Therapeutic Potential of Naturally Occurring Small Molecules to Target the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Wnt/β-Catenin Signaling Pathway
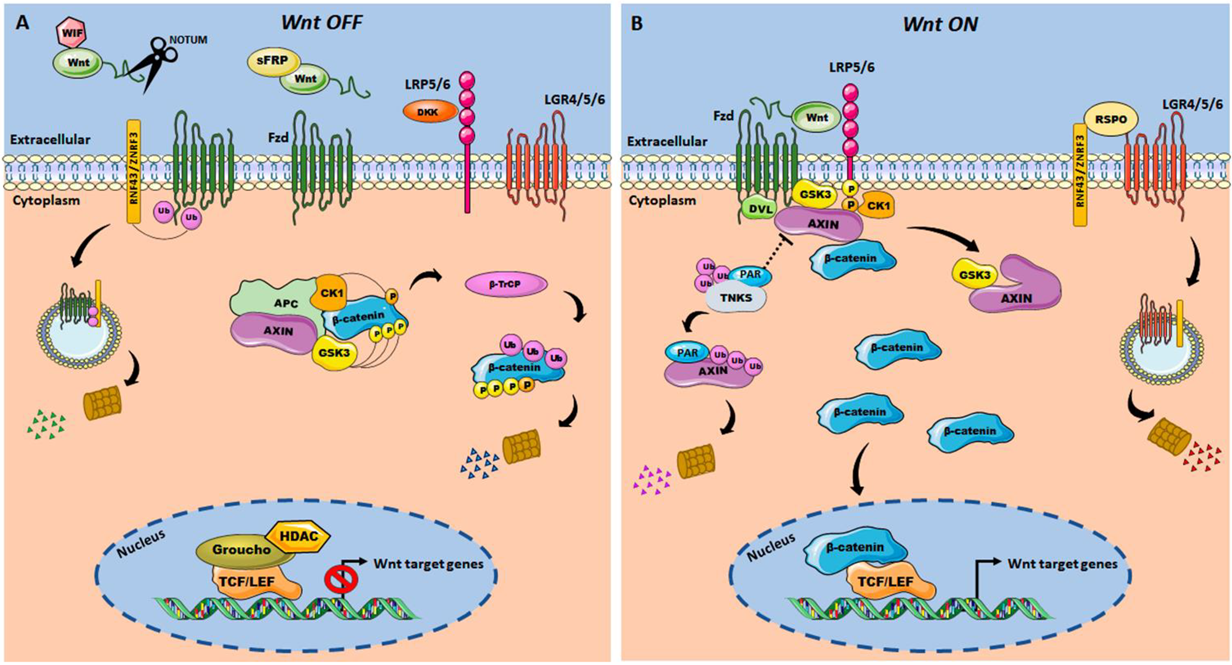
Wnt/β-Catenin Pathway in Colorectal Cancer
3. Systemic CRC Therapy and Wnt/β-Catenin Signaling Pathway
4. Wnt/β-Catenin Components as a Target for Natural-Derived Small Molecules-Based Therapy in CRC
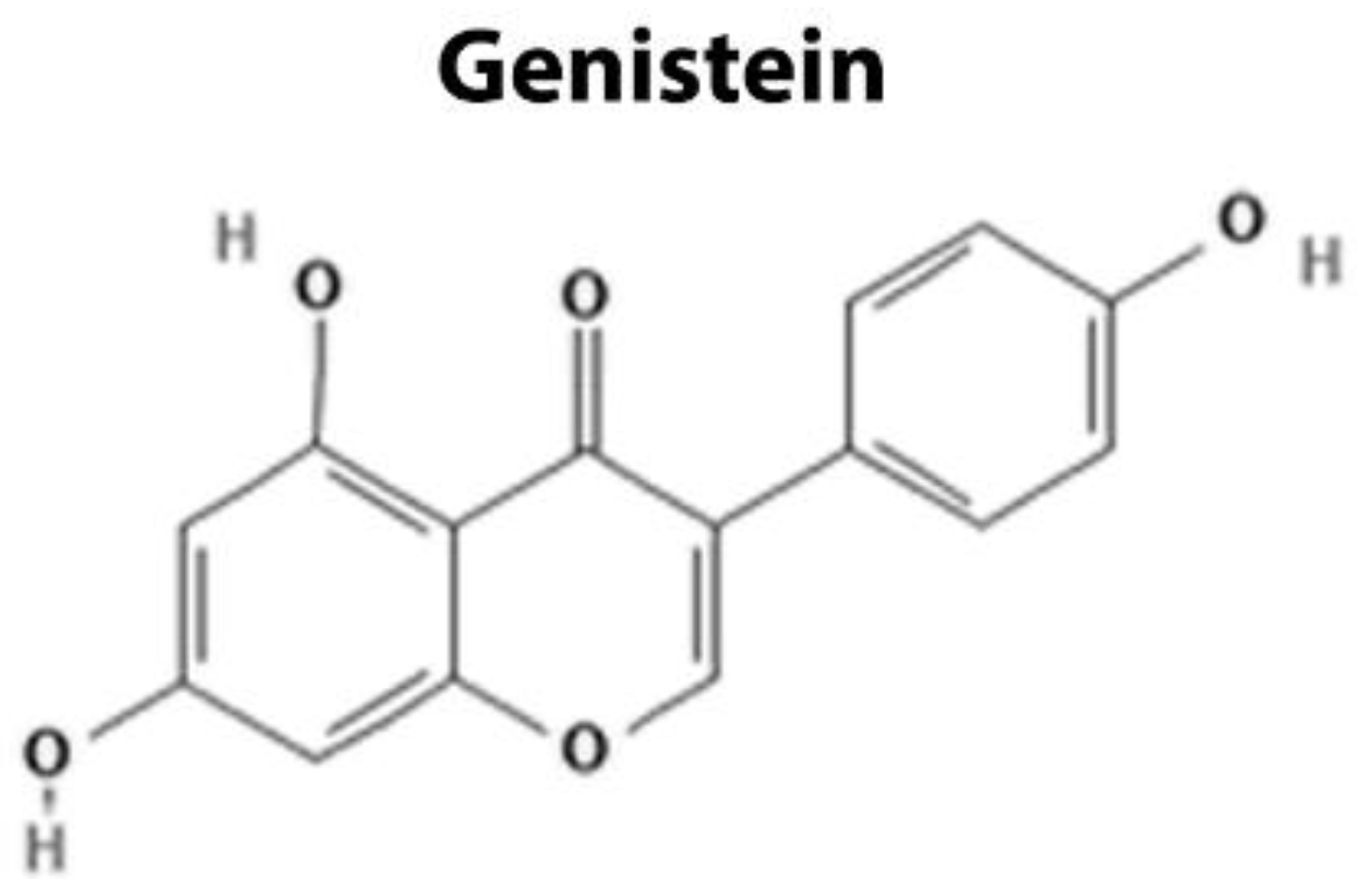
4.1. Targeting Extracellular Components
| Level | Agent | Target | Molecular Mechanism | Reference |
|---|---|---|---|---|
| Extracellular Domain | Genistein | DKK1 WIF1 sFRP Wnt5a | Upregulation of Wnt antagonists and impairment of the interaction between Wnt-ligand and its receptors | [101,102,103,104,105,106,107] |
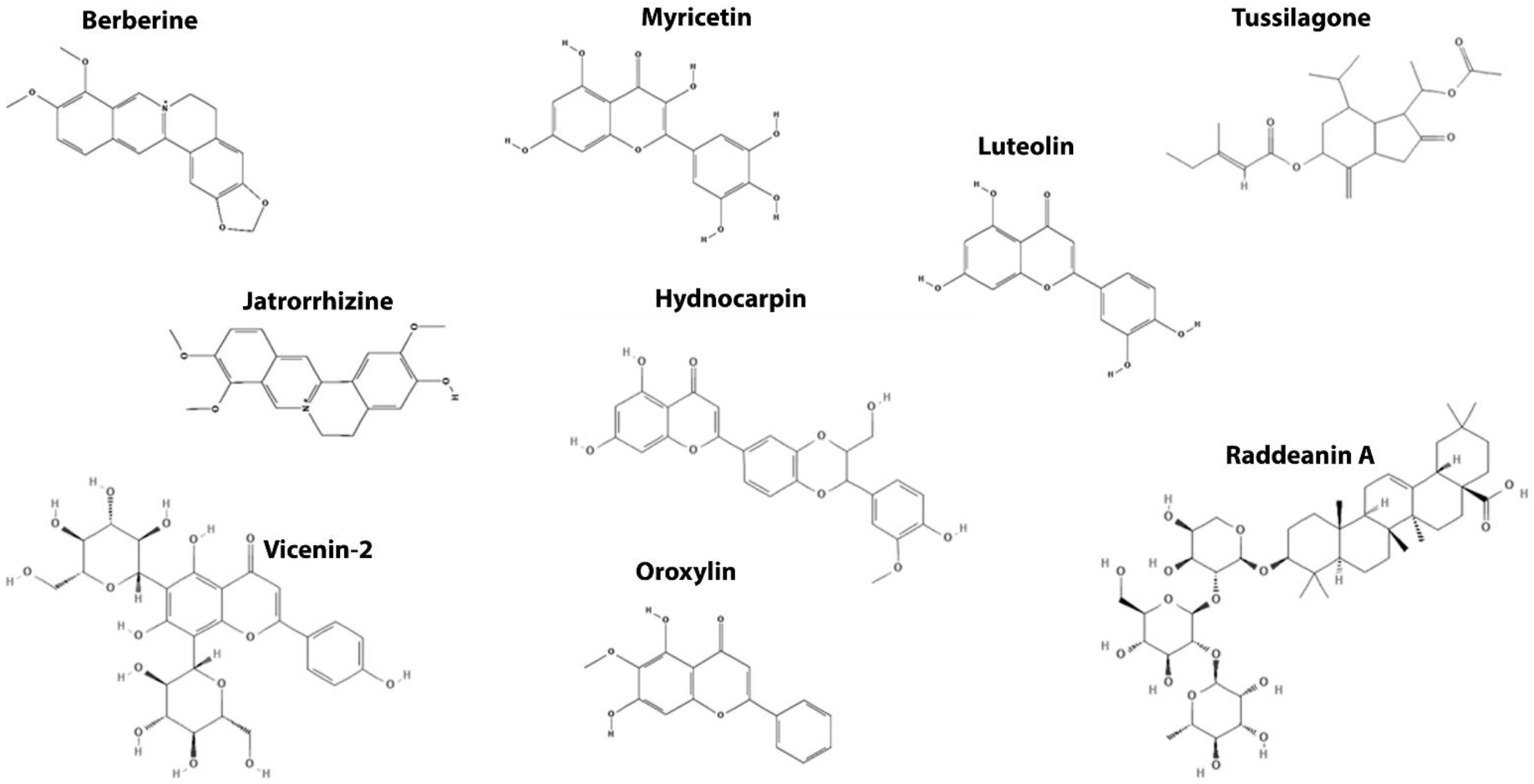
| Destruction Complex | Myricetin Oroxylin A Luteolin Vicenin-2 Jatrorrhizine Raddeanin A | GSK3 | Promotes β-catenin phosphorylation and degradation | [108,109] |
| [110,111] | ||||
| [110,111] | ||||
| [112,113] | ||||
| [113] | ||||
| [114] | ||||
| Hydnocarpin | AXIN | [115] | ||
| Berberine | APC | [116,117,118] | ||
| Tussilagone (TSL) | Unknown | [119] | ||
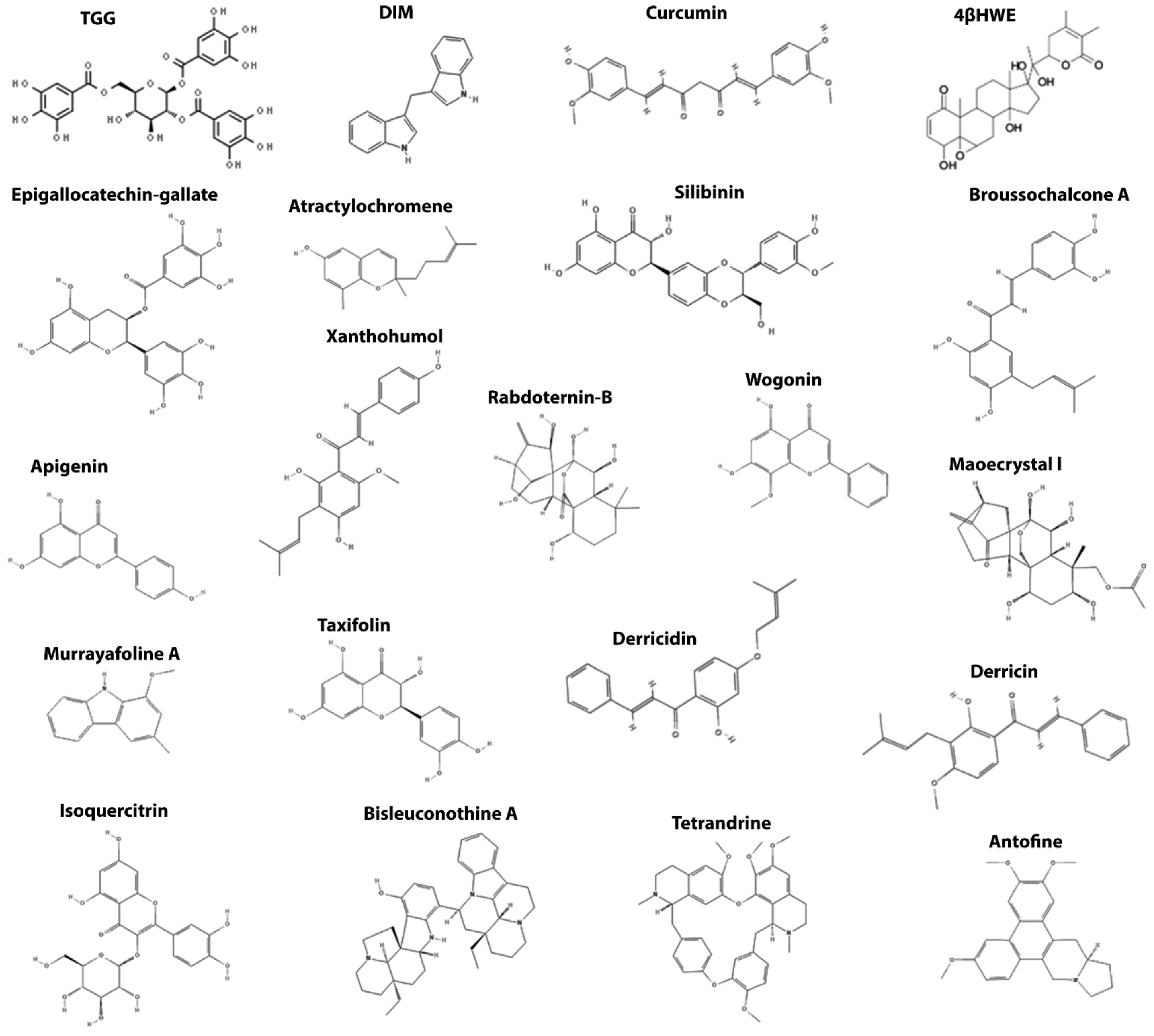
| β-catenin | TGG | β-catenin | Promotes β-catenin degradation and impairs its nuclear translocation | [120] |
| DIM | [121] | |||
| Curcumin | [122,123,124,125] | |||
| 4βHWE | [126] | |||
| EGCG | [127,128] | |||
| Atractylochromene | [129] | |||
| Silibinin | [130,131] | |||
| Broussochalcone | [132] | |||
| Apigenin | [133,134,135] | |||
| Xanthohumol | [136] | |||
| Rabdoternin B | [137] | |||
| Maoecrystal I | ||||
| Wogonin | [138] | |||
| Isoquercitrin | [139] | |||
| Taxifolin | [140,141] | |||
| Derricin | [142] | |||
| Derricidin | ||||
| Antofine | [143] | |||
| Bisleuconothine A | [144] | |||
| Murrayafoline A | [145] | |||
| Tetrandrine | [146] | |||
| Transcriptional Machinery | Magnolol Resveratrol Lonchocarpin Piperine Esculetin 2-Hydroxycinnamaldehyde | β-catenin/TCF | Disrupts β-catenin/TCF interaction | [147] |
| [148,149,150,151,152] | ||||
| [153] | ||||
| [154] | ||||
| [155] | ||||
| [156] | ||||
| Carnosic acid | β-catenin/BCL9 | Disrupts β-catenin/BCL9 interaction | [157] |
4.2. Targeting the Destruction Complex
4.3. Targeting β-Catenin
4.4. Targeting the β-Catenin Transcriptional Activity

5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 1–27. [Google Scholar] [CrossRef]
- Ghuman, S.; van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Wulaningsih, W. Serum Inflammatory Markers and Colorectal Cancer Risk and Survival. Br. J. Cancer 2017, 116, 1358–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; la Vecchia, C.; et al. Alcohol Drinking and Colorectal Cancer Risk: An Overall and Dose–Response Meta-Analysis of Published Studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
- Liang, P.S.; Chen, T.-Y.; Giovannucci, E. Cigarette Smoking and Colorectal Cancer Incidence and Mortality: Systematic Review and Meta-Analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Song, S.; Kim, Y.S.; Yang, S.Y.; Lee, J.E. The Association between Predicted Inflammatory Status and Colorectal Adenoma. Sci. Rep. 2020, 10, 2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Rametta Giuliano, S.; Tralongo, P. Early Colorectal Cancer: Diagnosis, Treatment and Survivorship Care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [Google Scholar] [CrossRef]
- Goodarzi, E.; Sohrabivafa, M.; Dehkordi, A.; Khazaei, Z. Worldwide Incidence and Mortality of Bladder Cancer and Human Development Index: An Ecological Study. Indian J. Med. Spec. 2020, 11, 88. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Mohammadian, M.; Mohammadian-Hafshejani, A.; Salehiniya, H. The Incidence and Mortality of Colorectal Cancer and Its Relationship with the Human Development Index in Asia. Ann. Glob. Health 2017, 82, 726. [Google Scholar] [CrossRef] [Green Version]
- Rafiemanesh, H.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Sepehri, Z.; Shamlou, R.; Salehiniya, H.; Towhidi, F.; Makhsosi, B.R. Incidence and Mortality of Colorectal Cancer and Relationships with the Human Development Index across the World. Asian Pac. J. Cancer Prev. 2016, 17, 2465–2473. [Google Scholar] [CrossRef]
- Colorectal Cancer Facts and Figures 2020–2022. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf (accessed on 1 January 2022).
- Cancer Treatment and Survivorship Facts and Figures 2019–2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf (accessed on 1 January 2022).
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer Stem Cells in Colorectal Cancer: A Review. J. Clin. Pathol. 2018, 71, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Neerven, S.M.; Vermeulen, L. The Interplay between Intrinsic and Extrinsic Wnt Signaling in Controlling Intestinal Transformation. Differentiation 2019, 108, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Clevers, H. Cell Fate Specification and Differentiation in the Adult Mammalian Intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Clevers, H. Regulation and Plasticity of Intestinal Stem Cells during Homeostasis and Regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Sabates–Bellver, J.; Oving, I.; Haegebarth, A.; de Palo, M.; Anti, M.; van Gijn, M.E.; Suijkerbuijk, S.; van de Wetering, M.; Marra, G.; et al. The Intestinal Wnt/TCF Signature. Gastroenterology 2007, 132, 628–632. [Google Scholar] [CrossRef]
