Role of Radiation Therapy in Mortality among Adolescents and Young Adults with Lymphoma: Differences According to Cause of Death
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Database
2.2. Definition of Variables from the SEER
2.3. Cause of Death Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Competing Risk Analyses
3.3. Comparison with the General Population
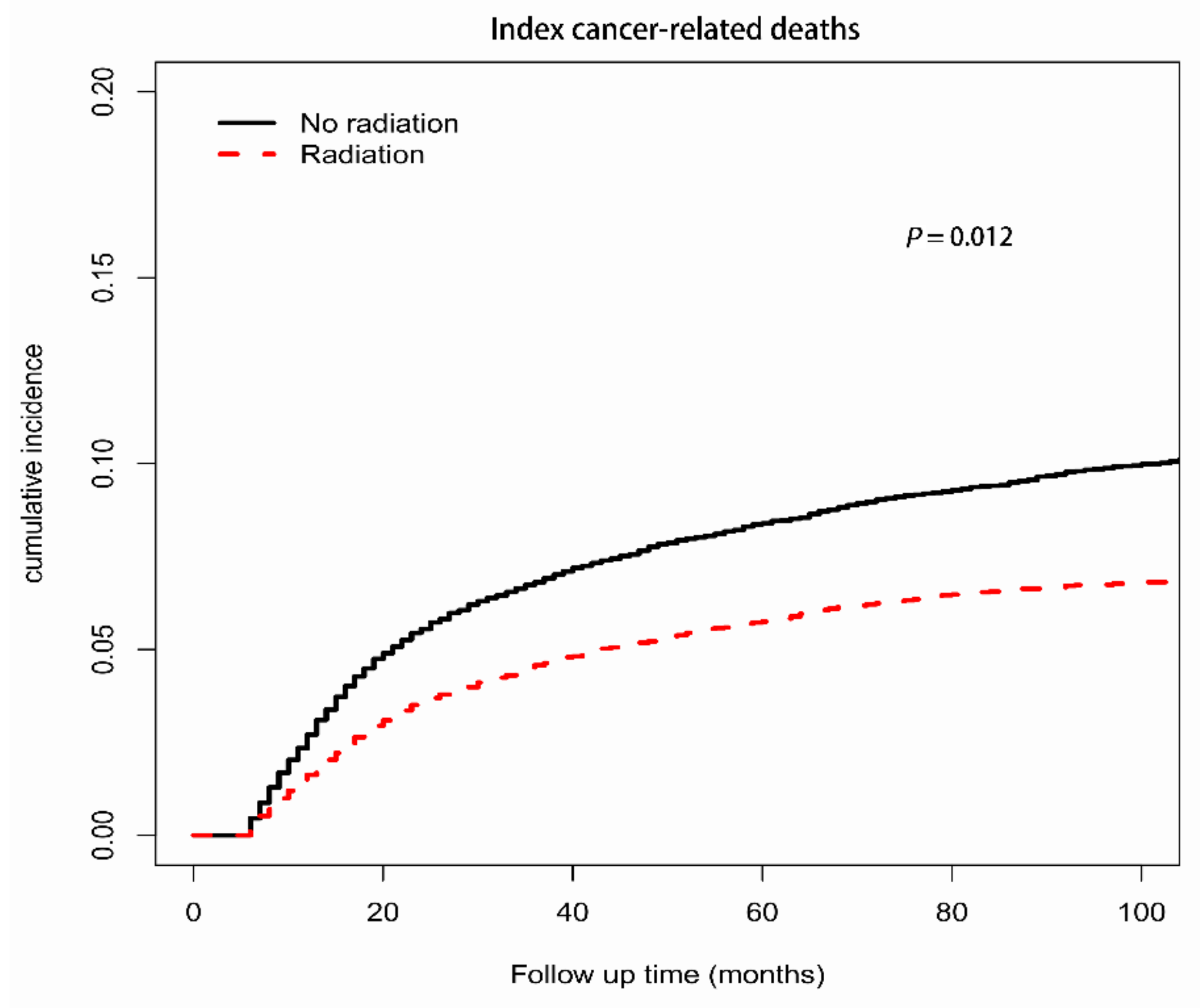
3.3.1. Index-Cancer-Related Deaths
3.3.2. SMN-Related Deaths
3.3.3. Noncancer-Related Deaths
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Close, A.G.; Dreyzin, A.; Miller, K.D.; Seynnaeve, B.K.N.; Rapkin, L.B. Adolescent and young adult oncology-past, present, and future. CA: A Cancer J. Clin. 2019, 69, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; Viny, A.; Barr, R. Cancer in 15- to 29-year-olds by primary site. Oncologist 2006, 11, 590–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Das, S.; Okwan-Duodu, D.; Esiashvili, N.; Flowers, C.; Chen, Z.; Wang, X.; Jiang, K.; Nastoupil, L.J.; Khan, M.K. Patterns of failure in advanced stage diffuse large B-cell lymphoma patients after complete response to R-CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Dores, G.M.; Curtis, R.E.; Lynch, C.F.; Stovall, M.; Hall, P.; Gilbert, E.S.; Hodgson, D.C.; Storm, H.H.; Johannesen, T.B.; et al. Stomach cancer risk after treatment for hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 3369–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; van Leeuwen, F.E. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Gilbert, E.S.; Stovall, M.; van Leeuwen, F.E.; Dores, G.M.; Lynch, C.F.; Hall, P.; Smith, S.A.; Weathers, R.E.; Storm, H.H.; et al. Risk of esophageal cancer following radiotherapy for Hodgkin lymphoma. Haematologica 2014, 99, e193–e196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maraldo, M.V.; Giusti, F.; Vogelius, I.R.; Lundemann, M.; van der Kaaij, M.A.E.; Ramadan, S.; Meulemans, B.; Henry-Amar, M.; Aleman, B.M.P.; Raemaekers, J.P.; et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015, 2, e492–e502. [Google Scholar] [CrossRef]
- Daniëls, L.A.; Krol, A.D.G.; Schaapveld, M.; Putter, H.; Jansen, P.M.; Marijt, E.W.A.; van Leeuwen, F.E.; Creutzberg, C.L. Long-term risk of secondary skin cancers after radiation therapy for Hodgkin’s lymphoma. Radiother. Oncol. 2013, 109, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.; van den Belt-Dusebout, A.W.; Klokman, W.J.; Veer, M.B.V.; Bartelink, H.; van Leeuwen, F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, M.V.; Brodin, N.P.; Aznar, M.C.; Vogelius, I.R.; Rosenschöld, P.M.a.; Petersen, P.M.; Specht, L. