The Evolution of Ovarian Carcinoma Subclassification
Abstract
:Simple Summary
Abstract
1. Introduction
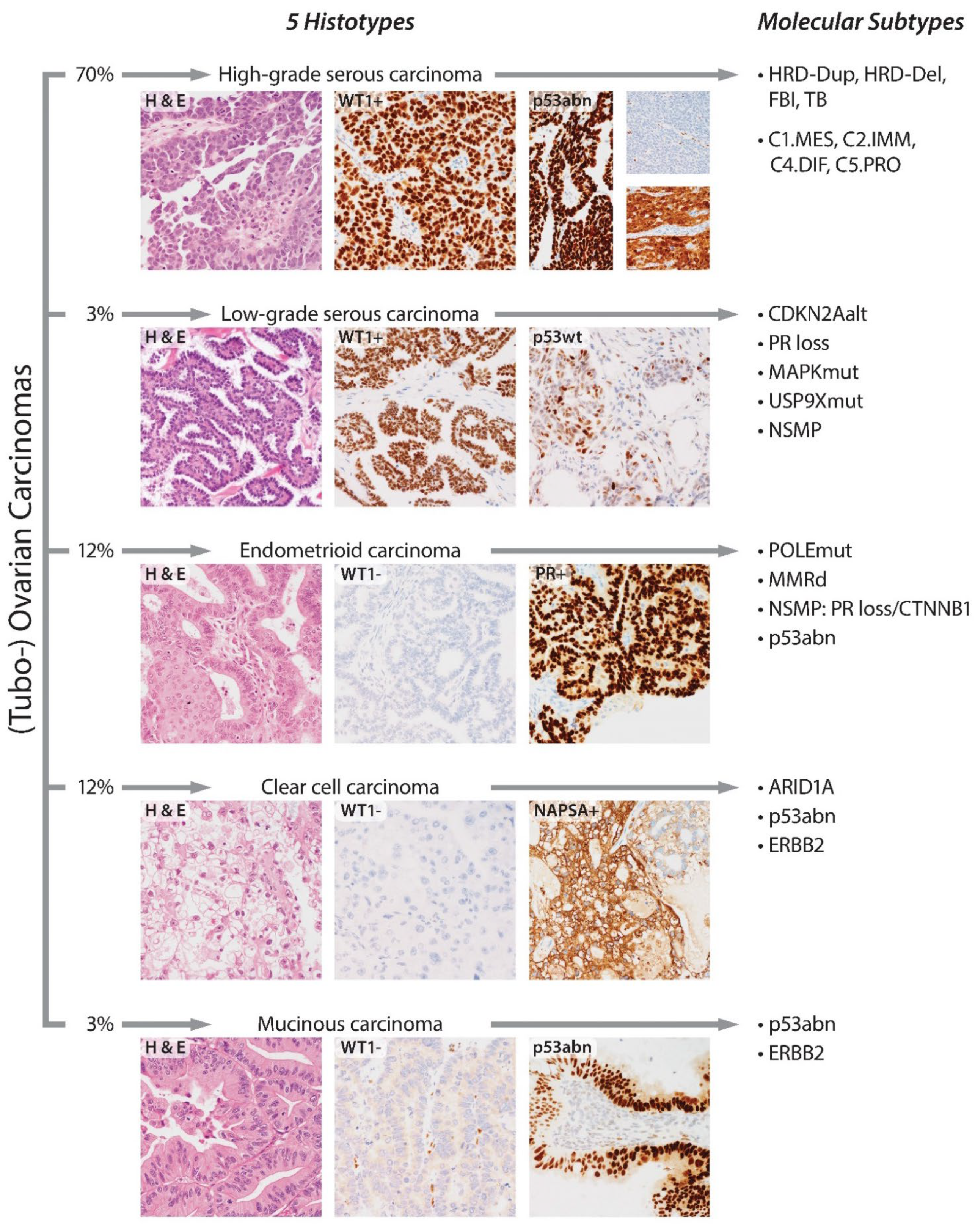
2. Evolution of Histotypes
3. Ancillary Immunohistochemical Testing to Confirm a Morphological Histotype Diagnosis
4. Molecular Subtypes of Ovarian Carcinomas
4.1. High-Grade Serous Carcinoma
4.2. Endometrioid Carcinoma
4.3. Clear Cell Carcinoma
4.4. Low-Grade Serous Carcinoma
4.5. Mucinous Carcinoma
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Köbel, M.; Rahimi, K.; Rambau, P.F.; Naugler, C.; Le Page, C.; Meunier, L.; de Ladurantaye, M.; Lee, S.; Leung, S.; Goode, E.L.; et al. An Immunohistochemical Algorithm for Ovarian Carcinoma Typing. Int. J. Gynecol. Pathol. 2016, 35, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Piek, J.M.; van Diest, P.J.; Zweemer, R.P.; Jansen, J.W.; Poort-Keesom, R.J.; Menko, F.H.; Gille, J.J.; Jongsma, A.P.; Pals, G.; Kenemans, P.; et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol. 2001, 195, 451–456. [Google Scholar] [CrossRef]
- Lee, Y.; Miron, A.; Drapkin, R.; Nucci, M.R.; Medeiros, F.; Saleemuddin, A.; Garber, J.; Birch, C.; Mou, H.; Gordon, R.W.; et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 2007, 211, 26–35. [Google Scholar] [CrossRef]
- Gilks, C.B.; Davidson, B.; Köbel, M.; Ledermann, J.A.; Lim, D.; Malpica, A.; Mikami, Y.; Singh, N.; Srinivasan, R.; Vang, R.; et al. Ovary, Fallopian Tube and Primary Peritoneal Carcinoma Histopathology Reporting Guide; International Collaboration on Cancer Reporting: Sydney, Australia, 2021. [Google Scholar]
- WHO. Classification of Tumours Editorial Board. In Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, D.R.; Tessier-Cloutier, B.; Lawrence, K.M.; Nazeran, T.; Karnezis, A.N.; Salamanca, C.; Cheng, A.S.; McAlpine, J.N.; Hoang, L.N.; Gilks, C.B.; et al. Clear cell and endometrioid carcinomas: Are their differences attributable to distinct cells of origin? J. Pathol. 2017, 243, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, S.; Fulmali, R.; McCluggage, W.G. Low-grade Serous Carcinoma Arising in Inguinal Nodal Endosalpingiosis: Report of 2 Cases and Literature Review. Int. J. Gynecol. Pathol. 2020, 39, 273–278. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Gilks, C.B.; Kalloger, S.; Longacre, T.A. Classification of Extraovarian Implants in Patients With Ovarian Serous Borderline Tumors (Tumors of Low Malignant Potential) Based on Clinical Outcome. Am. J. Surg. Pathol. 2016, 40, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Llaurado Fernandez, M.; Kim, H.; Elit, L.; Nourmoussavi, M.; Glaze, S.; Roberts, L.; Offman, S.L.; Rahimi, K.; Lytwyn, A.; et al. Low-grade serous carcinoma (LGSC): A Canadian multicenter review of practice patterns and patient outcomes. Gynecol. Oncol. 2020, 157, 36–45. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R.C.; Shwartz, L.E.; Haley, L.; Lin, M.T.; Shih Ie, M.; Kurman, R.J. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J. Pathol. 2015, 237, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Kommoss, F.K.F.; Cheasley, D.; Wakefield, M.J.; Scott, C.L.; Campbell, I.G.; Gilks, C.B.; Gorringe, K. Primary mucinous ovarian neoplasms rarely show germ cell histogenesis. Histopathology 2021, 78, 640–642. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A.; et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Serov, S.F.; Scully, R.E.; Sobin, L.H. International Classification of Tumours. Histological Typing of Ovarian Tumours, 1st ed.; WHO: Geneva, Switzerland, 1973. [Google Scholar]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; IARC: Lyon, France, 2014. [Google Scholar]