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt Pathway Is Involved in 5-FU Drug Resistance of Colorectal Cancer Cells. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.B.; Yan, C.; Mu, L.; Mi, Y.L.; Zhao, H.; Hu, H.; Li, X.L.; Tao, D.D.; Wu, Y.Q.; Gong, J.P.; et al. Exosomal Wnt-Induced Dedifferentiation of Colorectal Cancer Cells Contributes to Chemotherapy Resistance. Oncogene 2019, 38, 1951–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.-H.; Ro, E.J.; Yoon, J.-S.; Mizutani, T.; Kang, D.-W.; Park, J.-C.; il Kim, T.; Clevers, H.; Choi, K.-Y. 5-FU Promotes Stemness of Colorectal Cancer via P53-Mediated WNT/β-Catenin Pathway Activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, H.; Tan, S.; Li, Y.; Zhou, Y.; Wang, L.; Liu, C.; Li, Q.; Cen, X.; Yang, S.; et al. Synergistic Antitumor Effect of 5-Fluorouracil with the Novel LSD1 Inhibitor ZY0511 in Colorectal Cancer. Ther. Adv. Med. Oncol. 2020, 12, 175883592093742. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [Green Version]
- van Amerongen, R.; Nusse, R. Towards an Integrated View of Wnt Signaling in Development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Niehrs, C. The Complex World of WNT Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt Signaling in Development and Tissue Homeostasis. Development 2018, 145, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamos, J.L.; Weis, W.I. The β-Catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Behrens, J.; Jerchow, B.A.; Würtele, M.; Grimm, J.; Asbrand, C.; Wirtz, R.; Kühl, M.; Wedlich, D.; Birchmeier, W. Functional Interaction of an Axin Homolog, Conductin, with Beta-Catenin, APC, and GSK3beta. Science 1998, 280, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Munemitsu, S.; Albert, I.; Souza, B.; Rubinfeld, B.; Polakis, P. Regulation of Intracellular Beta-Catenin Levels by the Adenomatous Polyposis Coli (APC) Tumor-Suppressor Protein. Proc. Natl. Acad. Sci. USA 1995, 92, 3046–3050. [Google Scholar] [CrossRef] [Green Version]
- Amit, S.; Hatzubai, A.; Birman, Y.; Andersen, J.S.; Ben-Shushan, E.; Mann, M.; Ben-Neriah, Y.; Alkalay, I. Axin-Mediated CKI Phosphorylation of Beta-Catenin at Ser 45: A Molecular Switch for the Wnt Pathway. Genes Dev. 2002, 16, 1066–1076. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of GSK3beta to the APC-Beta-Catenin Complex and Regulation of Complex Assembly. Science 1996, 272, 1023–1026. [Google Scholar] [CrossRef]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A Dual-Kinase Mechanism for Wnt Co-Receptor Phosphorylation and Activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Kimelman, D.; Xu, W. β-Catenin Destruction Complex: Insights and Questions from a Structural Perspective. Oncogene 2006, 25, 7482–7491. [Google Scholar] [CrossRef] [Green Version]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. β-Catenin Is a Target for the Ubiquitin–Proteasome Pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, R.A.; Cox, R.T.; Moline, M.M.; Roose, J.; Polevoy, G.A.; Clevers, H.; Peifer, M.; Bejsovec, A. Drosophila Tcf and Groucho Interact to Repress Wingless Signalling Activity. Nature 1998, 395, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Roose, J.; Molenaar, M.; Peterson, J.; Hurenkamp, J.; Brantjes, H.; Moerer, P.; van de Wetering, M.; Destrée, O.; Clevers, H. The Xenopus Wnt Effector XTcf-3 Interacts with Groucho-Related Transcriptional Repressors. Nature 1998, 395, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Hingole, S.; Chaudhary, V. The Emerging Mechanisms of Wnt Secretion and Signaling in Development. Front. Cell Dev. Biol. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Takada, R.; Satomi, Y.; Kurata, T.; Ueno, N.; Norioka, S.; Kondoh, H.; Takao, T.; Takada, S. Monounsaturated Fatty Acid Modification of Wnt Protein: Its Role in Wnt Secretion. Dev. Cell 2006, 11, 791–801. [Google Scholar] [CrossRef] [Green Version]
- van den Heuvel, M.; Harryman-Samos, C.; Klingensmith, J.; Perrimon, N.; Nusse, R. Mutations in the Segment Polarity Genes Wingless and Porcupine Impair Secretion of the Wingless Protein. EMBO J. 1993, 12, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R.; Nusse, R. Wnt Proteins Are Lipid-Modified and Can Act as Stem Cell Growth Factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef]