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann. Oncol. 2013, 24, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, M.V.; Brodin, P.; Aznar, M.C.; Vogelius, I.R.; Rosenschöld, P.M.a.; Petersen, P.M.; Specht, L. Doses to carotid arteries after modern radiation therapy for Hodgkin lymphoma: Is stroke still a late effect of treatment? Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 297–303. [Google Scholar] [CrossRef]
- Eich, H.T.; Diehl, V.; Görgen, H.; Pabst, T.; Markova, J.; Debus, J.; Ho, A.; Dörken, B.; Rank, A.; Grosu, A.-L.; et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD11 trial. J. Clin. Oncol. 2010, 28, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- PHoskin, J.; Kirkwood, A.A.; Popova, B.; Smith, P.; Robinson, M.; Gallop-Evans, E.; Coltart, S.; Illidge, T.; Madhavan, K.; Brammer, C.; et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomised phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Lowry, L.; Smith, P.; Qian, W.; Falk, S.; Benstead, K.; Illidge, T.; Linch, D.; Robinson, M.; Jack, A.; Hoskin, P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother. Oncol. 2011, 100, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.C.; Costa, L.J. Changes in the use of radiation therapy for early classical Hodgkin lymphoma in adolescents and young adults: Implications for survival and second malignancies. Leuk. Lymphoma 2015, 56, 2339–2343. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.S.; Cho, W.K.; Lee, S.E.; Choi, B.O.; Jung, S.E.; Park, G.S.; Kim, S.H.; Yang, S.W.; Cho, S.G. Ophthalmologic outcomes after chemotherapy and/or radiotherapy in non-conjunctival ocular adnexal MALT lymphoma. Ann. Hematol. 2012, 91, 1393–1401. [Google Scholar] [CrossRef]
- Koshy, M.; Rich, S.E.; Mahmood, U.; Kwok, Y. Declining use of radiotherapy in stage I and II Hodgkin’s disease and its effect on survival and secondary malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, S.S. Finding the balance in pediatric Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3158–3159. [Google Scholar] [CrossRef]
- Purdy, J.A. From new frontiers to new standards of practice: Advances in radiotherapy planning and delivery. Front. Radiat. Ther. Oncol. 2007, 40, 18–39. [Google Scholar] [PubMed]
- Kumar, S. Second malignant neoplasms following radiotherapy. Int. J. Environ. Res. Public Health 2012, 9, 4744–4759. [Google Scholar] [CrossRef]
- Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: Current status and issues of interest. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 880–914. [Google Scholar] [CrossRef]
