Stereotactic Body Radiotherapy for Renal Cell Carcinoma in Patients with Von Hippel–Lindau Disease—Results of a Prospective Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Pre-Treatment Evaluations
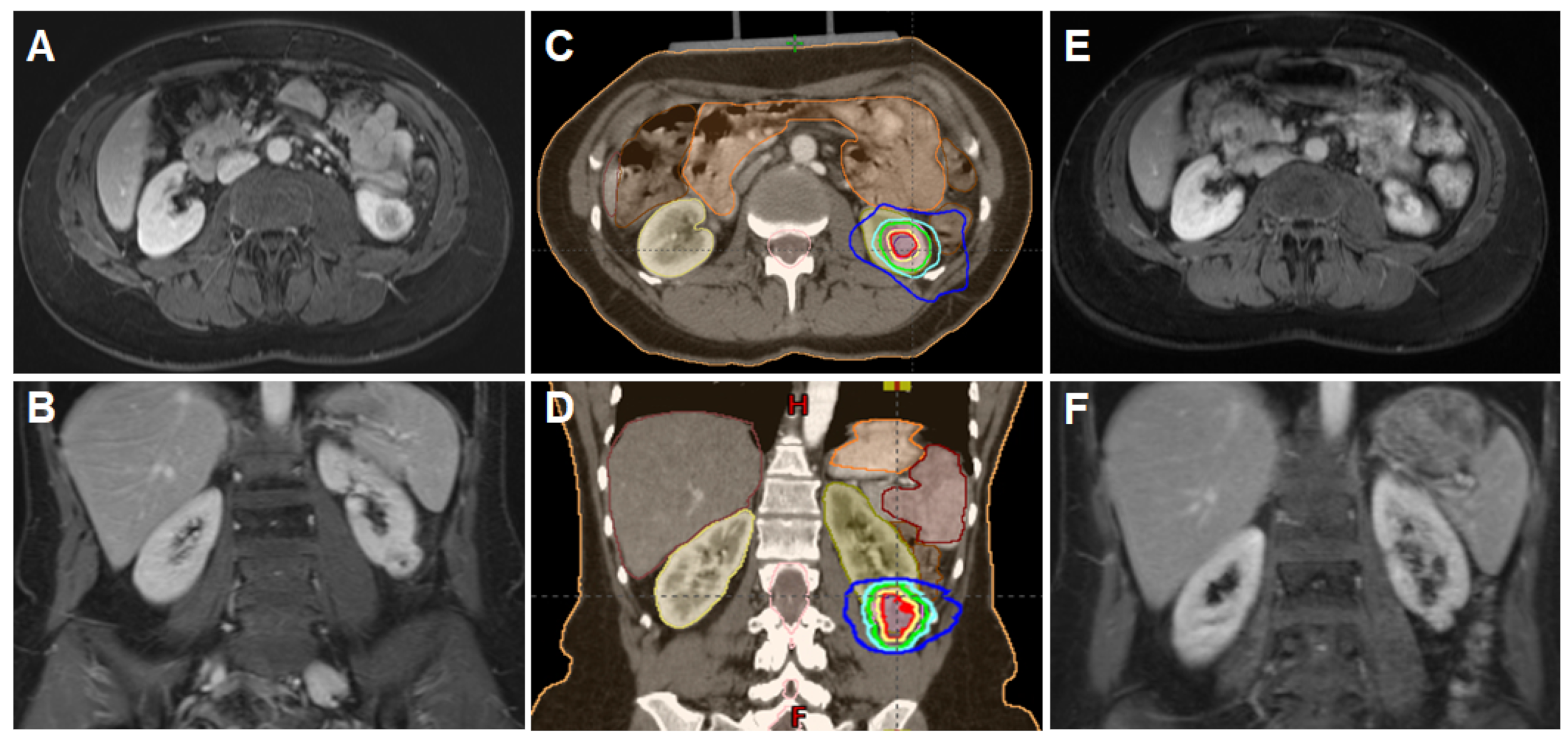
2.3. Immobilization, Planning and Delivery of Stereotactic Body Radiotherapy
2.4. Response Evaluation and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Outcomes and Toxicity
3.3. Effect on Kidney Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seizinger, B.R.; Rouleau, G.A.; Ozelius, L.J.; Lane, A.H.; Farmer, G.E.; Lamiell, J.M.; Haines, J.; Yuen, J.W.M.; Collins, D.; Majoor-Krakauer, D.; et al. Von Hippel–Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature 1988, 332, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.-M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Maher, E.R.; Iselius, L.; Yates, J.R.; Littler, M.; Benjamin, C.; Harris, R.; Sampson, J.; Williams, A.; Ferguson-Smith, M.A.; Morton, N. Von Hippel-Lindau disease: A genetic study. J. Med. Genet. 1991, 28, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Hippel, E.V. Über eine sehr seltene Erkrankung der Netzhaut. Albrecht Graefes Arch. Ophthalmol. 1904, 59, 83–106. [Google Scholar] [CrossRef] [Green Version]
- Lindau, A. Zur Frage der Angiomatosis retinae und ihrer Hirnkomplikationen. Acta Ophthalmol. 1926, 4, 193–226. [Google Scholar] [CrossRef]
- Varshney, N.; Kebede, A.A.; Owusu-Dapaah, H.; Lather, J.; Kaushik, M.; Bhullar, J.S. A Review of Von Hippel-Lindau Syndrome. J. Kidney Cancer VHL 2017, 4, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Chittiboina, P.; Lonser, R.R. Von Hippel-Lindau disease. Handb. Clin. Neurol. 2015, 132, 139–156. [Google Scholar] [PubMed] [Green Version]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. Von Hippel-Lindau disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Pantuck, A.J.; An, J.; Liu, H.; Rettig, M.B. NF-κB–Dependent Plasticity of the Epithelial to Mesenchymal Transition Induced by Von Hippel-Lindau Inactivation in Renal Cell Carcinomas. Cancer Res. 2010, 70, 752–761. [Google Scholar] [CrossRef]
- Jilg, C.A.; Neumann, H.P.; Gläsker, S.; Schäfer, O.; Leiber, C.; Ardelt, P.U.; Schwardt, M.; Schultze-Seemann, W. Nephron sparing surgery in von Hippel-Lindau associated renal cell carcinoma; clinicopathological long-term follow-up. Fam. Cancer 2012, 11, 387–394. [Google Scholar] [CrossRef]
- Van Poppel, H.; Becker, F.; Cadeddu, J.A.; Gill, I.S.; Janetschek, G.; Jewett, M.A.S.; Pilar Laguna, M.; Marberger, M.; Montorsi, F.; Polascik, T.J. Treatment of localised renal cell carcinoma. Eur. Urol. 2011, 60, 662–672. [Google Scholar] [CrossRef]
- Uhlig, J.; Strauss, A.; Rucker, G.; Seif Amir Hosseini, A.; Lotz, J.; Trojan, L.; Kim, H.S.; Uhlig, A. Partial nephrectomy versus ablative techniques for small renal masses: A systematic review and network meta-analysis. Eur. Radiol. 2019, 29, 1293–1307. [Google Scholar] [CrossRef]