- Rios-Esteves, J.; Resh, M.D. Stearoyl CoA Desaturase Is Required to Produce Active, Lipid-Modified Wnt Proteins. Cell Rep. 2013, 4, 1072–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartscherer, K.; Pelte, N.; Ingelfinger, D.; Boutros, M. Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein. Cell 2006, 125, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Bänziger, C.; Soldini, D.; Schütt, C.; Zipperlen, P.; Hausmann, G.; Basler, K. Wntless, a Conserved Membrane Protein Dedicated to the Secretion of Wnt Proteins from Signaling Cells. Cell 2006, 125, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Cong, F.; Schweizer, L.; Varmus, H. Casein Kinase Iε Modulates the Signaling Specificities of Dishevelled. Mol. Cell. Biol. 2004, 24, 2000–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauriello, D.V.F.; Jordens, I.; Kirchner, K.; Slootstra, J.W.; Kruitwagen, T.; Bouwman, B.A.M.; Noutsou, M.; Rudiger, S.G.D.; Schwamborn, K.; Schambony, A.; et al. Wnt/β-Catenin Signaling Requires Interaction of the Dishevelled DEP Domain and C Terminus with a Discontinuous Motif in Frizzled. Proc. Natl. Acad. Sci. USA 2012, 109, E812–E820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, K.; Zeng, X.; Liu, C.; Zhang, X.; Harada, Y.; Chang, Z.; He, X. A Mechanism for Wnt Coreceptor Activation. Mol. Cell 2004, 13, 149–156. [Google Scholar] [CrossRef]
- Davidson, G.; Wu, W.; Shen, J.; Bilic, J.; Fenger, U.; Stannek, P.; Glinka, A.; Niehrs, C. Casein Kinase 1 γ Couples Wnt Receptor Activation to Cytoplasmic Signal Transduction. Nature 2005, 438, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Shen, J.; Huang, Y.-L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell Cycle Control of Wnt Receptor Activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-E.; Huang, H.; Zhao, M.; Zhang, X.; Zhang, A.; Semonov, M.V.; MacDonald, B.T.; Zhang, X.; Abreu, J.G.; Peng, L.; et al. Wnt Stabilization of β-Catenin Reveals Principles for Morphogen Receptor-Scaffold Assemblies. Science 2013, 340, 867–870. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase Inhibition Stabilizes Axin and Antagonizes Wnt Signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Schuijers, J.; Mokry, M.; Hatzis, P.; Cuppen, E.; Clevers, H. Wnt-Induced Transcriptional Activation Is Exclusively Mediated by TCF/LEF. EMBO J. 2014, 33, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; van de Wetering, M.; van Es, J.H.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; et al. Tumour Suppressor RNF43 Is a Stem-Cell E3 Ligase That Induces Endocytosis of Wnt Receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef]
- Hao, H.-X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 Promotes Wnt Receptor Turnover in an R-Spondin-Sensitive Manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienz, M.; Clevers, H. Linking Colorectal Cancer to Wnt Signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, N.S. Serrated Pathway and APC (Conventional)-Type Colorectal Polyps. Am. J. Clin. Pathol. 2006, 125, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Stefanski, C.D.; Prosperi, J.R. Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response. Int. J. Mol. Sci. 2020, 21, 7844. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [CrossRef] [Green Version]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and Its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 8, djw332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodde, R.; Smits, R.; Clevers, H. APC, Signal Transduction and Genetic Instability in Colorectal Cancer. Nat. Rev. Cancer 2001, 1, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Béroud, C.; Soussi, T. APC gene: Database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996, 24, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Kinzler, K.W.; Vogelstein, B. Lessons from Hereditary Colorectal Cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The ABC of APC. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P.M. APC Mutations in Sporadic Colorectal Tumors: A Mutational “Hotspot” and Interdependence of the “Two Hits. ” Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.; Taketo, M.M. Adenomatous Polyposis Coli (APC): A Multi-Functional Tumor Suppressor Gene. J. Cell Sci. 2007, 120, 3327–3335. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, A.J.; Serra, S.; Chetty, R. Traditional Serrated Adenoma: An Overview of Pathology and Emphasis on Molecular Pathogenesis. BMJ Open Gastroenterol. 2019, 6, e000317. [Google Scholar] [CrossRef]
- Cefalì, M.; Epistolio, S.; Palmarocchi, M.C.; Frattini, M.; de Dosso, S. Research Progress on KRAS Mutations in Colorectal Cancer. J. Cancer Metastasis Treat. 2021, 7. [Google Scholar] [CrossRef]