- Yahalom, J.; Ryu, J.; Straus, D.J.; Gaynor, J.J.; Myers, J.; Caravelli, J.; Clarkson, B.D.; Fuks, Z. Impact of adjuvant radiation on the patterns and rate of relapse in advanced-stage Hodgkin’s disease treated with alternating chemotherapy combinations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.; Barrington, S.; Counsell, N.; Pettengell, R.; Johnson, P.; Wimperis, J.; Coltart, S.; Culligan, D.; Lister, A.; Bessell, E.; et al. Involved field radiotherapy versus no further treatment in patients with clinical stages IA and IIA Hodgkin lymphoma and a ‘Negative’ PET scan after 3 cycles ABVD. Results of the UK NCRI RAPID trial. Blood 2012, 120, 547. [Google Scholar] [CrossRef]
- Dhakal, S.; Biswas, T.; Liesveld, J.L.; Friedberg, J.W.; Phillips, G.L.; Constine, L.S. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin’s lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Kallam, A.; Armitage, J.O. Current and emerging treatment options for a patient with a second relapse of Hodgkin’s lymphoma. Expert Rev. Hematol. 2018, 11, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The childhood cancer survivor study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- Youn, P.; Milano, M.T.; Constine, L.S.; Travis, L.B. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer 2014, 120, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Andersson, M.; Gospodarowicz, M.; van Leeuwen, F.E.; Bergfeldt, K.; Lynch, C.F.; Curtis, R.E.; Kohler, B.A.; Wiklund, T.; Storm, H.; et al. Treatment-associated leukemia following testicular cancer. J. Natl. Cancer Inst. 2000, 92, 1165–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierard, G.E.; Pierard-Franchimont, C.; Paquet, P.; Quatresooz, P. Emerging therapies for ionizing radiation-associated skin field carcinogenesis. Expert Opin. Pharmacother. 2009, 10, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Mallick, S.; Lin, S.H.; Krishnan, S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit. Rev. Oncol. Hematol. 2018; 123, 42–51. [Google Scholar]
- O’Brien, M.M.; Donaldson, S.S.; Balise, R.R.; Whittemore, A.S.; Link, M.P. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1232–1239. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. New Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.L.; Connors, J.M.; Tyldesley, S.; Savage, K.J.; Campbell, B.A.; Zheng, Y.Y.; Hamm, J.; Pickles, T. Secondary Breast Cancer Risk by Radiation Volume in Women With Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bhuller, K.S.; Zhang, Y.; Li, D.; Sehn, L.H.; Goddard, K.; McBride, M.L.; Rogers, P.C. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: Report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br. J. Haematol. 2016, 172, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.; Swerdlow, A.J. Second primary breast cancer after Hodgkin’s disease. Br. J. Cancer 2004, 90, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Pugh, T.J.; Ballonoff, A.; Rusthoven, K.E.; McCammon, R.; Kavanagh, B.; Newman, F.; Rabinovitch, R. Cardiac mortality in patients with stage I and II diffuse large B-cell lymphoma treated with and without radiation: A surveillance, epidemiology, and end-results analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 845–849. [Google Scholar] [CrossRef]
- Ng, A.K.; Mauch, P.M. Role of radiation therapy in localized aggressive lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 757–759. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Advani, R.H.; Buadi, F.; Cabanillas, F.; Caligiuri, M.A.; Czuczman, M.S.; Damon, L.E.; Fayad, L.; Flinn, I.W.; Forero, A.; et al. Non-Hodgkin’s lymphoma. Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN 2006, 4, 258–310. [Google Scholar]
- von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Miller, T.P.; Dahlberg, S.; Cassady, J.R.; Adelstein, D.J.; Spier, C.M.; Grogan, T.M.; LeBlanc, M.; Carlin, S.; Chase, E.; Fisher, R.I. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N. Engl. J. Med. 1998, 339, 21–26. [Google Scholar] [CrossRef]

| Characteristic | Overall | Radiation | No Radiation | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Overall | 29,686 | 100 | 10,708 | 100 | 18,978 | 100 |
| Age, y | ||||||
| 15–24 | 9926 | 33.44 | 3944 | 36.83 | 5982 | 31.52 |
| 25–39 | 19,760 | 66.56 | 6764 | 63.17 | 12,996 | 68.48 |
| Sex | ||||||
| Male | 16,256 | 54.76 | 5583 | 52.14 | 10,673 | 56.24 |
| Female | 13,430 | 45.24 | 5125 | 47.86 | 8305 | 43.76 |
| Race | ||||||
| White | 23,648 | 79.66 | 8760 | 81.81 | 14,888 | 78.45 |
| Black | 3689 | 12.43 | 1050 | 9.81 | 2639 | 13.91 |
| Other | 2135 | 7.19 | 843 | 7.87 | 1292 | 6.81 |
| Unknown | 214 | 0.72 | 55 | 0.51 | 159 | 0.84 |
| Era of diagnosis, year | ||||||
| 1992–2001 | 8024 | 27.03 | 3303 | 30.85 | 4721 | 24.88 |
| 2002–2016 | 21,662 | 72.97 | 7405 | 69.15 | 14,257 | 75.12 |
| Marital status | ||||||
| Married | 10,413 | 35.08 | 3955 | 36.94 | 6458 | 34.03 |
| Unmarried | 18,210 | 61.34 | 6436 | 60.1 | 11,774 | 62.04 |
| Unknown | 1063 | 3.58 | 317 | 2.96 | 746 | 3.93 |
| Ann Arbor stage | ||||||
| I/II | 16,463 | 55.46 | 7888 | 73.66 | 8575 | 45.18 |
| III/IV | 11,442 | 38.54 | 2409 | 22.5 | 9033 | 47.6 |