- Allasia, M.; Soria, F.; Battaglia, A.; Gazzera, C.; Calandri, M.; Caprino, M.P.; Lucatello, B.; Velrti, A.; Maccario, M.; Pasini, B.; et al. Radiofrequency Ablation for Renal Cancer in Von Hippel–Lindau Syndrome Patients: A Prospective Cohort Analysis. Clin. Genitourin. Cancer 2018, 16, 28–34. [Google Scholar] [CrossRef]
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Teh, B.S.; McGarry, R.C.; Cardenes, H.R.; Timmerman, R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2009, 7, 44–54. [Google Scholar] [CrossRef]
- Correa, R.J.M.; Louie, A.V.; Zaorsky, N.G.; Lehrer, E.J.; Ellis, R.; Ponsky, L.; Kaplan, I.; Mahadevan, A.; Chu, W.; Swaminath, A.; et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2019, 5, 958–969. [Google Scholar] [CrossRef]
- Rühle, A.; Andratschke, N.; Siva, S.; Guckenberger, M. Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin. Transl. Radiat. Oncol. 2019, 18, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Siva, S.; Kothari, G.; Muacevic, A.; Louie, A.V.; Slotman, B.J.; Teh, B.S.; Lo, S.S. Radiotherapy for renal cell carcinoma: Renaissance of an overlooked approach. Nat. Rev. Urol. 2017, 14, 549–563. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The Renal nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kjaer, M.; Iversen, P.; Hvidt, V.; Bruun, E.; Skaarup, P.; Bech Hansen, J.; Frederiksen, P.L. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma: A study by the Copenhagen Renal Cancer Study Group. Scand. J. Urol. Nephrol. 1987, 21, 285–289. [Google Scholar] [CrossRef]
- Juusela, H.; Malmio, K.; Alfthan, O.; Oravisto, K.J. Preoperative Irradiation in the Treatment of Renal Adenocarcinoma. Scand. J. Urol. Nephrol. 1977, 11, 277–281. [Google Scholar] [CrossRef]
- Ali, M.; Mooi, J.; Lawrentschuk, N.; McKay, R.R.; Hannan, R.; Lo, S.S.; Hall, W.A.; Siva, S. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur. Urol. 2022. [Google Scholar] [CrossRef]
- Grubb, W.R.; Ponsky, L.; Lo, S.S.; Kharouta, M.; Traughber, B.; Sandstrom, K.; MacLennan, G.T.; Shankar, E.; Gupta, S.; Machtay, M.; et al. Final results of a dose escalation protocol of stereotactic body radiotherapy for poor surgical candidates with localized renal cell carcinoma. Radiother. Oncol. 2020, 155, 138–143. [Google Scholar] [CrossRef]
- Swaminath, A.; Cheung, P.; Glicksman, R.; Donovan, E.; Niglas, M.; Vesprini, D.; Kapoor, A.; Erler, D.; Chu, W. Patient-reported quality of life following stereotactic body radiation therapy for primary kidney cancer–results from a prospective cohort study. Clin. Oncol. 2021, 33, 468–475. [Google Scholar] [CrossRef]
- Margulis, V.; Freifeld, Y.; Pop, L.M.; Manna, S.; Kapur, P.; Pedrosa, I.; Christie, A.; Mohamad, O.; Mannala, S.; Singla, N.; et al. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus—Safety Lead-in Results of a Phase 2 Trial. Int. J. Radiat. Oncol. 2021, 110, 1135–1142. [Google Scholar] [CrossRef]