- Singer, G.; Oldt, R., 3rd; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih Ie, M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, M.A.; Llaurado Fernandez, M.; Langlais, E.; Tone, A.; Ghatage, P.; Glaze, S.; Provencher, D.; Rahimi, K.; Offman, S.L.; Scott, S.A.; et al. Low-grade serous ovarian carcinoma: Recommendation for efficient ancillary testing and standardized biomarker reporting from the Canadian LGSC community of practice. Can. J. Pathol. 2020, 12, 43–58. [Google Scholar]
- Kommoss, S.; Gilks, C.B.; du Bois, A.; Kommoss, F. Ovarian carcinoma diagnosis: The clinical impact of 15 years of change. Br. J. Cancer 2016, 115, 993–999. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Anglesio, M.; Wicklund, K.; Bentley, R.; Berchuck, A.; Kelemen, L.E.; Nazeran, T.M.; Gilks, C.B.; Harris, H.R.; et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol. Oncol. 2018, 151, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Luo, L.; Grevers, X.; Lee, S.; Brooks-Wilson, A.; Gilks, C.B.; Le, N.D.; Cook, L.S. Ovarian Carcinoma Histotype: Strengths and Limitations of Integrating Morphology With Immunohistochemical Predictions. Int. J. Gynecol. Pathol. 2019, 38, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.R.; Kardia, S.L.; Shedden, K.A.; Kuick, R.; Michailidis, G.; Taylor, J.M.; Misek, D.E.; Wu, R.; Zhai, Y.; Darrah, D.M.; et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002, 62, 4722–4729. [Google Scholar] [PubMed]
- Acs, G.; Pasha, T.; Zhang, P.J. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int. J. Gynecol. Pathol. 2004, 23, 110–118. [Google Scholar] [CrossRef]
- Soslow, R.A.; Han, G.; Park, K.J.; Garg, K.; Olvera, N.; Spriggs, D.R.; Kauff, N.D.; Levine, D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod. Pathol. 2012, 25, 625–636. [Google Scholar] [CrossRef]
- Köbel, M.; Kalloger, S.E.; Baker, P.M.; Ewanowich, C.A.; Arseneau, J.; Zherebitskiy, V.; Abdulkarim, S.; Leung, S.; Duggan, M.A.; Fontaine, D.; et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: A transcanadian study. Am. J. Surg. Pathol. 2010, 34, 984–993. [Google Scholar] [CrossRef]
- Köbel, M.; Bak, J.; Bertelsen, B.I.; Carpen, O.; Grove, A.; Hansen, E.S.; Levin Jakobsen, A.M.; Lidang, M.; Masback, A.; Tolf, A.; et al. Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathology 2014, 64, 1004–1013. [Google Scholar] [CrossRef]
- Rutgers, J.L.; Scully, R.E. Ovarian mixed-epithelial papillary cystadenomas of borderline malignancy of mullerian type. A clinicopathologic analysis. Cancer 1988, 61, 546–554. [Google Scholar] [CrossRef]
- Tavassoli, F.A.; Devilee, P. WHO Classification of Tumours. Tumors of the Breast and Female Genital Organs, 3rd ed.; IARC: Lyon, France, 2003. [Google Scholar]
- Taylor, J.; McCluggage, W.G. Ovarian seromucinous carcinoma: Report of a series of a newly categorized and uncommon neoplasm. Am. J. Surg. Pathol. 2015, 39, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Rambau, P.F.; McIntyre, J.B.; Taylor, J.; Lee, S.; Ogilvie, T.; Sienko, A.; Morris, D.; Duggan, M.A.; McCluggage, W.G.; Kobel, M. Morphologic Reproducibility, Genotyping, and Immunohistochemical Profiling Do Not Support a Category of Seromucinous Carcinoma of the Ovary. Am. J. Surg. Pathol. 2017, 41, 685–695. [Google Scholar] [CrossRef]