| Unknown | 1781 | 6 | 411 | 3.84 | 1370 | 7.22 |
| Status | ||||||
| Live | 25,391 | 85.53 | 9505 | 88.77 | 15,886 | 83.71 |
| Death | 4295 | 14.47 | 1203 | 11.23 | 3092 | 16.29 |
| Cause of death | ||||||
| Index cancer | 2439 | 56.79 | 712 | 59.19 | 1727 | 55.85 |
| SMNs | 961 | 22.37 | 222 | 18.45 | 739 | 23.9 |
| Noncancer causes | 895 | 20.84 | 269 | 22.36 | 626 | 20.25 |
| Cardiovascular disease | 208 | 4.84 | 70 | 5.82 | 138 | 4.46 |
| Lymphoma subtype | ||||||
| HL | 17,712 | 59.66 | 7447 | 69.55 | 10,265 | 54.09 |
| DLBCL | 7627 | 25.69 | 2578 | 24.08 | 5049 | 26.6 |
| BL | 1137 | 3.83 | 124 | 1.16 | 1013 | 5.34 |
| FL | 1636 | 5.51 | 281 | 2.62 | 1355 | 7.14 |
| MZL | 297 | 1 | 57 | 0.53 | 240 | 1.26 |
| MCL | 85 | 0.29 | 11 | 0.1 | 74 | 0.39 |
| CLL/SLL | 345 | 1.16 | 27 | 0.25 | 318 | 1.68 |
| PTCL | 847 | 2.85 | 183 | 1.71 | 664 | 3.5 |
| Index-Cancer-Related Mortality | SMN-Related Mortality | Noncancer-Disease-Related Mortality | ||||
|---|---|---|---|---|---|---|
| AHR (95% CI) | P | AHR (95% CI) | P | AHR (95% CI) | P | |
| Treatment | ||||||
| No radiation | Reference | - | Reference | - | Reference | - |
| Radiation | 0.89 (0.81–0.97) | 0.012 | 0.74 (0.63–0.88) | <0.001 | 0.77 (0.66–0.89) | <0.001 |
| Age, y | ||||||
| 15–24 | Reference | - | Reference | - | Reference | - |
| 25–39 | 1.12 (1.01–1.24) | 0.026 | 3.87 (3.17–4.72) | <0.001 | 1.47 (1.24–1.76) | <0.001 |
| Sex | ||||||
| Male | Reference | - | Reference | - | Reference | - |
| Female | 0.87 (0.80–0.94) | <0.001 | 0.49 (0.42–0.57) | <0.001 | 0.75 (0.65–0.86) | <0.001 |
| Year of diagnosis | ||||||
| 1992–2001 | Reference | - | Reference | - | Reference | - |
| 2002–2016 | 0.57 (0.52–0.61) | <0.001 | 0.45 (0.39–0.51) | <0.001 | 0.78 (0.68–0.9) | <0.001 |
| Race | ||||||
| White | Reference | - | Reference | - | Reference | - |
| Black | 1.32 (1.18–1.48) | <0.001 | 1.86 (1.6–2.18) | <0.001 | 1.21 (1–1.46) | 0.049 |
| Other | 1.34 (1.16–1.54) | <0.001 | 0.62 (0.45–0.87) | 0.005 | 0.98 (0.75–1.3) | 0.91 |
| Unknown | 0.24 (0.08–0.75) | 0.014 | 0.22 (0.03–1.59) | 0.13 | 0.23 (0.03–1.67) | 0.15 |
| Stage | ||||||
| I/II | Reference | - | Reference | - | Reference | - |
| III/IV | 2.01 (1.84–2.19) | <0.001 | 1.75 (1.52–2.03) | <0.001 | 1.42 (1.23–1.64) | <0.001 |
| Unknown | 1.47 (1.17–1.85) | 0.001 | 1.74 (1.33–2.29) | <0.001 | 1.18 (0.82–1.68) | 0.37 |
| Marital status | ||||||
| Married | Reference | - | Reference | - | Reference | - |
| Unmarried | 1.05 (0.96–1.15) | 0.31 | 2.61 (2.23–3.05) | <0.001 | 1.05 (0.91–1.23) | 0.50 |