- Fearon, E.R. Molecular Genetics of Colorectal Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Kaps, L.; Maderer, A.; Galle, P.R.; Moehler, M. The Role of P53 Dysfunction in Colorectal Cancer and Its Implication for Therapy. Cancers 2021, 13, 2296. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The Role of P53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Bar-Ephraim, Y.E.; Kretzschmar, K.; Clevers, H. Organoids in Immunological Research. Nat. Rev. Immunol. 2020, 20, 279–293. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, S.M.; de Groot, N.E.; Nijman, L.E.; Scicluna, B.P.; van Driel, M.S.; Lecca, M.C.; Warmerdam, D.O.; Kakkar, V.; Moreno, L.F.; Vieira Braga, F.A.; et al. Apc-Mutant Cells Act as Supercompetitors in Intestinal Tumour Initiation. Nature 2021, 594, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Yum, M.K.; Han, S.; Fink, J.; Wu, S.-H.S.; Dabrowska, C.; Trendafilova, T.; Mustata, R.; Chatzeli, L.; Azzarelli, R.; Pshenichnaya, I.; et al. Tracing Oncogene-Driven Remodelling of the Intestinal Stem Cell Niche. Nature 2021, 594, 442–447. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Pentinmikko, N.; Luopajärvi, K.; Willis, N.J.; Gilroy, K.; Raven, A.P.; Mcgarry, L.; Englund, J.I.; Webb, A.T.; Scharaw, S.; et al. NOTUM from Apc-Mutant Cells Biases Clonal Competition to Initiate Cancer. Nature 2021, 594, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The Inflammatory Pathogenesis of Colorectal Cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Heikkinen, T.; Msika, S.; Desvignes, G.; Schwandner, O.; Schiedeck, T.H.; Shekarriz, H.; Bloechle, C.H.; Baca, I.; Weiss, O.; Morino, M.; et al. Laparoscopic Surgery versus Open Surgery for Colon Cancer: Short-Term Outcomes of a Randomised Trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.G.M.; de Lange-de Klerk, E.S.M.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- van de Velde, C.J.H.; Boelens, P.G.; Tanis, P.J.; Espin, E.; Mroczkowski, P.; Naredi, P.; Pahlman, L.; Ortiz, H.; Rutten, H.J.; Breugom, A.J.; et al. Experts Reviews of the Multidisciplinary Consensus Conference Colon and Rectal Cancer 2012. Eur. J. Surg. Oncol. 2014, 40, 454–468. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, C.J.H.; Boelens, P.G.; Borras, J.M.; Coebergh, J.-W.; Cervantes, A.; Blomqvist, L.; Beets-Tan, R.G.H.; van den Broek, C.B.M.; Brown, G.; van Cutsem, E.; et al. EURECCA Colorectal: Multidisciplinary Management: European Consensus Conference Colon & Rectum. Eur. J. Cancer 2014, 50, 1.e1–1.e34. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- García-Alfonso, P.; Muñoz Martín, A.J.; Ortega Morán, L.; Soto Alsar, J.; Torres Pérez-Solero, G.; Blanco Codesido, M.; Calvo Ferrandiz, P.A.; Grasso Cicala, S. Oral Drugs in the Treatment of Metastatic Colorectal Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211009000. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Hatano, Y.; Niwa, M.; Hara, A.; Tomita, H. Heterogeneity of Colon Cancer Stem Cells. Adv. Exp. Med. Biol. 2019, 1139, 115–126. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, Y.-W.; Yang, C.-H.; Huang, K.-S. Drug Delivery Systems and Combination Therapy by Using Vinca Alkaloids. Curr. Top. Med. Chem. 2015, 15, 1491–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, G.J.; Damon, L.E.; Coutre, S.E.; Hsu, P.; Bhat, G.; Douer, D. High-Dose Vincristine Sulfate Liposome Injection, for Advanced, Relapsed, or Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia in an Adolescent and Young Adult Subgroup of a Phase 2 Clinical Trial. J. Adolesc. Young Adult Oncol. 2018, 7, 546–552. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.A.; Hart, J.; Seely, D. Cost Effectiveness of Natural Health Products: A Systematic Review of Randomized Clinical Trials. Evid. Based Complementary Altern. Med. 2009, 6, 297–304. [Google Scholar] [CrossRef]
- Manandhar, S.; Kabekkodu, S.P.; Pai, K.S.R. Aberrant Canonical Wnt Signaling: Phytochemical Based Modulation. Phytomedicine 2020, 76, 153243. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Neto, V.M.; Abreu, J.G. Flavonoids: Potential Wnt/Beta-Catenin Signaling Modulators in Cancer. Life Sci. 2011, 89, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Amado, N.; Predes, D.; Moreno, M.; Carvalho, I.; Mendes, F.; Abreu, J. Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies. Int. J. Mol. Sci. 2014, 15, 12094–12106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, H. Genistein Increases Gene Expression by Demethylation of WNT5a Promoter in Colon Cancer Cell Line SW1116. Anticancer Res. 2010, 30, 4537–4545. [Google Scholar]
- Zhang, Y.; Chen, H. Genistein Attenuates WNT Signaling by Up-Regulating SFRP2 in a Human Colon Cancer Cell Line. Exp. Biol. Med. 2011, 236, 714–722. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Chen, H. Genistein Affects Histone Modifications on Dickkopf-Related Protein 1 (DKK1) Gene in SW480 Human Colon Cancer Cell Line. PLoS ONE 2012, 7, e40955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Zhou, D.; Chen, H. Genistein, a Soya Isoflavone, Prevents Azoxymethane-Induced up-Regulation of WNT/β-Catenin Signalling and Reduces Colon Pre-Neoplasia in Rats. Br. J. Nutr. 2013, 109, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Ren, J.; Tang, L. Genistein Inhibits Invasion and Migration of Colon Cancer Cells by Recovering WIF1 Expression. Mol. Med. Rep. 2018, 17, 7265–7273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Q.; Chen, H. DNA Methylation and Histone Modifications of Wnt Genes by Genistein during Colon Cancer Development. Carcinogenesis 2013, 34, 1756–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, S.; Schrezenmeier, E.; Klare, S.; Zaade, D.; Seidel, K.; Schmitz, J.; Bernhard, S.; Lauer, D.; Slack, M.; Goldin-Lang, P.; et al. The (pro)Renin Receptor Mediates Constitutive PLZF-Independent pro-Proliferative Effects Which Are Inhibited by Bafilomycin but Not Genistein. Int. J. Mol. Med. 2014, 33, 795–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cui, S.-X.; Sun, S.-Y.; Shi, W.-N.; Song, Z.-Y.; Wang, S.-Q.; Yu, X.-F.; Gao, Z.-H.; Qu, X.-J. Chemoprevention of Intestinal Tumorigenesis by the Natural Dietary Flavonoid Myricetin in APCMin/+ Mice. Oncotarget 2016, 7, 60446–60460. [Google Scholar] [CrossRef] [Green Version]