- Mackenzie, R.; Talhouk, A.; Eshragh, S.; Lau, S.; Cheung, D.; Chow, C.; Le, N.; Cook, L.S.; Wilkinson, N.; McDermott, J.; et al. Morphologic and Molecular Characteristics of Mixed Epithelial Ovarian Cancers. Am. J. Surg. Pathol. 2015, 39, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Verhoef, L.; Aguirre-Hernandez, R.; Nazeran, T.M.; Tessier-Cloutier, B.; Praetorius, T.; Orr, N.L.; Noga, H.; Lum, A.; Khattra, J.; et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum. Reprod. 2019, 34, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Nazeran, T.M.; Tessier-Cloutier, B.; Aguirre-Hernandez, R.; Albert, A.; Lum, A.; Khattra, J.; Praetorius, T.; Mason, M.; Chiu, D.; et al. Oncogenic mutations in histologically normal endometrium: The new normal? J. Pathol. 2019, 249, 173–181. [Google Scholar] [CrossRef]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef]
- da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Da Cruz Paula, A.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef]
- Pors, J.; Segura, S.; Chiu, D.S.; Almadani, N.; Ren, H.; Fix, D.J.; Howitt, B.E.; Kolin, D.; McCluggage, W.G.; Mirkovic, J.; et al. Clinicopathologic Characteristics of Mesonephric Adenocarcinomas and Mesonephric-like Adenocarcinomas in the Gynecologic Tract: A Multi-institutional Study. Am. J. Surg. Pathol. 2021, 45, 498–506. [Google Scholar] [CrossRef]
- Kang, E.Y.; Rodriguez, M.; Lee, S.; Wiebe, N.; Liu, Y.; Cook, L.S.; Lee, C.H.; Karnezis, A.; Köbel, M. Abstracts from USCAP 2021: Gynecologic And Obstetric Pathology: Mesonephric-Like Carcinoma of the Ovary is a Rare and Aggressive Histotype of Ovarian Carcinoma (582). Lab. Investig. 2021, 101, 661–782. [Google Scholar] [CrossRef]
- Shen, S.; Rubinstein, M.M.; Park, K.J.; Konner, J.A.; Makker, V. Sustained response to lenvatinib and pembrolizumab in two patients with KRAS-mutated endometrial mesonephric-like adenocarcinoma. Gynecol. Oncol. Rep. 2021, 37, 100844. [Google Scholar] [CrossRef] [PubMed]
- Coatham, M.; Li, X.; Karnezis, A.N.; Hoang, L.N.; Tessier-Cloutier, B.; Meng, B.; Soslow, R.A.; Blake Gilks, C.; Huntsman, D.G.; Stewart, C.J.; et al. Concurrent ARID1A and ARID1B inactivation in endometrial and ovarian dedifferentiated carcinomas. Mod. Pathol. 2016, 29, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Tessier-Cloutier, B.; Coatham, M.; Carey, M.; Nelson, G.S.; Hamilton, S.; Lum, A.; Soslow, R.A.; Stewart, C.J.; Postovit, L.M.; Köbel, M.; et al. SWI/SNF-deficiency defines highly aggressive undifferentiated endometrial carcinoma. J. Pathol. Clin. Res. 2021, 7, 144–153. [Google Scholar] [CrossRef]
- Köbel, M.; Kalloger, S.E.; Huntsman, D.G.; Santos, J.L.; Swenerton, K.D.; Seidman, J.D.; Gilks, C.B. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 2010, 29, 203–211. [Google Scholar] [CrossRef]
- Assem, H.; Rambau, P.F.; Lee, S.; Ogilvie, T.; Sienko, A.; Kelemen, L.E.; Köbel, M. High-grade Endometrioid Carcinoma of the Ovary: A Clinicopathologic Study of 30 Cases. Am. J. Surg. Pathol. 2018, 42, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Kang, E.Y.; Farrington, K.; Cook, L.S.; Le, N.D.; Karnezis, A.N.; Lee, C.H.; Nelson, G.S.; Terzic, T.; Lee, S.; et al. Accurate Distinction of Ovarian Clear Cell From Endometrioid Carcinoma Requires Integration of Phenotype, Immunohistochemical Predictions, and Genotype: Implications for Lynch Syndrome Screening. Am. J. Surg. Pathol. 2021, 45, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rambau, P.F.; Kelemen, L.E.; Anglesio, M.S.; Leung, S.; Talhouk, A.; Köbel, M. Nuclear β-catenin and CDX2 expression in ovarian endometrioid carcinoma identify patients with favourable outcome. Histopathology 2019, 74, 452–462. [Google Scholar] [CrossRef]
- Dieters-Castator, D.Z.; Rambau, P.F.; Kelemen, L.E.; Siegers, G.M.; Lajoie, G.A.; Postovit, L.M.; Köbel, M. Proteomics-Derived Biomarker Panel Improves Diagnostic Precision to Classify Endometrioid and High-grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2019, 25, 4309–4319. [Google Scholar] [CrossRef] [Green Version]