| Unknown | 0.66 (0.50–0.87) | 0.0036 | 2.15 (1.52–3.04) | <0.001 | 0.91 (0.61–1.36) | 0.66 |
| Subtype | ||||||
| HL | Reference | - | Reference | - | Reference | - |
| DLBCL | 2.35 (2.14–2.57) | <0.001 | 2.14 (1.84–2.47) | <0.001 | 1.10 (0.94–1.29) | 0.25 |
| BL | 2.09 (1.72–2.53) | <0.001 | 3.91 (3.08–4.95) | <0.001 | 1.12 (0.79–1.58) | 0.53 |
| FL | 1.61 (1.37–1.88) | <0.001 | 0.54 (0.36–0.81) | 0.003 | 1.25 (0.97–1.61) | 0.078 |
| MZL | 1.27 (0.85–1.91) | 0.24 | 0.66 (0.27–1.59) | 0.35 | 1.25 (0.69–2.27) | 0.47 |
| MCL | 3.19 (2.08–4.88) | <0.001 | 0.95 (0.31–2.91) | 0.93 | 1.28 (0.48–3.39) | 0.62 |
| CLL/SLL | 1.01 (0.67–1.51) | 0.98 | 6.06 (4.5–8.16) | <0.001 | 2.07 (1.34–3.19) | <0.001 |
| PTCL | 3.21 (2.68–3.84) | <0.001 | 1.36 (0.9–2.05) | 0.14 | 0.73 (0.45–1.19) | 0.20 |
| Characteristic | Radiation | No Radiation |
|---|---|---|
| SMR (95% CI) | SMR (95% CI) | |
| Overall | 534.66 * (489.9–582.41) | 799.25 * (754.33–846.15) |
| Age, y | ||
| 15–24 | 806.26 * (679.11–950.31) | 1258.12 * (1113.29–1416.56) |
| 25–39 | 475.3 * (428.88–525.37) | 720.34 * (674.27–768.74) |
| Sex | ||
| Male | 473.24 * (421.36–529.74) | 738.42 * (687.05–792.62) |
| Female | 648.68 * (566.33–739.63) | 942.15 * (853.78–1037.18) |
| Race | ||
| White | 483.67 * (437.48–533.41) | 724.96 * (677.42–774.95) |
| Black | 633.48 * (493.83–800.37) | 829.34 * (717.84–953.25) |
| Other | 1274.98 * (957.81–1663.58) | 2482.04 * (2057.85–2967.91) |
| Latency periods, m | ||
| 0–11 | 3221.36 * (2639.98–3892.68) | 4981.89 * (4425.88–5588.43) |
| 12–59 | 1256.56 * (1119.7–1405.53) | 1684.83 * (1558.91–1818.21) |
| 60–119 | 282.24 * (222.38–353.27) | 438.62 * (375.52–509.3) |
| 120+ | 82.58 * (57.84–114.33) | 89.7 * (67.94–116.22) |
| Ann Arbor stage | ||
| I/II | 404.77 * (360.11–453.43) | 577.72 * (521.73–638.09) |
| III/IV | 937.95 * (814.81–1074.45) | 1018.5 * (946.9–1094.07) |
| Lymphoma subtype | ||
| HL | 385.96 * (338.58–438.12) | 583.55 * (529.99–641.05) |
| DLBCL | 782.51 * (682.08–893.57) | 1105.11 * (1006.2–1211.1) |
| BL | 1704.02 * (1025.93–2661.03) | 1327.53 * (1030.88–1682.96) |
| FL | 458.3 * (283.69–700.56) | 709.75 * (584.41–854.01) |
| MZL | 379.08 * (45.91–1369.35) | 755.29 * (431.72–1226.55) |
| MCL | 967.16 * (24.49–5388.68) | 2248.37 * (1161.76–3927.45) |
| CLL/SLL | 695.97 * (143.53–2033.93) | 438.53 * (264.03–684.82) |
| PTCL | 1331.76 * (834.61–2016.3) | 1544.74 * (1184.34–1980.28) |
| Radiation | No Radiation | |
|---|---|---|
| SMR (95% CI) | SMR (95% CI) | |
| Second cancer death | ||
| Oral cavity and pharynx | 4.28 (0.52–15.46) | 4.14 (0.85–12.09) |