- Ni, T.; He, Z.; Dai, Y.; Yao, J.; Guo, Q.; Wei, L. Oroxylin A Suppresses the Development and Growth of Colorectal Cancer through Reprogram of HIF1α-Modulated Fatty Acid Metabolism. Cell Death Dis. 2017, 8, e2865. [Google Scholar] [CrossRef] [Green Version]
- Pandurangan, A.K.; Dharmalingam, P.; Sadagopan, S.K.A.; Ramar, M.; Munusamy, A.; Ganapasam, S. Luteolin Induces Growth Arrest in Colon Cancer Cells Through Involvement of Wnt/β-Catenin/GSK-3β Signaling. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, P.; Sudhandiran, G. Luteolin Inhibits Cell Proliferation during Azoxymethane-Induced Experimental Colon Carcinogenesis via Wnt/ β-Catenin Pathway. Investig. New Drugs 2011, 29, 273–284. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Zhang, W.; Thamaraiselvan, R. Vicenin-2 Inhibits Wnt/β-Catenin Signaling and Induces Apoptosis in HT-29 Human Colon Cancer Cell Line. Drug Des. Dev. Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Gao, X.-Y.; Yang, S.-Q.; Sun, Z.; Dian, L.-L.; Qasim, M.; Thu Phyo, A.; Liang, Z.-S.; Sun, Y.-F. Jatrorrhizine Inhibits Colorectal Carcinoma Proliferation and Metastasis through Wnt/β-Catenin Signaling Pathway and Epithelial–Mesenchymal Transition. Drug Des. Dev. Ther. 2019, 13, 2235–2247. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bao, X.; Zhao, A.; Zhang, J.; Zhang, M.; Zhang, Q.; Ma, B. Raddeanin A Inhibits Growth and Induces Apoptosis in Human Colorectal Cancer through Downregulating the Wnt/β-Catenin and NF-ΚB Signaling Pathway. Life Sci. 2018, 207, 532–549. [Google Scholar] [CrossRef]
- Lee, M.-A.; Kim, W.K.; Park, H.J.; Kang, S.S.; Lee, S.K. Anti-Proliferative Activity of Hydnocarpin, a Natural Lignan, Is Associated with the Suppression of Wnt/β-Catenin Signaling Pathway in Colon Cancer Cells. Bioorganic Med. Chem. Lett. 2013, 23, 5511–5514. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q.; He, B. Berberine Inhibits the Proliferation of Colon Cancer Cells by Inactivating Wnt/β-Catenin Signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cao, H.; Zhang, B.; Cao, H.; Xu, X.; Ruan, H.; Yi, T.; Tan, L.; Qu, R.; Song, G.; et al. Berberine Potently Attenuates Intestinal Polyps Growth in ApcMin Mice and Familial Adenomatous Polyposis Patients through Inhibition of Wnt Signalling. J. Cell. Mol. Med. 2013, 17, 1484–1493. [Google Scholar] [CrossRef]
- Albring, K.F.; Weidemüller, J.; Mittag, S.; Weiske, J.; Friedrich, K.; Geroni, M.C.; Lombardi, P.; Huber, O. Berberine Acts as a Natural Inhibitor of Wnt/β-Catenin Signaling-Identification of More Active 13-Arylalkyl Derivatives. BioFactors 2013, 39, 652–662. [Google Scholar] [CrossRef]
- Li, H.; Lee, H.J.; Ahn, Y.H.; Kwon, H.J.; Jang, C.-Y.; Kim, W.-Y.; Ryu, J.-H. Tussilagone Suppresses Colon Cancer Cell Proliferation by Promoting the Degradation of β-Catenin. Biochem. Biophys. Res. Commun. 2014, 443, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.-J.; Wang, L.-Q.; Wu, J.; Dai, C.; Yuan, Y.-M.; Li, G.Q.; Yao, M.-C. A Tannin Compound from Sanguisorba Officinalis Blocks Wnt/β-Catenin Signaling Pathway and Induces Apoptosis of Colorectal Cancer Cells. Chin. Med. 2019, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Leem, S.-H.; Li, X.J.; Park, M.H.; Park, B.H.; Kim, S.M. Genome-Wide Transcriptome Analysis Reveals Inactivation of Wnt/β-Catenin by 3,3′-Diindolylmethane Inhibiting Proliferation of Colon Cancer Cells. Int. J. Oncol. 2015, 47, 918–926. [Google Scholar] [CrossRef]
- Hao, J.; Dai, X.; Gao, J.; Li, Y.; Hou, Z.; Chang, Z.; Wang, Y. Curcumin Suppresses Colorectal Tumorigenesis via the Wnt/Β-catenin Signaling Pathway by Downregulating Axin2. Oncol. Lett. 2021, 21, 186. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Xu, C.; Song, L.; Huang, L.; Lai, Y.; Wang, Y.; Chen, H.; Gu, D.; Ren, L.; et al. Curcumin Inhibits Tumor Epithelial-mesenchymal Transition by Downregulating the Wnt Signaling Pathway and Upregulating NKD2 Expression in Colon Cancer Cells. Oncol. Rep. 2016, 35, 2615–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Koh, G.Y.; Huang, Y.; Crott, J.W.; Bronson, R.T.; Mason, J.B. The Combination of Curcumin and Salsalate Is Superior to Either Agent Alone in Suppressing Pro-Cancerous Molecular Pathways and Colorectal Tumorigenesis in Obese Mice. Mol. Nutr. Food Res. 2019, 63, 1801097. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, S.; Qiu, X.; Cong, J.; Zhou, J.; Miu, W. Curcumin Inhibits Cell Viability and Increases Apoptosis of SW620 Human Colon Adenocarcinoma Cells via the Caudal Type Homeobox-2 (CDX2)/Wnt/β-Catenin Pathway. Med. Sci. Monit. 