- Heinze, K.; Nazeran, T.M.; Lee, S.; Krämer, P.; Cairns, E.S.; Chiu, D.S.; Leung, S.C.Y.; Kang, E.Y.; Meagher, N.S.; Kennedy, C.J.; et al. Validated biomarker assays confirm ARID1A loss is confounded with MMR deficiency, CD8 TIL infiltration, and provides no independent prognostic value in endometriosis-associated ovarian carcinomas. J. Pathol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Altman, A.D.; Nelson, G.S.; Ghatage, P.; McIntyre, J.B.; Capper, D.; Chu, P.; Nation, J.G.; Karnezis, A.N.; Han, G.; Kalloger, S.E.; et al. The diagnostic utility of TP53 and CDKN2A to distinguish ovarian high-grade serous carcinoma from low-grade serous ovarian tumors. Mod. Pathol. 2013, 26, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Köbel, M.; Kalloger, S.E.; Carrick, J.; Huntsman, D.; Asad, H.; Oliva, E.; Ewanowich, C.A.; Soslow, R.A.; Gilks, C.B. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am. J. Surg. Pathol. 2009, 33, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Woodbeck, R.; Kelemen, L.E.; Köbel, M. Ovarian Endometrioid Carcinoma Misdiagnosed as Mucinous Carcinoma: An Underrecognized Problem. Int. J. Gynecol. Pathol. 2019, 38, 568–575. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kang, E.Y. The Many Uses of p53 Immunohistochemistry in Gynecological Pathology: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual9 Meeting. Int. J. Gynecol. Pathol. 2021, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Meagher, N.S.; Wang, L.; Rambau, P.F.; Intermaggio, M.P.; Huntsman, D.G.; Wilkens, L.R.; El-Bahrawy, M.A.; Ness, R.B.; Odunsi, K.; Steed, H.; et al. A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ovarian mucinous tumors from colorectal and appendiceal metastases. Mod. Pathol. 2019, 32, 1834–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, Y.; Hatano, K.; Tamada, M.; Morishige, K.I.; Tomita, H.; Yanai, H.; Hara, A. A Comprehensive Review of Ovarian Serous Carcinoma. Adv. Anat. Pathol. 2019, 26, 329–339. [Google Scholar] [CrossRef]
- Murakami, R.; Matsumura, N.; Mandai, M.; Yoshihara, K.; Tanabe, H.; Nakai, H.; Yamanoi, K.; Abiko, K.; Yoshioka, Y.; Hamanishi, J.; et al. Establishment of a Novel Histopathological Classification of High-Grade Serous Ovarian Carcinoma Correlated with Prognostically Distinct Gene Expression Subtypes. Am. J. Pathol. 2016, 186, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; George, J.; Wang, C.; Budden, T.; Tan, T.Z.; Chiu, D.S.; Kommoss, S.; Leong, H.S.; Chen, S.; Intermaggio, M.P.; et al. Development and Validation of the Gene Expression Predictor of High-grade Serous Ovarian Carcinoma Molecular SubTYPE (PrOTYPE). Clin. Cancer Res. 2020, 26, 5411–5423. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Tamayo, P.; Yang, J.Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H.; et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2013, 123, 517–525. [Google Scholar] [CrossRef]
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.S.; Chen, S.; Elatre, W.; Gilks, B.; et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Tumor Tissue Analysis Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [Green Version]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Etemadmoghadam, D.; deFazio, A.; Beroukhim, R.; Mermel, C.; George, J.; Getz, G.; Tothill, R.; Okamoto, A.; Raeder, M.B.; Harnett, P.; et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin. Cancer Res. 2009, 15, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.M.; Enwere, E.; McIntyre, J.B.; Wilson, H.; Nwaroh, C.; Wiebe, N.; Ou, Y.; Liu, S.; Wiedemeyer, K.; Rambau, P.F.; et al. Combined CCNE1 high-level amplification and overexpression is associated with unfavourable outcome in tubo-ovarian high-grade serous carcinoma. J. Pathol. Clin. Res. 2020, 6, 252–262. [Google Scholar] [CrossRef]
