
| cT1a | 18 (52.9%) |
| cT1b | 15 (44.1%) |
| cT2 | 1 (2.9%) |
| c N classification | |
| cN0 | 32 (94.1%) |
| cN1 | 2 (5.9%) |
| c Stage | |
| Ia | 30 (88.2%) |
| Ib | 4 (11.8%) |
| Variable | Value [Number (%)] |
|---|---|
| Reflux esophagitis in EGD | |
| No | 33 (97.1%) |
| LA-A | 0 (0%) |
| LA-B | 1 (2.9%) |
| LA-C | 0 (0%) |
| Bile reflux in EGD | |
| Grade 0 | 28 (82.4%) |
| Grade 1 | 6 (17.6%) |
| Residual food in EGD | |
| Grade 0 | 18 (52.9%) |
| Grade 1 | 3 (8.8%) |
| Grade 2 | 3 (8.8%) |
| Grade 3 | 10 (29.4%) |
| Reflux symptoms | |
| No symptom | 28 (82.4%) |
| Mild symptom | 5 (14.7%) |
| Moderate symptom | 1 (2.9%) |
| Severe symptom | 0 (0%) |
| Variable | Value [Number (%)] |
|---|---|
| Operating time † (min) | 245.4 ± 42.2 (175–340) |
| Estimated blood loss ‡ (ml) | 30.0 (10.0–100.0) |
| Postoperative hospital stay ‡ (day) | 7.0 (7.0–8.0) |
| Stage | |
| Ia | 21 (61.8%) |
| Ib | 9 (26.5%) |
| IIa | 3 (8.8%) |
| IIb | 1 (2.9%) |
| Postoperative complications | |
| No | 25 (73.5%) |
| Anastomotic stricture | 5 (14.7%) |
| Postoperative ileus | 4 (11.8%) |
| Clavien Dindo classification | |
| II | 4 (11.8%) |
| IIIa | 5 (14.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Khalayleh, H.; Kim, S.G.; Eom, S.S.; Merei, F.; Ryu, J.; Kim, Y.-W. Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series. Cancers 2022, 14, 379. https://doi.org/10.3390/cancers14020379
Park SH, Khalayleh H, Kim SG, Eom SS, Merei F, Ryu J, Kim Y-W. Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series. Cancers. 2022; 14(2):379. https://doi.org/10.3390/cancers14020379
Chicago/Turabian StylePark, Sin Hye, Harbi Khalayleh, Sung Gon Kim, Sang Soo Eom, Fahed Merei, Junsun Ryu, and Young-Woo Kim. 2022. "Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series" Cancers 14, no. 2: 379. https://doi.org/10.3390/cancers14020379
APA StylePark, S. H., Khalayleh, H., Kim, S. G., Eom, S. S., Merei, F., Ryu, J., & Kim, Y.-W. (2022). Spade-Shaped Anastomosis after Laparoscopic Proximal Gastrectomy Using Double Suture Anchoring between the Posterior Wall of the Esophagus and the Anterior Wall of the Stomach (SPADE Operation): A Case Series. Cancers, 14(2), 379. https://doi.org/10.3390/cancers14020379