2019, 25, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-N.; Yuan, F.; Liu, J.-Q.; Peng, X.-R.; An, T.; Li, X.; Kong, L.-M.; Qiu, M.-H.; Li, Y. Physalis Peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules 2019, 24, 1146. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.-Q.; Zhang, Q.; Zhu, J.-Y.; Li, Y.; Xie, C.-F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green Tea Polyphenol EGCG Suppresses Wnt/β-Catenin Signaling by Promoting GSK-3β- and PP2A-Independent β-Catenin Phosphorylation/Degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Shim, A.-R.; Dong, G.; Lee, H.J.; Ryu, J.-H. Atractylochromene Is a Repressor of Wnt/β-Catenin Signaling in Colon Cancer Cells. Biomol. Ther. 2015, 23, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Sangeetha, N.; Aranganathan, S.; Panneerselvam, J.; Shanthi, P.; Rama, G.; Nalini, N. Oral Supplementation of Silibinin Prevents Colon Carcinogenesis in a Long Term Preclinical Model. Eur. J. Pharmacol. 2010, 643, 93–100. [Google Scholar] [CrossRef]
- Sangeetha, N.; Viswanathan, P.; Balasubramanian, T.; Nalini, N. Colon Cancer Chemopreventive Efficacy of Silibinin through Perturbation of Xenobiotic Metabolizing Enzymes in Experimental Rats. Eur. J. Pharmacol. 2012, 674, 430–438. [Google Scholar] [CrossRef]
- Shin, S.; Son, Y.; Liu, K.-H.; Kang, W.; Oh, S. Cytotoxic Activity of Broussochalcone a against Colon and Liver Cancer Cells by Promoting Destruction Complex-Independent β-Catenin Degradation. Food Chem. Toxicol. 2019, 131, 110550. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Chen, H.-H.; Lin, C.-A.; Wu, H.-C.; Sheu, J.J.-C.; Chen, H.-J. Apigenin-Induced Lysosomal Degradation of β-Catenin in Wnt/β-Catenin Signaling. Sci. Rep. 2017, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin Suppresses Colorectal Cancer Cell Proliferation, Migration and Invasion via Inhibition of the Wnt/β-Catenin Signaling Pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef] [Green Version]
- Ozbey, U.; Attar, R.; Romero, M.A.; Alhewairini, S.S.; Afshar, B.; Sabitaliyevich, U.Y.; Hanna-Wakim, L.; Ozcelik, B.; Farooqi, A.A. Apigenin as an Effective Anticancer Natural Product: Spotlight on TRAIL, WNT/β-Catenin, JAK-STAT Pathways, and MicroRNAs. J. Cell. Biochem. 2018, 120, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol Protects against Azoxymethane-induced Colorectal Cancer in Sprague-Dawley Rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.-M.; Zhan, R.; Ye, Z.-N.; Pu, J.-X.; Sun, H.-D.; Li, Y. Two Natural Ent-Kauranoids as Novel Wnt Signaling Inhibitors. Nat. Prod. Bioprospecting 2014, 4, 135–140. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin Induced G1 Cell Cycle Arrest by Regulating Wnt/β-Catenin Signaling Pathway and Inactivating CDK8 in Human Colorectal Cancer Carcinoma Cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin Suppresses Colon Cancer Cell Growth in Vitro by Targeting the Wnt/β-Catenin Signaling Pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [Green Version]
- Manigandan, K.; Manimaran, D.; Jayaraj, R.L.; Elangovan, N.; Dhivya, V.; Kaphle, A. Taxifolin Curbs NF-ΚB-Mediated Wnt/β-Catenin Signaling via up-Regulating Nrf2 Pathway in Experimental Colon Carcinogenesis. Biochimie 2015, 119, 103–112. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a Natural Flavonoid Interacts with Cell Cycle Regulators Causes Cell Cycle Arrest and Causes Tumor Regression by Activating Wnt/ β -Catenin Signaling Pathway. BMC Cancer 2018, 18, 1043. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, B.F.; Predes, D.; Cerqueira, D.M.; Reis, A.H.; Amado, N.G.; Cayres, M.C.L.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Derricin and Derricidin Inhibit Wnt/β-Catenin Signaling and Suppress Colon Cancer Cell Growth In Vitro. PLoS ONE 2015, 10, e0120919. [Google Scholar] [CrossRef] [Green Version]
- Min, H.-Y.; Chung, H.-J.; Kim, E.-H.; Kim, S.; Park, E.-J.; Lee, S.K. Inhibition of Cell Growth and Potentiation of Tumor Necrosis Factor-α (TNF-α)-Induced Apoptosis by a Phenanthroindolizidine Alkaloid Antofine in Human Colon Cancer Cells. Biochem. Pharmacol. 2010, 80, 1356–1364. [Google Scholar] [CrossRef]
- Kong, L.-M.; Feng, T.; Wang, Y.-Y.; Li, X.-Y.; Ye, Z.-N.; An, T.; Qing, C.; Luo, X.-D.; Li, Y. Bisleuconothine A, a Bisindole Alkaloid, Inhibits Colorectal Cancer Cell in Vitro and in Vivo Targeting Wnt Signaling. Oncotarget 2016, 7, 10203–10214. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Gwak, J.; Cho, M.; Ryu, M.-J.; Lee, J.-H.; Kim, S.K.; Kim, Y.H.; Lee, G.W.; Yun, M.-Y.; Cuong, N.M.; et al. Murrayafoline A Attenuates the Wnt/β-Catenin Pathway by Promoting the Degradation of Intracellular β-Catenin Proteins. Biochem. Biophys. Res. Commun. 2010, 391, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.R.; Nargizyan, T.; Meliton, V.; Nachtergaele, S.; Rohatgi, R.; Stappenbeck, F.; Jung, M.E.; Johnson, J.S.; Aghdasi, B.; Tian, H.; et al. A Novel Osteogenic Oxysterol Compound for Therapeutic Development to Promote Bone Growth: Activation of Hedgehog Signaling and Osteogenesis through Smoothened Binding. J. Bone Miner. Res. 2014, 29, 1872–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.