- Siva, S.; Jackson, P.; Kron, T.; Bressel, M.; Lau, E.; Hofman, M.; Shaw, M.; Chander, S.; Pham, D.; Lawrentschuk, N.; et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose–response relationship. Radiother. Oncol. 2016, 118, 540–546. [Google Scholar] [CrossRef]
- Siva, S.; Pham, D.; Kron, T.; Bressel, M.; Lam, J.; Tan, T.H.; Chesson, B.; Shaw, M.; Chander, S.; Gill, S.; et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: A prospective clinical trial. Br. J. Urol. 2017, 120, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Maher, E.R.; Neumann, H.P.; Richard, S. von Hippel–Lindau disease: A clinical and scientific review. Eur. J. Hum. Genet. 2011, 19, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Walther, M.M.; Lubensky, I.A.; Venzon, D.; Zbar, B.; Linehan, W.M. Prevalence of Microscopic lesions in Grossly Normal Renal Parenchyma from Patients with von Hippel-Lindau Disease, Sporadic Renal Cell Carcinoma and No Renal Disease. J. Urol. 1995, 154, 2010–2014. [Google Scholar] [CrossRef]
- Yang, B.; Autorino, R.; Remer, E.; Laydner, H.; Hillyer, S.; Altunrende, F.; White, M.A.; Khanna, R.; Stein, R.J.; Haber, G.-P.; et al. Probe ablation as salvage therapy for renal tumors in von Hippel-Lindau patients: The Cleveland Clinic experience with 3 years follow-up. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 686–692. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, C.K. Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: Preliminary results. J. Urol. 2010, 183, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Donovan, E.K.; Xie, F.; Louie, A.V.; Chu, W.; Siva, S.; Kapoor, A.; Swaminath, A. Cost Effectiveness Analysis of Radiofrequency Ablation (RFA) Versus Stereotactic Body Radiotherapy (SBRT) for Early Stage Renal Cell Carcinoma (RCC). Clin. Genitourin. Cancer 2022, 20, e353–e361. [Google Scholar] [CrossRef] [PubMed]
- Staehler, M.; Bader, M.; Schlenker, B.; Casuscelli, J.; Karl, A.; Roosen, A.; Stief, C.G.; Bex, A.; Wowra, B.; Muacevic, A. Single Fraction Radiosurgery for the Treatment of Renal Tumors. J. Urol. 2015, 193, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Louie, A.V.; Warner, A.; Muacevic, A.; Gandhidasan, S.; Ponsky, L.; Ellis, R.; Kaplan, I.; Mahadevan, A.; Chu, W.; et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer 2018, 124, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Siva, S.; Chesson, B.; Bressel, M.; Pryor, D.; Higgs, B.; Reynolds, H.M.; Hardcastle, N.; Montgomery, R.; Vanneste, B.; Khoo, V.; et al. TROG 15.03 phase II clinical trial of Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney—FASTRACK II. BMC Cancer 2018, 18, 1030. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.R.M.; Brook, A.; Powell, M.F.; Kaliannan, K.; Wagner, A.A.; Kaplan, I.D.; Pedrosa, I. Effect of Stereotactic Body Radiotherapy on the Growth Kinetics and Enhancement Pattern of Primary Renal Tumors. Am. J. Roentgenol. 2016, 206, 544–553. [Google Scholar] [CrossRef]