- Kang, E.Y.; Millstein, J.; Popovic, G.; Meagher, N.S.; Bolithon, A.; Talhouk, A.; Chiu, D.S.; Anglesio, M.S.; Leung, B.; Tang, K.; et al. MCM3 is a novel proliferation marker associated with longer survival for patients with tubo-ovarian high-grade serous carcinoma. Virchows Arch. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Garsed, D.W.; Alsop, K.; Fereday, S.; Emmanuel, C.; Kennedy, C.J.; Etemadmoghadam, D.; Gao, B.; Gebski, V.; Gares, V.; Christie, E.L.; et al. Homologous Recombination DNA Repair Pathway Disruption and Retinoblastoma Protein Loss Are Associated with Exceptional Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2018, 24, 569–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macintyre, G.; Goranova, T.E.; De Silva, D.; Ennis, D.; Piskorz, A.M.; Eldridge, M.; Sie, D.; Lewsley, L.A.; Hanif, A.; Wilson, C.; et al. Copy number signatures and mutational processes in ovarian carcinoma. Nat. Genet. 2018, 50, 1262–1270. [Google Scholar] [CrossRef]
- Vázquez-García, I.; Uhlitz, F.; Ceglia, N.; Lim, J.L.P.; Wu, M.; Mohibullah, N.; Ruiz, A.E.B.; Boehm, K.M.; Bojilova, V.; Fong, J.F.; et al. Immune and malignant cell phenotypes of ovarian cancer are determined by distinct mutational processes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Virani, S.; Baiocchi, G.; Bowtell, D.; Cabasag, C.J.; Cho, K.R.; Fortner, R.T.; Fujiwara, K.; Kim, J.W.; Köbel, M.; Kurtz, J.E.; et al. Joint IARC/NCI International Cancer Seminar Series Report: Expert consensus on future directions for ovarian carcinoma research. Carcinogenesis 2021, 42, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Funingana, I.G.; Reinius, M.A.V.; Petrillo, A.; Ang, J.E.; Brenton, J.D. Can integrative biomarker approaches improve prediction of platinum and PARP inhibitor response in ovarian cancer? Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, L.M.; Vermeulen, S.; Meijers, M.; van Diest, M.F.; Ter Haar, N.T.; de Jonge, M.M.; Solleveld-Westerink, N.; van Wezel, T.; van Gent, D.C.; Kroep, J.R.; et al. The RECAP Test Rapidly and Reliably Identifies Homologous Recombination-Deficient Ovarian Carcinomas. Cancers 2020, 12, 2805. [Google Scholar] [CrossRef]
- van Wijk, L.M.; Kramer, C.J.H.; Vermeulen, S.; Ter Haar, N.T.; de Jonge, M.M.; Kroep, J.R.; de Kroon, C.D.; Gaarenstroom, K.N.; Vrieling, H.; Bosse, T.; et al. The RAD51-FFPE Test; Calibration of a Functional Homologous Recombination Deficiency Test on Diagnostic Endometrial and Ovarian Tumor Blocks. Cancers 2021, 13, 2994. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; Weir, B.A.; Au-Yeung, G.; Alsop, K.; Mitchell, G.; George, J.; Davis, S.; D'Andrea, A.D.; Simpson, K.; Hahn, W.C.; et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc. Natl. Acad. Sci. USA 2013, 110, 19489–19494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, P.; Talhouk, A.; Brett, M.A.; Chiu, D.S.; Cairns, E.S.; Scheunhage, D.A.; Hammond, R.F.L.; Farnell, D.; Nazeran, T.M.; Grube, M.; et al. Endometrial Cancer Molecular Risk Stratification is Equally Prognostic for Endometrioid Ovarian Carcinoma. Clin. Cancer Res. 2020, 26, 5400–5410. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Parra-Herran, C.; Lerner-Ellis, J.; Xu, B.; Khalouei, S.; Bassiouny, D.; Cesari, M.; Ismiil, N.; Nofech-Mozes, S. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod. Pathol. 2017, 30, 1748–1759. [Google Scholar] [CrossRef]
- Leskela, S.; Romero, I.; Rosa-Rosa, J.M.; Caniego-Casas, T.; Cristobal, E.; Pérez-Mies, B.; Gutierrez-Pecharroman, A.; Santón, A.; Ojeda, B.; López-Reig, R.; et al. Molecular Heterogeneity of Endometrioid Ovarian Carcinoma: An Analysis of 166 Cases Using the Endometrial Cancer Subrogate Molecular Classification. Am. J. Surg. Pathol. 2020, 44, 982–990. [Google Scholar] [CrossRef]