-J.; Park, H.J.; Chung, H.-J.; Min, H.-Y.; Park, E.J.; Lee, M.A.; Shin, Y.; Lee, S.K. Wnt/β-Catenin Signaling Mediates the Antitumor Activity of Magnolol in Colorectal Cancer Cells. Mol. Pharmacol. 2012, 82, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Lee, J.; Lee, S.-H. TCF4 Is a Molecular Target of Resveratrol in the Prevention of Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 10411–10425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nana, A.W.; Chin, Y.-T.; Lin, C.-Y.; Ho, Y.; Bennett, J.A.; Shih, Y.-J.; Chen, Y.-R.; Changou, C.A.; Pedersen, J.Z.; Incerpi, S.; et al. Tetrac Downregulates β-Catenin and HMGA2 to Promote the Effect of Resveratrol in Colon Cancer. Endocr. Relat. Cancer 2018, 25, 279–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Hsu, L.-S.; Shia, Y.-T.; Lin, M.-W.; Lin, C.-M. The β-Catenin/TCF Complex as a Novel Target of Resveratrol in the Wnt/β-Catenin Signaling Pathway. Biochem. Pharmacol. 2012, 84, 1143–1153. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wu, K.; Huang, J.; Liu, Y.; Wang, X.; Meng, Z.-J.; Yuan, S.-X.; Wang, D.-X.; Luo, J.-Y.; Zuo, G.-W.; et al. The PTEN/PI3K/Akt and Wnt/β-Catenin Signaling Pathways Are Involved in the Inhibitory Effect of Resveratrol on Human Colon Cancer Cell Proliferation. Int. J. Oncol. 2014, 45, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Predes, D.; Oliveira, L.F.; Ferreira, L.S.S.; Maia, L.A.; Delou, J.M.A.; Faletti, A.; Oliveira, I.; Amado, N.G.; Reis, A.H.; Fraga, C.; et al. The Chalcone Lonchocarpin Inhibits Wnt/β-Catenin Signaling and Suppresses Colorectal Cancer Proliferation. Cancers 2019, 11, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine Suppresses the Wnt/β-Catenin Pathway and Has Anti-Cancer Effects on Colorectal Cancer Cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lim, T.-G.; Chen, H.; Jung, S.K.; Lee, H.-J.; Lee, M.-H.; Kim, D.J.; Shin, A.; Lee, K.W.; Bode, A.M.; et al. Esculetin Suppresses Proliferation of Human Colon Cancer Cells by Directly Targeting β-Catenin. Cancer Prev. Res. 2013, 6, 1356–1364. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Park, H.J.; Chung, H.-J.; Kim, W.K.; Lee, S.K. Antitumor Activity of 2-Hydroxycinnamaldehyde for Human Colon Cancer Cells through Suppression of β-Catenin Signaling. J. Nat. Prod. 2013, 76, 1278–1284. [Google Scholar] [CrossRef]
- de la Roche, M.; Rutherford, T.J.; Gupta, D.; Veprintsev, D.B.; Saxty, B.; Freund, S.M.; Bienz, M. An Intrinsically Labile α-Helix Abutting the BCL9-Binding Site of β-Catenin Is Required for Its Inhibition by Carnosic Acid. Nat. Commun. 2012, 3, 680. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-Tumor Effects and Associated Molecular Mechanisms of Myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, K.; Wang, Y.; Xue, D.; Ning, N.; Zou, Z.; Ye, X.; Li, X.; Wang, D.; Pang, J. The Antihypercholesterolemic Effect of Jatrorrhizine Isolated from Rhizoma Coptidis. Phytomedicine 2014, 21, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Davidson, L.A.; Zoh, R.S.; Hensel, M.E.; Salinas, M.L.; Patil, B.S.; Jayaprakasha, G.K.; Callaway, E.S.; Allred, C.D.; Turner, N.D.; et al. Rapidly Cycling Lgr5+ Stem Cells Are Exquisitely Sensitive to Extrinsic Dietary Factors That Modulate Colon Cancer Risk. Cell Death Dis. 2016, 7, e2460. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, L.A.; Velloso, I.; Abreu, J.G. Advances in the Use of Xenopus for Successful Drug Screening. Expert Opin. Drug Discov. 2017, 12, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- He, B.-C.; Gao, J.-L.; Zhang, B.-Q.; Luo, Q.; Shi, Q.; Kim, S.H.; Huang, E.; Gao, Y.; Yang, K.; Wagner, E.R.; et al. Tetrandrine Inhibits Wnt/β-Catenin Signaling and Suppresses Tumor Growth of Human Colorectal Cancer. Mol. Pharmacol. 2011, 79, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Zhou, M.; Wu, Q.-X.; Yuan, S.-X.; Wang, D.-X.; Jin, J.-L.; Huang, J.; Yang, J.-Q.; Sun, W.-J.; Wan, L.-H.; et al. The Role of IGFBP-5 in Mediating the Anti-Proliferation Effect of Tetrandrine in Human Colon Cancer Cells. Int. J. Oncol. 2015, 46, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, N.; Zeng, J.; Wang, Y.; Cui, H. The Versatile Roles of Cancer-Associated Fibroblasts in Colorectal Cancer and Therapeutic Implications. Front. Cell Dev. Biol. 2021, 9, 2772. [Google Scholar] [CrossRef] [PubMed]
- Brommage, R.; Liu, J.; Vogel, P.; Mseeh, F.; Thompson, A.Y.; Potter, D.G.; Shadoan, M.K.; Hansen, G.M.; Jeter-Jones, S.; Cui, J.; et al. NOTUM Inhibition Increases Endocortical Bone Formation and Bone Strength. Bone Res. 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kariv, R.; Caspi, M.; Fliss-Isakov, N.; Shorer, Y.; Shor, Y.; Rosner, G.; Brazowski, E.; Beer, G.; Cohen, S.; Rosin-Arbesfeld, R. Resorting the Function of the Colorectal Cancer Gatekeeper Adenomatous Polyposis Coli. Int. J. Cancer 2020, 146, 1064–1074. [Google Scholar] [CrossRef]