| Digestive system | 2.28 * (1.21–3.9) | 2.54 * (1.59–3.85) |
| Respiratory system | 2.67 * (1.33–4.78) | 1.58 (0.76–2.91) |
| Bones and joints | 5.46 (0.14–30.4) | 11.87 * (2.45–34.68) |
| Soft tissue including heart | 11.11 * (3.61–25.93) | 1.56 (0.04–8.67) |
| Melanoma | 1.38 (0.03–7.7) | 0.97 (0.02–5.4) |
| Breast | 1.75 (0.57–4.09) | 1.85 (0.74–3.81) |
| Gynecologic and female genital | 1.75 (0.36–5.13) | 3.63 * (1.57–7.16) |
| Testicular and male genital | 3.35 (0.08–18.67) | 4.12 (0.5–14.87) |
| Urinary system | 2.72 (0.33–9.81) | 0.87 (0.02–4.87) |
| Brain and other nervous system | 0.71 (0.02–3.97) | 1.99 (0.54–5.1) |
| Hematopoietic # | 16.52 * (10.35–25.01) | 45.84 * (36.81–56.41) |
| Myeloma | 4.42 (0.11–24.62) | 5.49 (0.66–19.83) |
| Leukemia | 19 * (11.76–29.04) | 55.16 * (44.18–68.04) |
| ALL | 12.92 * (2.66–37.75) | 34.59 * (17.27–61.89) |
| AML | 19.51 * (9.36–35.89) | 22.2 * (12.69–36.06) |
| CLL | 97 * (26.43–248.37) | 632.21 * (455.64–854.57) |
| CML | 0 (0–43.79) | 23.06 * (4.76–67.4) |
| Noncancer deaths | ||
| Infection | 14.67 * (12.01–17.74) | 33.04 * (29.85–36.48) |
| CVD | 2.42 * (1.82–3.16) | 2.8 * (2.28–3.42) |
| Suicide and self-inflicted injury | 1.08 (0.56–1.89) | 1.2 (0.72–1.88) |
| Diabetes mellitus | 1.31 (0.36–3.35) | 1.49 (0.6–3.06) |
| Chronic liver disease and cirrhosis | 0.46 (0.06–1.65) | 0.62 (0.17–1.58) |
| Chronic obstructive pulmonary disease and allied conditions | 1.61 (0.33–4.71) | 2.51 * (1.01–5.16) |
| Nephritis, nephrotic syndrome, and nephrosis | 0.95 (0.02–5.3) | 6.6 * (3.29–11.81) |
| Accidents and adverse effects | 0.62 * (0.36–0.99) | 1.02 (0.73–1.39) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; You, L.; Hu, X. Role of Radiation Therapy in Mortality among Adolescents and Young Adults with Lymphoma: Differences According to Cause of Death. Cancers 2022, 14, 5067. https://doi.org/10.3390/cancers14205067
Yin X, You L, Hu X. Role of Radiation Therapy in Mortality among Adolescents and Young Adults with Lymphoma: Differences According to Cause of Death. Cancers. 2022; 14(20):5067. https://doi.org/10.3390/cancers14205067
Chicago/Turabian StyleYin, Xuejiao, Liangshun You, and Xuelian Hu. 2022. "Role of Radiation Therapy in Mortality among Adolescents and Young Adults with Lymphoma: Differences According to Cause of Death" Cancers 14, no. 20: 5067. https://doi.org/10.3390/cancers14205067
APA StyleYin, X., You, L., & Hu, X. (2022). Role of Radiation Therapy in Mortality among Adolescents and Young Adults with Lymphoma: Differences According to Cause of Death. Cancers, 14(20), 5067. https://doi.org/10.3390/cancers14205067