- Rambau, P.; Kelemen, L.E.; Steed, H.; Quan, M.L.; Ghatage, P.; Kobel, M. Association of Hormone Receptor Expression with Survival in Ovarian Endometrioid Carcinoma: Biological Validation and Clinical Implications. Int. J. Mol. Sci. 2017, 18, 515. [Google Scholar] [CrossRef] [Green Version]
- Rambau, P.F.; Duggan, M.A.; Ghatage, P.; Warfa, K.; Steed, H.; Perrier, R.; Kelemen, L.E.; Kobel, M. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology 2016, 69, 288–297. [Google Scholar] [CrossRef]
- Hollis, R.L.; Stanley, B.; Iida, Y.; Thomson, J.; Churchman, M.; Rye, T.; Mackean, M.; Nussey, F.; Gourley, C.; Herrington, C.S. Hormone receptor expression patterns define clinically meaningful subgroups of endometrioid ovarian carcinoma. Gynecol. Oncol. 2019, 155, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Hollis, R.L.; Thomson, J.P.; Stanley, B.; Churchman, M.; Meynert, A.M.; Rye, T.; Bartos, C.; Iida, Y.; Croy, I.; Mackean, M.; et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat. Commun. 2020, 11, 4995. [Google Scholar] [CrossRef]
- Hollis, R.L.; Stanley, B.; Thomson, J.P.; Churchman, M.; Croy, I.; Rye, T.; Bartos, C.; Nussey, F.; Mackean, M.; Meynert, A.M.; et al. Integrated molecular characterisation of endometrioid ovarian carcinoma identifies opportunities for stratification. NPJ Precis. Oncol. 2021, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Takenaka, M.; Okamoto, A.; Bowtell, D.D.L.; Kohno, T. Treatment Strategies for ARID1A-Deficient Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 1769. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.M.; Winham, S.J.; Wang, C.; Weigelt, B.; Fu, Z.; Armasu, S.M.; McCauley, B.M.; Brand, A.H.; Chiew, Y.E.; Elishaev, E.; et al. DNA Methylation Profiles of Ovarian Clear Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 31, 132–141. [Google Scholar] [CrossRef]
- Tan, D.S.; Iravani, M.; McCluggage, W.G.; Lambros, M.B.; Milanezi, F.; Mackay, A.; Gourley, C.; Geyer, F.C.; Vatcheva, R.; Millar, J.; et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin. Cancer Res. 2011, 17, 1521–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambau, P.F.; Vierkant, R.A.; Intermaggio, M.P.; Kelemen, L.E.; Goodman, M.T.; Herpel, E.; Pharoah, P.D.; Kommoss, S.; Jimenez-Linan, M.; Karlan, B.Y.; et al. Association of p16 expression with prognosis varies across ovarian carcinoma histotypes: An Ovarian Tumor Tissue Analysis consortium study. J. Pathol. Clin. Res. 2018, 4, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zeng, Z.; Afsharpad, M.; Lin, C.; Wang, S.; Yang, H.; Liu, S.; Kelemen, L.E.; Xu, W.; Ma, W.; et al. Overexpression of IGF2BP3 as a Potential Oncogene in Ovarian Clear Cell Carcinoma. Front. Oncol. 2019, 9, 1570. [Google Scholar] [CrossRef]
- Takenaka, M.; Kobel, M.; Garsed, D.W.; Fereday, S.; Pandey, A.; Etemadmoghadam, D.; Hendley, J.; Kawabata, A.; Noguchi, D.; Yanaihara, N.; et al. Survival Following Chemotherapy in Ovarian Clear Cell Carcinoma Is Not Associated with Pathological Misclassification of Tumor Histotype. Clin. Cancer Res. 2019, 25, 3962–3973. [Google Scholar] [CrossRef] [Green Version]
- Wiedemeyer, K.; Wang, L.; Kang, E.Y.; Liu, S.; Ou, Y.; Kelemen, L.E.; Feil, L.; Anglesio, M.S.; Glaze, S.; Ghatage, P.; et al. Prognostic and Theranostic Biomarkers in Ovarian Clear Cell Carcinoma. Int. J. Gynecol. Pathol. 2021. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wen, K.C.; Sung, P.L.; Chou, Y.T.; Liew, P.L.; Chen, L.Y.; Huang, R.L.; Lai, H.C.; Chang, L.T. Complete remission of heavily treated ovarian clear cell carcinoma with ARID1A mutations after pembrolizumab and bevacizumab combination therapy: A case report. J. Ovarian Res. 2020, 13, 143. [Google Scholar] [CrossRef]