- Zilberberg, A.; Lahav, L.; Rosin-Arbesfeld, R. Restoration of APC Gene Function in Colorectal Cancer Cells by Aminoglycoside- and Macrolide-Induced Read-through of Premature Termination Codons. Gut 2010, 59, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Dow, L.E.; O’Rourke, K.P.; Simon, J.; Tschaharganeh, D.F.; van Es, J.H.; Clevers, H.; Lowe, S.W. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell 2015, 161, 1539–1552. [Google Scholar] [CrossRef] [Green Version]
- Angers, S. Wnt Signaling Inhibition Confers Induced Synthetic Lethality to PARP Inhibitors. EMBO Mol. Med. 2021, 13, e14002. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 Study of Single-Agent WNT974, a First-in-Class Porcupine Inhibitor, in Patients with Advanced Solid Tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef]
- Wu, X.; Luo, F.; Li, J.; Zhong, X.; Liu, K. Tankyrase 1 Inhibitior XAV939 Increases Chemosensitivity in Colon Cancer Cell Lines via Inhibition of the Wnt Signaling Pathway. Int. J. Oncol. 2016, 48, 1333–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjaneh, R.M.; Rahmani, F.; Hassanian, S.M.; Rezaei, N.; Hashemzehi, M.; Bahrami, A.; Ariakia, F.; Fiuji, H.; Sahebkar, A.; Avan, A.; et al. Phytosomal Curcumin Inhibits Tumor Growth in Colitis-associated Colorectal Cancer. J. Cell. Physiol. 2018, 233, 6785–6798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low Curcumin Concentration Enhances the Anticancer Effect of 5-Fluorouracil against Colorectal Cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, R.; Zhang, X.; Zhang, B.; Yao, Q. Curcumin May Reverse 5-Fluorouracil Resistance on Colonic Cancer Cells by Regulating TET1-NKD-Wnt Signal Pathway to Inhibit the EMT Progress. Biomed. Pharmacother. 2020, 129, 110381. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and Quercetin Synergistically Inhibit Cancer Cell Proliferation in Multiple Cancer Cells and Modulate Wnt/β-Catenin Signaling and Apoptotic Pathways in A375 Cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Ndreshkjana, B.; Çapci, A.; Klein, V.; Chanvorachote, P.; Muenzner, J.K.; Huebner, K.; Steinmann, S.; Erlenbach-Wuensch, K.; Geppert, C.I.; Agaimy, A.; et al. Combination of 5-Fluorouracil and Thymoquinone Targets Stem Cell Gene Signature in Colorectal Cancer Cells. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Amerizadeh, F.; Rezaei, N.; Rahmani, F.; Hassanian, S.M.; Moradi-Marjaneh, R.; Fiuji, H.; Boroumand, N.; Nosrati-Tirkani, A.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Crocin Synergistically Enhances the Antiproliferative Activity of 5-flurouracil through Wnt/PI3K Pathway in a Mouse Model of Colitis-associated Colorectal Cancer. J. Cell. Biochem. 2018, 119, 10250–10261. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic Effect of Kaempferol and 5-Fluorouracil on the Growth of Colorectal Cancer Cells by Regulating the PI3K/Akt Signaling Pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib Synergistically Inhibit Colon Cancer Cell Proliferation via Modulating Wnt/β-Catenin Signaling Pathway. Phytomedicine 2021, 84, 153484. [Google Scholar] [CrossRef]
- Unson, S.; Kongsaden, C.; Wonganan, P. Cepharanthine Combined with 5-Fluorouracil Inhibits the Growth of P53-Mutant Human Colorectal Cancer Cells. J. Asian Nat. Prod. Res. 2020, 22, 370–385. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, M.; Wu, X.; Yang, P.; Yin, C. Discovery of a Potent β-Catenin Destabilizer for Overcoming the Resistance of 5-Fluorouracil in Colorectal Cancer. Bioorganic Med. Chem. 2021, 30, 115929. [Google Scholar] [CrossRef] [PubMed]
- Zargar, P.; Ghani, E.; Jalali Mashayekhi, F.; Ramezani, A.; Eftekhar, E. Acriflavine Enhances the Antitumor Activity of the Chemotherapeutic Drug 5-Fluorouracil in Colorectal Cancer Cells. Oncol. Lett. 2018, 15, 10084–10090. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin Enhances 5-Fluorouracil-Induced Apoptosis in MSI Colorectal Cancer Cells through P53 Modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Turdo, A.; Glaviano, A.; Pepe, G.; Calapà, F.; Raimondo, S.; Fiori, M.E.; Carbone, D.; Basilicata, M.G.; di Sarno, V.; Ostacolo, C.; et al. Nobiletin and Xanthohumol Sensitize Colorectal Cancer Stem Cells to Standard Chemotherapy. Cancers 2021, 13, 3927. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.F.S.; Predes, D.; Borges, H.L.; Abreu, J.G. Therapeutic Potential of Naturally Occurring Small Molecules to Target the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer. Cancers 2022, 14, 403. https://doi.org/10.3390/cancers14020403
Oliveira LFS, Predes D, Borges HL, Abreu JG. Therapeutic Potential of Naturally Occurring Small Molecules to Target the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer. Cancers. 2022; 14(2):403. https://doi.org/10.3390/cancers14020403
Chicago/Turabian StyleOliveira, Luiz F. S., Danilo Predes, Helena L. Borges, and Jose G. Abreu. 2022. "Therapeutic Potential of Naturally Occurring Small Molecules to Target the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer" Cancers 14, no. 2: 403. https://doi.org/10.3390/cancers14020403