- Frick, M.A.; Chhabra, A.M.; Lin, L.; Simone, I.C.B. First Ever Use of Proton Stereotactic Body Radiation Therapy Delivered with Curative Intent to Bilateral Synchronous Primary Renal Cell Carcinomas. Cureus 2017, 9, e1799. [Google Scholar] [CrossRef] [PubMed]
- Svedman, C.; Karlsson, K.; Rutkowska, E.; Sandström, P.; Blomgren, H.; Lax, I.; Wersäll, P. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol. 2008, 47, 1578–1583. [Google Scholar] [CrossRef]



| Median (Range) | |||
|---|---|---|---|
| Age at radiotherapy [years] | 44 (36–56) | ||
| Body Mass Index | 23.3 (20.3–25.8) | ||
| n | % | ||
| Gender | male | 3 | 42.9 |
| female | 4 | 57.1 | |
| ECOG performance status | 0 | 4 | 57.1 |
| 1 | 3 | 42.9 | |
| Age-adjusted Charlson Comorbiditiy Index | 2 | 6 | 85.7 |
| 3 | 1 | 14.3 | |
| Prior kidney surgeries | partial nephrectomy | 3 | 42.9 |
| adrenalectomy | 1 | 14.3 | |
| none | 3 | 42.9 | |
| Kidney cysts | multiple bilateral cysts | 5 | 71.4 |
| solitary unilateral cyst | 1 | 14.3 | |
| no cysts | 1 | 14.3 | |
| Laterality | left-sided | 4 | 57.1 |
| right-sided | 3 | 42.9 | |
| Relative function of the treated kidney [%] | 49 (21–55) | ||
| Median (range) | |||
| Tumor size, largest diameter [cm] | 2.8 cm (1.9–3.5) | ||
| Renal Nephrometry Score | 8.5 (4–10) | ||
| n | % | ||
| Renal Nephrometry Score (Complexity) | low | 2 | 25.0 |
| intermediate | 4 | 50.0 | |
| high | 2 | 25.0 | |
| Median (Range) | |||
|---|---|---|---|
| Radiotherapy dose EQD2 (α/β = 10) [Gy] | 85.9 (83.3–87.5) | ||
| Radiotherapy dose BED [Gy] | 103.0 (103.0–123.8) | ||
| GTV [ccm] | 9.3 (3.5–14.3) | ||
| ITV [ccm] | 13.2 (5.6–22.1) | ||
| PTV [ccm] | 27.0 (15.6–44.0) | ||
| n | |||
| Fractionation | 5 × 10 Gy | 7 | |
| 8 × 7.5 Gy | 1 | ||
| Acute | n | % | |
|---|---|---|---|
| CTCAE grade 0 | 4 | 57.1 | |
| CTCAE grade 1 | 3 | 42.9 | |
| CTCAE grade 2–5 | 0 | 0 | |
| Chronic | n | % | |
| CTCAE grade 0 | 7 | 0 | |
| CTCAE grade 1–5 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirste, S.; Rühle, A.; Zschiedrich, S.; Schultze-Seemann, W.; Jilg, C.A.; Neumann-Haefelin, E.; Lo, S.S.; Grosu, A.-L.; Kim, E. Stereotactic Body Radiotherapy for Renal Cell Carcinoma in Patients with Von Hippel–Lindau Disease—Results of a Prospective Trial. Cancers 2022, 14, 5069. https://doi.org/10.3390/cancers14205069
Kirste S, Rühle A, Zschiedrich S, Schultze-Seemann W, Jilg CA, Neumann-Haefelin E, Lo SS, Grosu A-L, Kim E. Stereotactic Body Radiotherapy for Renal Cell Carcinoma in Patients with Von Hippel–Lindau Disease—Results of a Prospective Trial. Cancers. 2022; 14(20):5069. https://doi.org/10.3390/cancers14205069
Chicago/Turabian StyleKirste, Simon, Alexander Rühle, Stefan Zschiedrich, Wolfgang Schultze-Seemann, Cordula A. Jilg, Elke Neumann-Haefelin, Simon S. Lo, Anca-Ligia Grosu, and Emily Kim. 2022. "Stereotactic Body Radiotherapy for Renal Cell Carcinoma in Patients with Von Hippel–Lindau Disease—Results of a Prospective Trial" Cancers 14, no. 20: 5069. https://doi.org/10.3390/cancers14205069