- Bennett, J.A.; Morales-Oyarvide, V.; Campbell, S.; Longacre, T.A.; Oliva, E. Mismatch Repair Protein Expression in Clear Cell Carcinoma of the Ovary: Incidence and Morphologic Associations in 109 Cases. Am. J. Surg. Pathol. 2016, 40, 656–663. [Google Scholar] [CrossRef]
- Sue, A.Q.R.; Patel, P.G.; Shakfa, N.; Nyi, M.N.; Afriyie-Asante, A.; Kang, E.Y.; Köbel, M.; Koti, M. Prognostic significance of T cells, PD-L1 immune checkpoint and tumour associated macrophages in clear cell carcinoma of the ovary. Gynecol. Oncol. 2021, 162, 421–430. [Google Scholar] [CrossRef]
- Khalique, S.; Nash, S.; Mansfield, D.; Wampfler, J.; Attygale, A.; Vroobel, K.; Kemp, H.; Buus, R.; Cottom, H.; Roxanis, I.; et al. Quantitative Assessment and Prognostic Associations of the Immune Landscape in Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- Cheasley, D.; Nigam, A.; Zethoven, M.; Hunter, S.; Etemadmoghadam, D.; Semple, T.; Allan, P.; Carey, M.S.; Fernandez, M.L.; Dawson, A.; et al. Genomic analysis of low-grade serous ovarian carcinoma to identify key drivers and therapeutic vulnerabilities. J. Pathol. 2021, 253, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Llaurado Fernandez, M.; Dawson, A.; Kim, H.; Lam, N.; Russell, H.; Bruce, M.; Bittner, M.; Hoenisch, J.; Scott, S.A.; Talhouk, A.; et al. Hormone receptor expression and outcomes in low-grade serous ovarian carcinoma. Gynecol. Oncol. 2020, 157, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Llaurado Fernandez, M.; Dawson, A.; Hoenisch, J.; Volik, S.; Lin, Y.Y.; Anderson, S.; Kim, H.; Haegert, A.M.; Colborne, S.; et al. Multiomics Characterization of Low-Grade Serous Ovarian Carcinoma Identifies Potential Biomarkers of MEK Inhibitor Sensitivity and Therapeutic Vulnerability. Cancer Res. 2021, 81, 1681–1694. [Google Scholar] [CrossRef]
- Chui, M.H.; Chang, J.C.; Zhang, Y.; Zehir, A.; Schram, A.M.; Konner, J.; Drilon, A.E.; Da Cruz Paula, A.; Weigelt, B.; Grisham, R.N. Spectrum of BRAF Mutations and Gene Rearrangements in Ovarian Serous Carcinoma. JCO Precis. Oncol. 2021, 5, 1480–1492. [Google Scholar] [CrossRef]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Anglesio, M.S.; Au-Yeung, G.; Bohm, M.; et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.Y.; Cheasley, D.; LePage, C.; Wakefield, M.J.; da Cunha Torres, M.; Rowley, S.; Salazar, C.; Xing, Z.; Allan, P.; Bowtell, D.D.L.; et al. Refined cut-off for TP53 immunohistochemistry improves prediction of TP53 mutation status in ovarian mucinous tumors: Implications for outcome analyses. Mod. Pathol. 2021, 34, 194–206. [Google Scholar] [CrossRef]
- Gorringe, K.L.; Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Bowtell, D.D.L.; Christie, M.; et al. Therapeutic options for mucinous ovarian carcinoma. Gynecol. Oncol. 2020, 156, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.Y.; Wiebe, N.J.; Aubrey, C.; Lee, C.H.; Anglesio, M.S.; Tilley, D.; Ghatage, P.; Nelson, G.S.; Lee, S.; Köbel, M. Selection of endometrial carcinomas for p53 immunohistochemistry based on nuclear features. J. Pathol. Clin. Res. 2022, 8, 19–32. [Google Scholar] [CrossRef]
- Santandrea, G.; Piana, S.; Valli, R.; Zanelli, M.; Gasparini, E.; De Leo, A.; Mandato, V.D.; Palicelli, A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics 2021, 11, 199. [Google Scholar] [CrossRef] [PubMed]

| WHO 1973, 1st ed. | WHO 2003, 3rd ed. | WHO 2014, 4th ed. | WHO 2020, 5th ed. |
|---|---|---|---|
| Serous | Serous | High-grade serous | High-grade serous |
| Low-grade serous | Low-grade serous | ||
| Mucinous | Mucinous | Mucinous | Mucinous |
| Seromucinous | |||
| Endometrioid | Endometrioid | Endometrioid | Endometrioid |
| Clear cell | Clear cell | Clear cell | Clear cell |
| Brenner | Transitional cell | Brenner | Brenner |
| Squamous | |||
| Mesonephric-like | |||
| Undifferentiated | Undifferentiated | Undifferentiated | Undifferentiated |
| Carcinosarcoma | |||
| Mixed | Mixed | Mixed | |
| Unclassified epithelial |
| Histotype 1 | Histotype 2 | First-Line Panel | Second-Line Panel | Reference(s) |
|---|---|---|---|---|
| HGSC | EC | WT1/p53: WT1+/p53abn combination is 99% specific for HGSC. WT1-/p53 wild type is highly specific for EC. Note: 10–15% of ECs can be either WT1+ or p53abn (rarely, both). | MMR and ARID1A have limited sensitivity (12% and 25%, respectively) for EC but are specific. PR, ELAPOR1 have limited discriminatory values as they are present in 85% of ECs versus 40% of HGSCs. Nuclear CTNNB1 expression is specific for ECs and present in ~50%, mostly low-grade ECs with squamous differentiation. Consider testing for somatic BRCA1/2 or HRD. | [3,44,45,46,47,48] |
| HGSC | LGSC | p53: p53abn excludes LGSC (100% specific); however, 2–4% of HGSCs can show p53 wild type staining despite harboring a TP53 mutation due to a non-functional but expressed protein. | p16: in the context of p53 wild type staining, if p16 shows normal patchy/heterogeneous expression, the probability of LGSC is 84%; if p16 is block diffuse, the probability of HGSC is 88%. Rare cases of p53 wild type, p16 block diffuse LGSC do exist, but they seem to carry an adverse outcome. Consider sequencing for MAPK pathway mutations. | [49] |
| HGSC | CCC | WT1, napsin A, ER: WT1+/ER+ confirms HGSC. WT1-/napsin A+ confirms CCC. | HNF1B, ARID1A: some napsin A- CCCs are HNF1B+. ARID1A is lost in 42% of CCCs. | [3,48,50] |
| HGSC | MC | WT1: WT1+ confirms HGSC. | [3,23] | |
| EC | LGSC | WT1: WT1+ alone has perfect sensitivity for LGSC but is expressed in 10–15% of ECs. | Specific markers for EC (CTNNB1, ARID1A, MMR). | [3] |
| EC | CCC | Napsin A, HNF1B, PR: napsin A+/HNF1B diffuse +/PR- supports CCC (note that areas of cytoplasmic clearing in EC can show this profile). Napsin A-/HNF1B non-diffuse/PR+ confirms EC. | ELAPOR1, CDX2, AMACR: ELAPOR1+, CDX2+, AMACR- support EC. Further, ambiguous or mixed EC/CCC or tumors with diffuse intratumoral stromal inflammation should be tested for MMR, and, if deficient, consider EC. | [45] |
| EC | MC | PR+ confirms EC, although 15% of ECs are PR-. Presence of any vimentin expression supports EC. | ER is usually negative in MC. | [51] |
| LGSC | CCC/MC | WT1: WT1+ in LGSC, WT1- in CCC/MC. | [3] | |
| CCC | MC | Napsin A, mucin stain: napsin A+/mucin- in CCC. Napsin A-/mucin+ in MC. | [3] | |
| EC | Meso- Nephric-like | GATA3, TTF1, ER, PR: GATA3+ and/or TTF1+ with ER-/PR- confirms mesonephric-like adenocarcinoma. | [38,39] | |
| EC | DDC | ARID1B, BRG1, INI1: loss of any of these markers confirms DDC. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köbel, M.; Kang, E.Y. The Evolution of Ovarian Carcinoma Subclassification. Cancers 2022, 14, 416. https://doi.org/10.3390/cancers14020416
Köbel M, Kang EY. The Evolution of Ovarian Carcinoma Subclassification. Cancers. 2022; 14(2):416. https://doi.org/10.3390/cancers14020416
Chicago/Turabian StyleKöbel, Martin, and Eun Young Kang. 2022. "The Evolution of Ovarian Carcinoma Subclassification" Cancers 14, no. 2: 416. https://doi.org/10.3390/cancers14020416
APA StyleKöbel, M., & Kang, E. Y. (2022). The Evolution of Ovarian Carcinoma Subclassification. Cancers, 14(2), 416. https://doi.org/10.3390/cancers14020416






